
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Julia Jerzykiewicz | + 3857 word(s) | 3857 | 2022-01-06 04:48:38 | | | |
| 2 | Amina Yu | Meta information modification | 3857 | 2022-01-10 03:32:19 | | |
Video Upload Options
Lipopolyplexes based on poliethylenimine are an interesting platform for future anti-cancer gene therapies. The carrier consists of nucleic acids condensed with poliethylenimine chains and enclosed in lipid vesicles. Lipopolyplexes could be very versatile, what enables tailoring the carrier for specific thereapeutic needs, however the preparation process is a multistage and fairly sensitive one, which additionally requires a specific balance to be maintained between its stability in the body, which would allow the appropriate dose of the preparation to reach the target site, and the ability to release nucleic acid at the right place and time.
1. Polyethyleneimine-Based Lipopolyplexes as Nucleic Acid Carriers
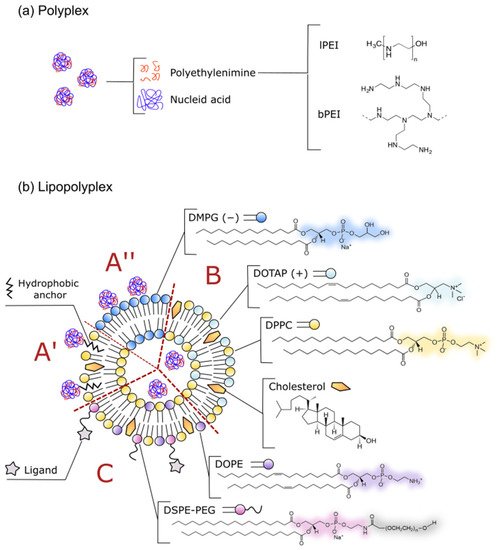
2. Composition of an Effective Lipopolyplex Based on Polyethyleneimine
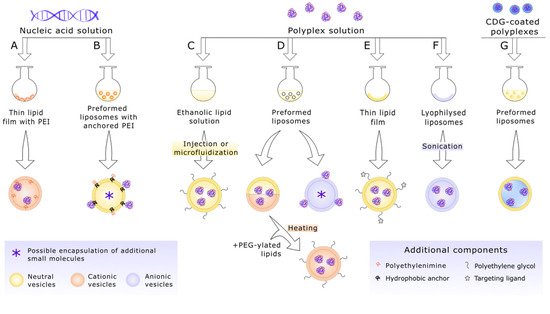
3. Selected In Vivo Studies on Anticancer PEI-Based Lipopolyplexes
4. Summary
The preparation of polyethylenimine-based lipopolyplexes is a multistage and fairly sensitive process, which additionally requires a specific balance to be maintained between its stability in the body, which would allow the appropriate dose of the preparation to reach the target site, and the ability to release nucleic acid at the right place and time. However, as the above examples of research show, such a carrier is a promising platform for future anti-cancer gene therapies, especially due to its versatility. The possibility to select from a broad array of components (DNA in the form of plasmid or oligonucleotides, small RNAs, polyethylenimines of various length and topology, lipids and/or additional targeting ligands) and exchange or modify them without affecting the overall carrier’s functionality makes it possible to tailor a lipopolyplex suitable for a given application. Moreover, lipopolyplexes’ performance in vivo is superior to bare polyplexes or nucleic acids, and even though no formulation has yet reached the transfection effectiveness of viral carriers, they are able to safely deliver therapeutic nucleic acids, notwithstanding the manner in which these molecules are associated with the carrier (i.e. inside a lipid vesicle or on its surface). Finally, the repeatedly emphasized modularity and flexibility of lipopolyplexes allow considerable room for improvement of these carriers in the coming years.
References
- Ewe, A.; Schaper, A.; Barnert, S.; Schubert, R.; Temme, A.; Bakowsky, U.; Aigner, A. Storage stability of optimal liposome-polyethylenimine complexes (lipopolyplexes) for DNA or siRNA delivery. Acta Biomater. 2014, 10, 2663–2673.
- Hanzlíková, M.; Soininen, P.; Lampela, P.; Männistö, P.T.; Raasmaja, A. The role of PEI structure and size in the PEI/liposome-mediated synergism of gene transfection. Plasmid 2009, 61, 15–21.
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286.
- Ewe, A.; Panchal, O.; Pinnapireddy, S.R.; Bakowsky, U.; Przybylski, S.; Temme, A.; Aigner, A. Liposome-polyethylenimine complexes (DPPC-PEI lipopolyplexes) for therapeutic siRNA delivery in vivo. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 209–218.
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315.
- Needham, D.; McIntosh, T.J.; Lasic, D.D. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. BBA Biomembr. 1992, 1108, 40–48.
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207.
- Linder, B.; Weirauch, U.; Ewe, A.; Uhmann, A.; Seifert, V.; Mittelbronn, M.; Harter, P.N.; Aigner, A.; Kögel, D. Therapeutic targeting of stat3 using lipopolyplex nanoparticle-formulated sirna in a syngeneic orthotopic mouse glioma model. Cancers 2019, 11, 333.
- Toporkiewicz, M.; Meissner, J.; Matusewicz, L.; Czogalla, A.; Sikorski, A.F. Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: Principles, hopes, and challenges. Int. J. Nanomed. 2015, 10, 1399–1414.
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640.
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574.
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931.
- Bofinger, R.; Zaw-Thin, M.; Mitchell, N.J.; Patrick, P.S.; Stowe, C.; Gomez-Ramirez, A.; Hailes, H.C.; Kalber, T.L.; Tabor, A.B. Development of lipopolyplexes for gene delivery: A comparison of the effects of differing modes of targeting peptide display on the structure and transfection activities of lipopolyplexes. J. Pept. Sci. 2018, 24, e3131.
- Shabana, A.M.; Xu, B.; Schneiderman, Z.; Ma, J.; Chen, C.C.; Kokkoli, E. Targeted liposomes encapsulating mir-603 complexes enhance radiation sensitivity of patient-derived glioblastoma stem-like cells. Pharmaceutics 2021, 13, 1115.
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370.
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2020, 124, 13–26.
- Ganesh, K.; Basnet, H.; Kaygusuz, Y.; Laughney, A.M.; He, L.; Sharma, R.; O’Rourke, K.P.; Reuter, V.P.; Huang, Y.-H.; Turkekul, M.; et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 2020, 1, 28–45.
- Gao, D.; Mittal, V.; Ban, Y.; Lourenco, A.R.; Yomtoubian, S.; Lee, S. Metastatic tumor cells—Genotypes and phenotypes. Front. Biol. 2018, 13, 277–286.
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28.
- Dai, Z.; Wu, C. How does DNA complex with polyethylenimine with different chain lengths and topologies in their aqueous solution mixtures? Macromolecules 2012, 45, 4346–4353.
- Choosakoonkriang, S.; Lobo, B.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Biophysical characterization of PEI/DNA complexes. J. Pharm. Sci. 2003, 92, 1710–1722.
- Chytła, A.; Gajdzik-Nowak, W.; Biernatowska, A.; Sikorski, A.F.; Czogalla, A. High-level expression of palmitoylated MPP1 recombinant protein in mammalian cells. Membranes 2021, 11, 715.
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-viral in vitro gene delivery: It is now time to set the bar! Pharmaceutics 2020, 12, 183.
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual salt and pH induced changes in polyethylenimine solutions. PLoS ONE 2016, 11, e0158147.
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275.
- Wagner, M.; Rinkenauer, A.C.; Schallon, A.; Schubert, U.S. Opposites attract: Influence of the molar mass of branched poly(ethylene imine) on biophysical characteristics of siRNA-based polyplexese. RSC Adv. 2013, 3, 12774–12785.
- Lampela, P.; Soininen, P.; Urtti, A.; Männistö, P.T.; Raasmaja, A. Synergism in gene delivery by small PEIs and three different nonviral vectors. Int. J. Pharm. 2004, 270, 175–184.
- Lampela, P.; Elomaa, M.; Ruponen, M.; Urtti, A.; Männistö, P.T.; Raasmaja, A. Different synergistic roles of small polyethylenimine and Dosper in gene delivery. J. Control. Release 2003, 88, 173–183.
- Kunath, K.; von Harpe, A.; Fischer, D.; Petersen, H.; Bickel, U.; Voigt, K.; Kissel, T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: Comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J. Control. Release 2003, 89, 113–125.
- Xun, M.M.; Xiao, Y.P.; Zhang, J.; Liu, Y.H.; Peng, Q.; Guo, Q.; Wu, W.-X.; Xu, Y.; Yu, X.-Q. Low molecular weight PEI-based polycationic gene vectors via Michael addition polymerization with improved serum-tolerance. Polymer 2015, 65, 45–54.
- Eigner, A.; Fischer, D.; Merdan, T.; Brus, C.; Kissel, T.; Czubayko, F. Delivery of unmodified bioactive ribozymes by an RNA stabilizing polyethylenimine LMW PEI efficiently down regulates gene expression. Gene Ther. 2002, 9, 1700–1707.
- Itaka, K.; Harada, A.; Yamasaki, Y.; Nakamura, K.; Kawaguchi, H.; Kataoka, K. In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J. Gene Med. 2004, 6, 76–84.
- Dai, Z.; Gjetting, T.; Mattebjerg, M.A.; Wu, C.; Andresen, T.L. Elucidating the interplay between DNA-condensing and free polycations in gene transfection through a mechanistic study of linear and branched PEI. Biomaterials 2011, 32, 8626–8634.
- Dunlap, D.D.; Maggi, A.; Soria, M.R.; Monaco, L. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997, 25, 3095–3101.
- Reisinger, H.; Steinfellner, W.; Katinger, H.; Kunert, R. Serum-free transfection of CHO cells with chemically defined transfection systems and investigation of their potential for transient and stable transfection. Cytotechnology 2009, 60, 115–123.
- Sundaram, S.; Lee, L.K.; Roth, C.M. Interplay of polyethyleneimine molecular weight and oligonucleotide backbone chemistry in the dynamics of antisense activity. Nucleic Acids Res. 2007, 35, 4396–4408.
- Lehner, R.; Wang, X.; Hunziker, P. Plasmid linearization changes shape and efficiency of transfection complexes. Eur. J. Nanomed. 2013, 5, 205–212.
- Kwok, A.; Hart, S.L. Comparative structural and functional studies of nanoparticle formulations for DNA and siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 210–219.
- Juszkiewicz, K.; Sikorski, A.F.; Czogalla, A. Building blocks to design liposomal delivery systems. Int. J. Mol. Sci. 2020, 21, 9559.
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schäfer, J.; Bakowsky, U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101.
- Wang, L.L.; Feng, C.L.; Zheng, W.S.; Huang, S.; Zhang, W.X.; Wu, H.N.; Zhan, Y.; Han, Y.-X.; Wu, S.; Jiang, J.-D. Tumor-selective lipopolyplex encapsulated small active RNA hampers colorectal cancer growth in vitro and in orthotopic murine. Biomaterials 2017, 141, 13–28.
- Heyes, J.; Palmer, L.; Chan, K.; Giesbrecht, C.; Jeffs, L.; MacLachlan, I. Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Mol. Ther. 2007, 15, 713–720.
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoellerbauer, P.; Mims, A.; Klisovic, R.; Walker, A.R.; Chan, K.K.; et al. Targeted delivery of microRNA-29b by transferrin-conjugated anionic lipopolyplex nanoparticles: A novel therapeutic strategy in acute myeloid leukemia. Clin. Cancer Res. 2013, 19, 2355–2367.
- Perche, F.; Clemençon, R.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Pichon, C. Neutral lipopolyplexes for in vivo delivery of conventional and replicative RNA vaccine. Mol. Ther. Nucleic Acids 2019, 17, 767–775.
- Ko, Y.T.; Bhattacharya, R.; Bickel, U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J. Control. Release 2009, 133, 230–237.
- Schäfer, J.; Höbel, S.; Bakowsky, U.; Aigner, A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials 2010, 31, 6892–6900.
- Du, Z.; Munye, M.M.; Tagalakis, A.D.; Manunta, M.D.I.; Hart, S.L. The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci. Rep. 2014, 4, 7107.
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control. Release 2016, 236, 1–14.
- Jilek, J.L.; Zhang, Q.Y.; Tu, M.J.; Ho, P.Y.; Duan, Z.; Qiu, J.X.; Yu, A.M. Bioengineered let-7c inhibits orthotopic hepatocellular carcinoma and improves overall survival with minimal immunogenicity. Mol. Ther. Nucleic Acids 2019, 14, 498–508.
- Song, H.; Wang, G.; He, B.; Li, L.; Li, C.; Lai, Y.; Xu, X.; Gu, Z. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int. J. Nanomed. 2012, 7, 4637–4648.
- Petrek, H.; Ho, P.Y.; Batra, N.; Tu, M.J.; Zhang, Q.; Qiu, J.X.; Yu, A.M. Single bioengineered ncRNA molecule for dual-targeting toward the control of non-small cell lung cancer patient-derived xenograft tumor growth. Biochem. Pharmacol. 2021, 189, 114392.
- Zhang, Q.-Y.; Ho, P.Y.; Tu, M.J.; Jilek, J.L.; Chen, Q.X.; Zeng, S.; Yu, A.M. Lipidation of polyethylenimine-based polyplex increases serum stability of bioengineered RNAi agents and offers more consistent tumoral gene knockdown in vivo. Int. J. Pharm. 2018, 547, 537.
- García, L.; Buñuales, M.; Düzgüneş, N.; de Ilarduya, C.T. Serum-resistant lipopolyplexes for gene delivery to liver tumour cells. Eur. J. Pharm. Biopharm. 2007, 67, 58–66.
- Meissner, J.M.; Toporkiewicz, M.; Czogalla, A.; Matusewicz, L.; Kuliczkowski, K.; Sikorski, A.F. Novel antisense therapeutics delivery systems: In vitro and in vivo studies of liposomes targeted with anti-CD20 antibody. J. Control. Release 2015, 220, 515–528.
- Penacho, N.; Simões, S.; de Lima, M.C.P. Polyethylenimine of various molecular weights as adjuvant for transfection mediated by cationic liposomes. Mol. Membr. Biol. 2009, 26, 249–263.
- Ahmed, S.; Salmon, H.; Distasio, N.; Do, H.D.; Scherman, D.; Alhareth, K.; Tabrizian, M.; Mignet, N. Viscous core liposomes increase siRNA encapsulation and provides gene inhibition when slightly positively charged. Pharmaceutics 2021, 13, 479.
- Schwabe, K.; Ewe, A.; Kohn, C.; Loth, T.; Aigner, A.; Hacker, M.C.; Schulz-Siegmund, M. Sustained delivery of siRNA poly- and lipopolyplexes from porous macromer-crosslinked gelatin gels. Int. J. Pharm. 2017, 526, 178–187.
- Zhupanyn, P.; Ewe, A.; Büch, T.; Malek, A.; Rademacher, P.; Müller, C.; Reinert, A.; Jaimes, Y.; Aigner, A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J. Control. Release 2020, 319, 63–76.
- Pelisek, J.; Gaedtke, L.; DeRouchey, J.; Walker, G.F.; Nikol, S.; Wagner, E. Optimized lipopolyplex formulations for gene transfer to human colon carcinoma cells under in vitro conditions. J. Gene Med. 2006, 8, 186–197.
- Cho, S.K.; Dang, C.; Wang, X.; Ragan, R.; Kwon, Y.J. Mixing-sequence-dependent nucleic acid complexation and gene transfer efficiency by polyethylenimine. Biomater. Sci. 2015, 3, 1124–1133.
- Xue, Y.; Feng, J.; Liu, Y.; Che, J.; Bai, G.; Dong, X.; Wu, F.; Jin, T. A synthetic carrier of nucleic acids structured as a neutral phospholipid envelope tightly assembled on polyplex surface. Adv. Healthc. Mater. 2020, 9, 1901705.
- Kumar, K.; Vulugundam, G.; Kondaiah, P.; Bhattacharya, S. Co-liposomes of redox-active alkyl-ferrocene modified low MW branched PEI and DOPE for efficacious gene delivery in serum. J. Mater. Chem. B 2015, 3, 2318–2330.
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163.
- Sabín, J.; Vázquez-Vázquez, C.; Prieto, G.; Bordi, F.; Sarmiento, F. Double charge inversion in polyethylenimine-decorated liposomes. Langmuir 2012, 28, 10534–10542.
- Opanasopit, P.; Paecharoenchai, O.; Rojanarata, T.; Ngawhirunpat, T.; Ruktanonchai, U. Type and composition of urfactants mediating gene transfection of polyethylenimine-coated liposomes. Int. J. Nanomed. 2011, 6, 975–983.
- Ma, K.; Shen, H.; Shen, S.; Xie, M.; Mao, C.; Qiu, L.; Jin, Y. Development of a successive targeting liposome with multi-ligand for efficient targeting gene delivery. J. Gene Med. 2011, 13, 290–301.
- Samaddar, S.; Mazur, J.; Boehm, D.; Thompson, D.H. Development and in vitro characterization of bladder tumor cell targeted lipid-coated polyplex for dual delivery of plasmids and small molecules. Int. J. Nanomed. 2019, 14, 9547–9561.
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746.
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397.
- Wang, Y.; Wang, R.; Wu, S.; An, J.; Liang, Y.; Hou, L.; Zhang, Z. Self-responsive co-delivery system for remodeling tumor intracellular microenvironment to promote PTEN-mediated anti-tumor therapy. Nanoscale 2020, 12, 9392–9403.
- Mendes, L.P.; Sarisozen, C.; Luther, E.; Pan, J.; Torchilin, V.P. Surface-engineered polyethyleneimine-modified liposomes as novel carrier of siRNA and chemotherapeutics for combination treatment of drug-resistant cancers. Drug Deliv. 2019, 26, 443.




