
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christine Dinh | + 5458 word(s) | 5458 | 2021-12-06 02:40:21 | | | |
| 2 | Conner Chen | -175 word(s) | 5283 | 2022-01-10 01:46:00 | | | | |
| 3 | Conner Chen | -175 word(s) | 5283 | 2022-01-10 01:48:32 | | |
Video Upload Options
Head and neck cancers are the 6th most common cancers in the United States and responsible for nearly 2% of all deaths related to cancer. Approximately 95% of all head and neck cancers are squamous cell carcinoma (SCC); of which, oral cavity SCC is the most common (excluding non-melanoma cutaneous cancers). The incidence of oral cavity cancer has gradually increased over the past 20 years. Globally, there were more than 375,000 new cases of oral cavity cancers (including cancers of the lip) and more than 175,000 deaths in 2020.
1. Anatomy and Innervation of the Oral Cavity
For oncologic purposes, the oral cavity is divided into distinct subsites: upper and lower mucosal lip, oral tongue (anterior two-thirds), floor of mouth (FOM), buccal mucosa, hard palate, maxillary and mandibular alveolar ridge, and retromolar trigone. Consistent with its importance in taste, swallowing, and speech, the oral cavity has abundant motor, sensory, and special sensory innervation (Figure 1) [1] . The oral tongue receives sensory innervation from the lingual nerve (cranial nerve V3) and special sensory gustatory innervation from the chorda tympani (cranial nerve VII). Motor innervation of the intrinsic and extrinsic musculature of the oral tongue – responsible for tongue mobility - is predominantly provided by the hypoglossal nerve (cranial nerve XII), except for the palatoglossus which is innervated by the vagus nerve (cranial nerve X). Sensory innervation to the rest of the oral cavity is supplied by branches of the trigeminal nerve (cranial nerve V). The hard palate, upper teeth, upper gingiva, and upper lip are supplied by several nerves that originate from the maxillary nerve (cranial nerve V2), while the mandibular nerve (cranial nerve V3) provides sensory innervation to the retromolar trigone and buccal mucosa via the buccal nerves, the lower teeth via the inferior alveolar nerves, and the lower gingiva and mucosal lip via the mental nerves [2][3]. Given this rich innervation, it is unsurprising that perineural invasion (PNI) in oral cavity SCC can manifest with a variety of sensory and/or motor deficits. These include oral pain or paresthesia, taste disturbances, tongue weakness or atrophy, changes in speech, dysphagia, and facial palsy.
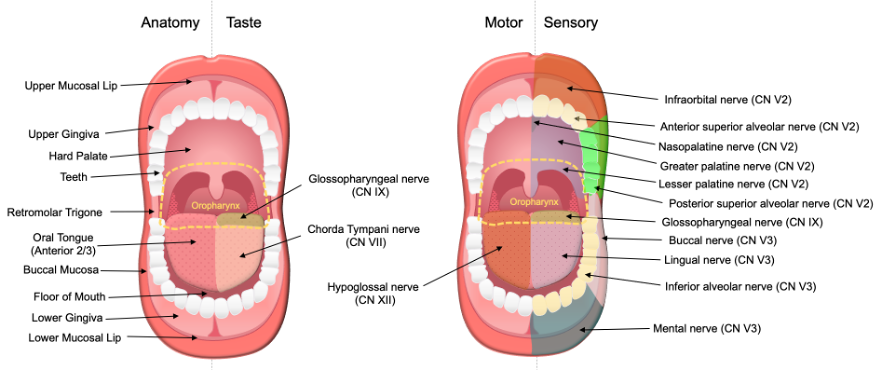
Figure 1. Anatomy and Innervation of the Oral Cavity. The oral cavity cancer site consists of several subsites: oral tongue (anterior two-thirds), floor of mouth, hard palate, retromolar trigone, buccal mucosa, upper and lower mucosal lip, and gingiva. The oral tongue (anterior two-thirds) receives taste innervation from the chorda tympani (CN VII). The posterior 1/3 of the tongue belongs to the oropharynx cancer site and receives taste and sensory innervations by the glossopharyngeal nerve (CN IX). Motor innervation of the tongue is primarily supplied by the hypoglossal nerves (CN XII). Sensory innervation of the oral cavity is supplied by the maxillary (CN V2) and mandibular (CN V3) nerves. Reprinted from Misztal CI, Green C, Mei C, et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers (Basel). 2021;13(23) [1].
2. Epidemiology
Other than cutaneous cancers, oral cavity SCC is the most common malignancy of the head and neck [4]. Within the oral cavity, incidence of SCC by subsite differs worldwide in part due to the impact of varying cultural practices resulting in different environmental exposures. In Western society, the tongue is the most common site, with a majority of SCC occurring along the lateral and ventrolateral aspects. The next most common site is the floor of the mouth, with the buccal mucosa, gingiva, and hard palate being less common [4]. However, in areas where betel quid chewing (a practice similar to chewing tobacco) is customary such as Southeast Asia, oral cavity SCC of the buccal mucosa is most common [5].
The incidence of oral cavity cancer has unfortunately increased over the past 20 years. In the United States alone, the estimated number of new cases of oral cavity and pharyngeal cancer in 2021 was 54,010 people, with approximately 10,850 deaths, making them the 8th leading source of new cancer cases and deaths in the United States [6]. Worldwide, there were more than 375,000 new cases of oral cavity cancers in 2020, with more than 175,000 deaths [7].
3. Risk Factors
Tobacco consumption is the most significant risk factor for oral cavity squamous cell carcinoma. Tobacco intake via smoking, as well as smokeless tobacco, can cause a plethora of intracellular changes leading to unregulated cell proliferation and ultimately SCC [8]. A pooled analysis from the International Head and Neck Cancer Epidemiology Consortium in 2013 reported increased head and neck cancer risk among exclusive cigarette, exclusive cigar, and exclusive pipe use, with odds ratios (OR) of 3.93 (95% Confidence Interval [CI]: 3.47, 4.22), 3.49 (95%CI: 2.58, 4.73), and 3.71 (95%CI: 2.59, 5.33), respectively [9]. Although cigarette consumption decreased 38.7% over the past two decades, cigarettes remain the most common combustible tobacco product used . In contrast, there has been a 117% increase in large cigar and pipe tobacco use and a 23.1% increase in smokeless tobacco in the United States [10].
Alcohol consumption is also strongly associated with an increased risk of oral and pharyngeal cancer. In a systematic review, the relative risk of developing oral and pharyngeal cancer with alcohol consumption (> 4 drinks per day) ranged from 3.2 to 9.2, when adjusted for potential confounders, including tobacco smoking. There is also a strong dose-response relationship between intensity of alcohol use and risk of oral cavity and pharyngeal SCC [11]. It is important to note that the combination of tobacco and alcohol consumption exhibits a strong synergistic effect, meaning that the risk of oral cavity SCC for individuals who smoke tobacco and drink alcohol is more than the sum of their risks alone. Mello et al. conducted a systematic review of 33 published manuscripts and a meta-analysis consisting of 15 of those publications to determine the risk of developing oral SCC by alcohol and tobacco consumption. The odds of developing oral SCC were greatly increased with synergistic consumption of alcohol, smoked tobacco, and smokeless tobacco (OR=16.17, 95%CI: 7.97, 32.79; p<0.01), when compared to alcohol alone (OR=1.58, 95%CI: 0.77, 3.25; p=0.22), smoked tobacco alone (OR=2.68, 95%CI: 1.90, 3.78; p<0.01), or smokeless tobacco alone (OR=7.00, 95%CI: 4.18, 11.74; p<0.01) [12].
Outside of the United States, another significant risk factor to consider is betel quid (paan), which is frequently chewed in parts of India and southeast Asia. Betel quid is often mixed with other carcinogens such as tobacco and crushed areca nut (also known as betel nut). When combined with smoking tobacco and alcohol, betel quid has a synergistic effect on the risk of oral cancer occurrence [13]. A meta-analysis of observational studies in Southeast Asia by Petti et al. reported the ORs to be 2.20 (95%CI: 1.62, 2.98) for alcohol consumption, 3.63 (95%CI: 1.94, 7.04) for tobacco smoking, and 7.90 (95%CI: 6.71, 9.30) for betel quid chewing. Combined alcohol-tobacco-betel quid use had a significant synergistic effect on oral cancer risk (OR=40.09, 95%CI: 35.06, 45.83) [13]. Thus, it is important to determine regional risk factors when evaluating patients with oral cavity masses. Figure 2 demonstrates the major risk factors for oral cavity SCC.
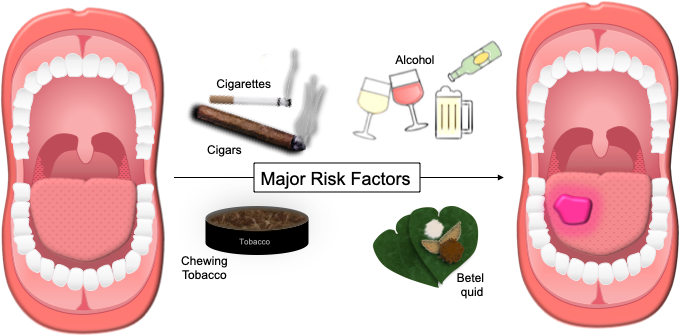
Figure 2. Major Risk Factors for Oral SCC. Tobacco (smoking and smokeless), alcohol consumption, and betel quid are the most significant risks factors associated with the development of oral cavity SCC. Synergistic effects have been demonstrated for combined alcohol-tobacco-betel quid use and alcohol-smokeless tobacco-smoking tobacco use.
Human Papilloma Virus (HPV) infection is another factor worth mentioning as the incidence of oral cavity HPV infection has risen with time [14]. Although HPV is an established cause of oropharyngeal SCC (currently classified as HPV related or non HPV related), its role in oral cavity SCC is less clear [15]. Risk factors for oropharyngeal HPV+ SCC include earlier age of sexual behaviors, increased number of sexual partners, and increased lifetime exposures to these behavior [16] . Although vaccination against HPV has not yet been shown to prevent oropharyngeal SCC, HPV vaccination can protect against oral transmission of HPV infection that causes oropharyngeal SCC [17][18]. In a large systematic review and meta-analysis evaluating the prevalence of HPV in head and neck SCC, Ndiaye et al. showed that 6.8% of oral cavity SCCs express both p16 (surrogate marker for HPV) and HPV DNA (deoxyribonucleic acid), compared to 39.7% in oropharyngeal SCC [14]. HPV+ and HPV- oropharyngeal SCCs are two distinct entities, and the overall and disease-free survival outcomes for advanced stage HPV+ oropharyngeal SCC are higher than HPV- oropharyngeal SCC [4][19]. However, survival in oral cavity SCC is not dependent on HPV status, and staging and treatment of oral cavity SCC is not tailored to HPV status like oropharyngeal cancer [19].
Although tobacco and alcohol are the major risks factors for oral cavity SCC, other potential risk factors are: (1) diet low in fruits and vegetables and high in animal products, (2) vitamin D deficiency, (3) immunosuppressed individuals, such as those with HIV (human deficiency virus) or organ transplantation, (4) exposures to carcinogenic heavy metals, and (5) rare genetic disorders, such as Fanconi anemia [4] and Li Fraumeni [20]. Chronic trauma due to local irritation from poorly fitted dentures, poor dental hygiene, or trauma has been proposed to play a role in oral carcinogenesis, however there is a lack of high quality evidence to support this association [21].
4. Clinical Presentation
Oral cavity SCC can present as a variety of distinctive lesions. White or red mucosal changes - referred to as leukoplakia or erythroplakia respectively – are commonly seen. Although often premalignant in nature, these lesions require biopsy due to their potential for malignant transformation. These lesions can be found throughout the oral cavity, involving the tongue, floor of mouth, gums, hard palate, or buccal mucosa [22]. Other presentations include lumps, mucosal thickening, or persistent sores or ulcers that fail to resolve or grow over time [23]. Patients with oral cavity cancer can also present with a variety of symptomatic complaints resulting from tumor mass effect, local tissue invasion, nerve involvement, and tumor associated metabolic changes. These include severe oral pain, loosening of the teeth, dysgeusia, tongue fixation, trismus, changes in speech, otalgia, weight loss, oral bleeding, neck masses, and sensory or motor deficits in the distributions of cranial nerves V, VII, X, and XII including muscular weakness and atrophy, numbness, and paresthesia [2][3][24].
5. Diagnosis
Diagnosing oral cavity SCC begins with obtaining a thorough history. The history should elicit information about lesion onset, evolution with time, associated symptoms, patient risk factors, and prior oncologic history. Next, a methodical head and neck physical exam should be performed. Critical elements include (1) direct visual inspection and palpation of the lesion of interest to gauge its extent and involvement of surrounding structures, (2) a cranial nerve exam to assess for nerve involvement, and (3) palpation of the neck to assess for cervical lymphadenopathy suspicious for regional metastasis. Depending on the location of the lesion, flexible fiberoptic laryngoscopy is also utilized to better assess the full extent of the lesion and its proximity to other structures and the airway for surgical planning, as well as to rule out additional primary lesions in the upper aerodigestive tract. This latter role is especially important in patients who use alcohol and tobacco because they have higher rates of synchronous malignancies due to field cancerization [25][26].
There are a variety of benign oral cavity lesions that may appear similar to SCC, including aphthous ulcers, oral lichen planus, hairy leukoplakia, pyogenic granulomas and necrotizing sialometaplasia [27][28]. However, persistent lesions that fail to resolve spontaneously, progressively worsen over a period of weeks, or are accompanied by concerning symptoms such as sensory or motor deficits should be biopsied to rule out malignancy. In patients with palpable neck masses, fine needle aspiration with cytologic analysis may be diagnostic and can help distinguish between neoplastic and inflammatory processes.
After biopsy, oral SCC is classified microscopically by assessment of the degree of keratinization (i.e., keratin pearls), cellular and nuclear pleomorphism and mitotic activity, characterizing tumor grade as follows: grade 1 (well differentiated, 75% keratinization), grade 2 (moderately differentiated, 25% - 75% keratinization), or grade 3 (poorly differentiated, less than 25% keratinization) [29]. Well- and moderately-differentiated tumors are grouped together as low grade neoplasms while poorly differentiated and undifferentiated tumors are considered high grade neoplasms [29]. A variety of histological subtypes of SCC exist based on morphology such as basaloid, verrucous, spindle cell, papillary, adenosquamous, acantholytic, and cuniculatum [30]. On fine needle aspiration, a cervical lymph node positive for oral SCC may show squamous cells with pyknotic hyperchromatic nuclei, small amounts of keratinized cytoplasm, nuclear atypia, and pleomorphism in low-grade disease. Giant cells, keratin plaques, and necrosis can be observed in high-grade disease [31]. Figure 3 shows a histologic section of oral cavity squamous cell carcinoma stained with hematoxylin and eosin.
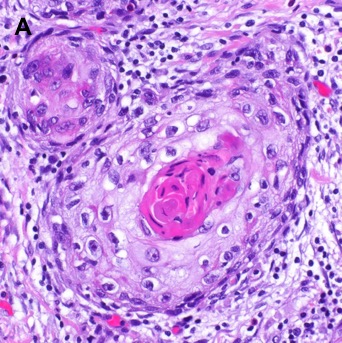
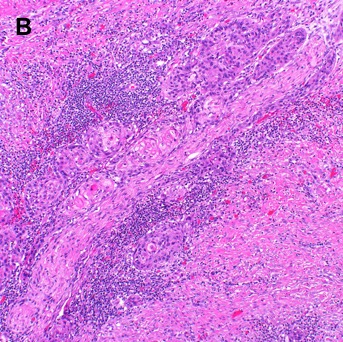
Figure 3. Histological Section of Oral Cavity Squamous Cell Carcinoma with Hematoxylin and Eosin Staining. [A] Moderately differentiated squamous cell carcinoma shows islands of malignant epithelial cells with keratin pearl formation. A lymphocytic infiltrate is noted associated with small tumor nests (60x). [B] Moderately differentiated squamous cell carcinoma associated with stromal desmoplasia and a lymphocytic infiltrate is noted associated with small tumor nests surrounding a nerve (20x).
Molecular hallmarks of oral SCC include deleterious changes in tumor biomarkers cyclin D1, p53, retinoblastoma, epidermal growth factor receptor, signal transducer and activator of transcription 3, and vascular endothelial growth factor [32][33]. Increased tumor proliferation marker Ki-67 expression is strongly associated with increased rate of recurrence and decreased survival [34][35][36]. The histological presence or absence of perineural, bone, or lympho-vascular invasion must be reported as they are associated with poorer outcomes and influence choice of adjuvant therapy [37][38][39]. Positive p16 staining, which is routinely performed for oropharyngeal SCC due to significant differences in clinical outcomes between p16- and HPV associated p16+ subtypes, is less relevant for oral cavity SCC due to low rates of p16 and HPV positivity and unclear clinical significance [19].
To better evaluate the extent of the primary lesion, its proximity to critical structures such as major vessels, and to assess for lymphadenopathy not appreciable on physical exam, computed tomography (CT) scan of the head and neck with contrast is commonly ordered as part of the initial workup. Compared to other imaging modalities, CT is especially sensitive for detecting osseous involvement, particularly cortical erosions of the mandible and maxilla [40]. Significant bone involvement in oral cancer affects staging and surgical planning. Figure 4 shows CT images of a FOM SCC with tumor enhancement and cortical erosion of the mandible.
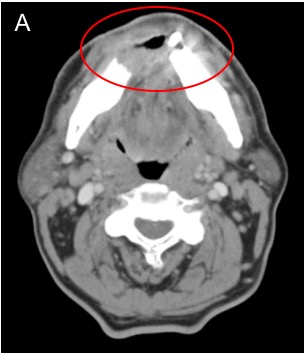
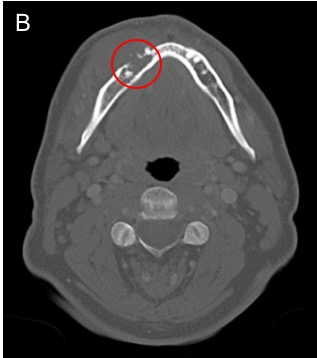
Figure 4. Computed Tomography (CT) of Oral Cavity Squamous Cell Carcinoma (SCC). [A] Axial CT head with contrast demonstrates an ill-defined enhancing mass involving the anterior floor of mouth. [B] Axial CT head windowed for bone shows osseous erosion through the cortex of the mandible from alveolar SCC.
Compared to CT, Magnetic resonance (MR) imaging is better at characterizing soft tissue involvement. As such MRI is the imaging modality of choice for evaluating nerve involvement – with findings such as nerve enlargement and enhancement suggesting PNI – and for better delineating extent of soft tissue invasion [40][41]. CT of the chest with or without contrast or positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with CT (18F-FDG PET/CT) are tests utilized for detecting distant metastases in patients with advanced nodal disease or identifying primary lung cancers in smokers, both of which have implications for treatment planning [42]. Figure 5 demonstrates radiographic evidence of PNI on MR imaging in a patient with left buccal SCC [1].
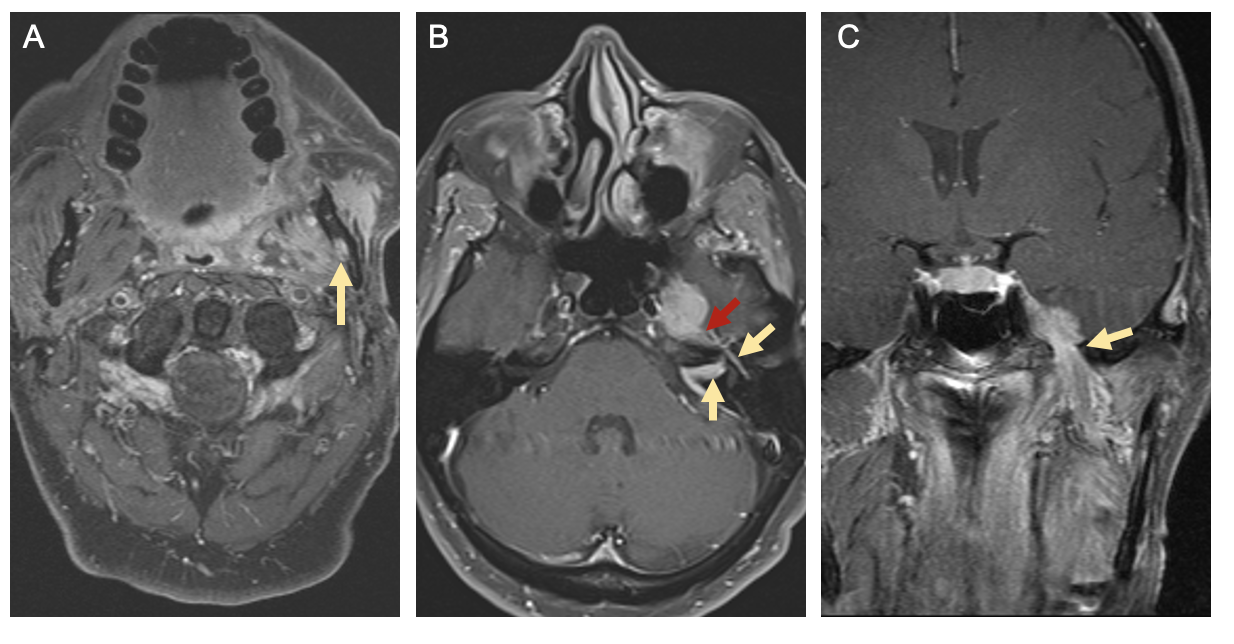
Figure 5. Radiographic Signs of Perineural Spread. T1-weighted magnetic resonance (MR) images with gadolinium from a patient with left buccal squamous cell carcinoma and perineural invasion. [A] Axial image showing enlargement and enhancement of the left inferior alveolar canal (yellow arrow). [B] Axial image showing enhancement of the intracanalicular and tympanic segments of the facial nerve (yellow arrows) as well as the greater superficial petrosal nerve (red arrow). [C] Coronal image showing enlargement and tumor involvement of the mandibular branch of the left trigeminal nerve at the foramen ovale (yellow arrow). Reprinted from Misztal CI, Green C, Mei C, et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers (Basel). 2021;13(23) [1].
Finally, examination under general anesthesia - involving direct laryngoscopy, bronchoscopy, and esophagoscopy - can be useful for some patients. It provides further clarity regarding the boundaries of the tumor and its extension into neighboring subsites, improves assessment of the upper aerodigestive tract in patients at high risk for synchronous malignancy, and enables biopsy in patients for whom in office biopsies were non-diagnostic or unable to be obtained [43][44].
6. Staging
Consensus staging criteria for oral cavity SCC comes from The American Joint Committee on Cancer (AJCC) Staging of Oral Cavity Cancers, the 8th and most recent edition of which was published in 2018 (Table 1) [45]. Compared to the 7th edition, a number of new criteria have been added to the staging guidelines in order to reflect the latest research on factors which effect disease specific survival. For primary tumor (T) staging, depth of invasion (DOI) is now included as an equally important factor along with maximum tumor dimension [46][47]. For nodal (N) staging, the presence or absence of extracapsular nodal extension (ENE) is now included because of its association with worse disease-specific survival [48]. Notably, N staging categories are now also separated for patients treated without cervical lymph node dissection (clinical, cN) and those treated with cervical lymph node dissection (pathological, pN) [45]. Based on T, N, and M stages, oral cavity SCC can be further categorized into prognostic staging groups.
Table 1. American Joint Committee on Cancer (AJCC) 8th Edition Staging Criteria for Oral Cavity Cancer
|
Primary tumor (T) |
|||
|
TX |
Primary tumor cannot be assessed |
||
|
Tis |
Carcinoma in situ |
||
|
T1 |
Tumor ≤2 cm and DOI ≤5 mm |
||
|
T2 |
Tumor ≤2 cm, DOI >5 mm and ≤10 mm or Tumor > 2 cm but ≤ 4 cm and DOI ≤ 10 mm |
||
|
T3 |
Tumor > 4 cm or Any tumor with DOI > 10 mm but ≤ 20 mm |
||
|
T4a |
Moderately Advanced Local Disease: Tumor invades adjacent structures only (e.g., through cortical bone of the mandible or maxilla, or involves the maxillary sinus or skin of the face) or Extensive tumor with bilateral tongue involvement or DOI > 20 mm |
||
|
T4b |
Very advanced local disease: Tumor invades masticator space, pterygoid plates, or skull base and/or encases the internal carotid artery |
||
|
Regional Lymph Nodes - Clinical (cN) |
|||
|
NX |
Regional lymph nodes cannot be assessed |
||
|
N0 |
No regional lymph node metastasis |
||
|
N1 |
Metastasis in a single ipsilateral lymph node, ≤3 cm and ENE– |
||
|
N2a |
Metastasis in a single ipsilateral node, >3 cm and ≤6 cm and ENE– |
||
|
N2b |
Metastases in multiple ipsilateral nodes, ≤6 cm and ENE– |
||
|
N2c |
Metastases in bilateral or contralateral lymph nodes, ≤6 cm and ENE– |
||
|
N3a |
Metastasis in a lymph node >6 cm and ENE– |
||
|
N3b |
Metastasis in any lymph node(s) with ENE+ clinically or Metastasis in a single ipsilateral node, >3 cm and ENE+ or Metastasis to multiple ipsilateral, contralateral, or bilateral nodes and ENE+ |
||
|
Regional Lymph Nodes - Pathologic (pN) |
|||
|
NX |
Regional lymph nodes cannot be assessed |
||
|
N0 |
No regional lymph node metastasis |
||
|
N1 |
Metastasis in a single ipsilateral lymph node, ≤3 cm and ENE– |
||
|
N2a |
Metastasis in a single ipsilateral node, <3 cm and ENE+ or Metastasis in a single ipsilateral node >3 cm and ≤6 cm and ENE– |
||
|
N2b |
Metastases in multiple ipsilateral nodes, <6 cm and ENE– |
||
|
N2c |
Metastases in bilateral or contralateral lymph nodes, <6 cm and ENE– |
||
|
N3a |
Metastasis in a lymph node >6 cm and ENE– |
||
|
N3b |
Metastasis in a single ipsilateral node >3 cm with ENE+ or Metastasis to multiple ipsilateral, contralateral, or bilateral nodes, any with ENE+ or Metastasis to a single contralateral node of any size and ENE+ |
||
|
Distant Metastasis (M) |
|||
|
M0 |
No distant metastases |
||
|
M1 |
Distant metastases |
||
|
Prognostic Stage Groups |
|||
|
Stage 0 |
Tis |
N0 |
M0 |
|
Stage I |
T1 |
N0 |
M0 |
|
Stage II |
T2 |
N0 |
M0 |
|
Stage III |
T3 |
N0 |
M0 |
|
T1-T3 |
N1 |
M0 |
|
|
Stage IVa |
T4a |
N0 |
M0 |
|
T4a |
N1 |
M0 |
|
|
T1-T4a |
N2 |
M0 |
|
|
Stage IVb |
Any T |
N3 |
M0 |
|
T4b |
Any N |
M0 |
|
|
Stage IVc |
Any T |
Any N |
M1 |
|
Abbreviations: DOI, depth of invasion; ENE, extranodal extension. Adapted from Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Chicago, IL: American College of Surgeons; 2017 45. |
|||
7. Treatment Overview
The main treatment options utilized for patients with oral cavity SCC are surgery, radiation therapy, chemotherapy, and combinations thereof. Based on the National Comprehensive Cancer Center Guidelines, appropriate primary and adjuvant treatment depends largely on the clinical stage of the cancer and presence of adverse pathological features [49]. Adverse features include ENE, positive margins, close margins (less than 5mm), pT3 or pT4 primary tumors, pN2 or pN3 nodal disease, nodal disease in levels IV or V, vascular invasion, lymphatic invasion, and PNI.
Early stage, localized tumors (T1-2, N0) can be treated with either surgical resection or definitive radiotherapy. However, surgery is generally preferred because of the high rate of osteoradionecrosis of the mandible with definitive radiotherapy that in turn may require surgical management. As such radiotherapy is generally reserved for patients who are poor surgical candidates (with significant medical co-morbidities) or when surgical resection would lead to unacceptably severe functional impairment [50][51][52]. In patients with clinically N0 necks, management depends in part on T staging. In early stage tumors (T1-T2), elective neck dissection involving levels I-III or I-IV (ipsilateral or bilateral depending on the location of the primary tumor), sentinel lymph node biopsy, or close observation may be considered, with the final decision being influenced by tumor location, depth of invasion, imaging and biopsy findings, and patient preference [49][53].
More advanced tumors (T3-T4a) are usually treated with definitive surgical resection of the primary lesion with ipsilateral and/or bilateral neck dissections for levels I-IV, as are any tumors with clinically apparent nodal disease [49][53][54]. For advanced tumors with no adverse features, adjuvant therapy with radiotherapy alone may be considered versus surgical management alone. In the case of ENE and/or positive margins after surgical resection, adjuvant therapy generally consists of concurrent chemoradiation. For other adverse features such as PNI or advanced nodal disease, adjuvant therapy may consist of either radiotherapy alone or concurrent chemoradiation [49]. The decision for adjuvant therapy should be made using a multidisciplinary team approach that includes specialists in head and neck surgical, medical, and radiation oncology.
There are multiple options for the treatment of very advanced tumors without distant metastasis (T4b, any N; unresectable nodal disease). These include concurrent chemoradiation, induction chemotherapy followed by radiation alone or chemoradiation, definitive radiotherapy, or palliative radiation or chemotherapy, with the choice of therapy in part dependent on patient performance status [49]. Other systemic therapies including immunotherapy may also be utilized both within and outside clinical trials.
8. Treatment Considerations
Appropriate surgical resection depends in part on the location of the primary tumor. In general, an oncologically sound resection consists of 1 cm clinical margins around the tumor where possible. Surgical resection may also require marginal mandibulectomy if the tumor abuts the mandible periosteum, or segmental mandibulectomy if it invades through periosteum into bone. Similarly, maxillectomy may be necessary for tumors that invade the upper gums or hard palate [55]. Furthermore, clearing nerve margins with PNI may require more extensive resection at the skull base even if the primary tumor itself is clear, often with neurosurgery on standby if such involvement is suspected preoperatively.
It is also critical to have a reconstructive plan prior to surgery to ensure maximal restoration of function and tissue coverage of critical structures after the anticipated oncologic resection. Reconstruction is approached as a ladder of escalating interventions, beginning with primary closure, healing by secondary intention, or skin grafts / skin substitute grafts for smaller defects, and moving up to local tissue transfers and/or free tissue transfer with or without vascularized bone in order to restore function and cosmesis in the case of larger defects [56][57][58].
The need for and choice of adjuvant therapy for the primary tumor and neck is determined largely on the final pathological evaluation of surgical specimens, with the decision made by a multidisciplinary team that includes head and neck surgical, medical, and radiation oncology specialists. Adjuvant radiotherapy or chemoradiation is utilized postoperatively to treat possible microscopic disease and decrease risk of locoregional recurrence. Dosing for post-operative radiation – delivered either via intensity-modulated radiation therapy (IMRT) or three-dimensional conformal radiation therapy – can vary, with dose at each region decided based on a risk assessment. High risk areas with adverse features such as positive margins receive 60-66 Gy in 2 Gy fractions, while low-to-intermediate risk areas of suspected subclinical spread may receive between 44-50 Gy in 2 Gy fractions up to 54-63 Gy in 1.6-1.8 Gy fractions [49]. High-dose cisplatin is the preferred chemotherapeutic agent for post-operative chemoradiation for oral cavity SCC [59][60][61]. Other chemotherapeutic agents, including other platinum-based (carboplatin), taxane-based (docetaxel, paclitaxel), and pyrimidine-based treatments (5-fluorouracil), are also utilized, though less commonly as adjuvant therapy [49].
For locally advanced disease not amenable to surgical resection, concurrent chemoradiation with high dose cisplatin is the most common treatment modality. Compared to adjuvant therapy total radiation dose is generally higher – ranging from 66 Gy in 2.2 Gy fractions to 70 Gy in 2 Gy fractions. Hyperfractionation or accelerated fractionation protocols may also be utilized. Induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil is sometimes used, though the benefits over conventional chemoradiation have not been established [49][62][63].
In addition to chemotherapy and radiation, the last several decades have brought significant developments in targeted therapies for head and neck SCC, with the goal of improving overall survival while having fewer side effects than conventional therapies. Cetuximab is an anti-EGFR immunoglobulin G1 monoclonal antibody approved for the treatment of head and neck SCC. EGFR are cell surface receptors that are overexpressed in up to 90% of head and neck SCC, with over expression of EGFR associated with poorer disease-free and cause-specific survival outcomes [64][65]. Cetuximab is utilized both as a radiosensitizer for the treatment of locoregionally advanced head and neck SCC, and as a monotherapy for patients with recurrent or metastatic cancer who have failed cisplatin chemotherapy [66][67]. However, several studies have found inferior overall survival in patients with locally advanced head and neck SCC treated with cetuximab and radiotherapy compared to those treated with conventional concurrent chemoradiation [68][69].
Another class of targeted therapy utilized in the treatment of oral SCC are called checkpoint inhibitors, which target immune checkpoints and play key roles in regulating the immune system [70]. Oral SCC can express the checkpoint molecule PD-1 and its ligand PD-L1, with higher expression associated with increased disease progression and decreased survival [70]. As such, the PD-1 inhibitor nivolumab and the PD-L1 inhibitor pembrolizumab have begun to be used in the treatment of oral SCC. Currently, pembrolizumab and nivolumab are approved for the treatment of cisplatin-refractory recurrent or metastatic head and neck SCC, and pembrolizumab is also approved as a first-line therapy for PD-L1+ recurrent or metastatic SCC [71][72]. Work is ongoing on the use of checkpoint inhibitors as neoadjuvant therapy prior to surgical treatment of oral SCC [73][74].
Given variable response among patients treated with targeted therapies, there is significant interest in identifying biomarkers that may predict expected efficacy in order to better guide treatment decision making. To date, no molecular marker including level of EGFR expression has been validated as a predictor for clinical response to cetuximab, though a metanalysis of 13 clinical trials found that development of cetuximab-induced skin rash is associated with improved overall and progression free survival [75][76][77]. Level of PD-L1 expression has been clinically validated as a biomarker for efficacy of treatment with pembrolizumab, with use of pembrolizumab as first-line monotherapy in recurrent or metastatic head and neck SCC approved only in patients with a PD-L1 combined positive score >1 [71][72]. However, studies have found significant heterogeneity in measured PD-L1 expression both intratumorally within different tumor cores, and between different testing protocols, underscoring the difficulties in implementing biomarkers clinically [78][79].
9. Prognosis
The relative 5-year survival rate for oral cavity and oropharyngeal cancers in the United States has improved significantly over time as documented by the Surveillance, Epidemiology and End Results (SEER) database, from 53% in 1975-1977 to 67% in 2011-2017 [80]. This likely reflects the substantial changes in management of oral cavity and pharyngeal SCC in the past few decades, with advances in imaging, the introduction of 18F-FDG-PET imaging, the widespread use of microvascular free flap reconstruction, and significant progress in adjuvant therapy protocols [46]. However, prognosis for oral cavity SCC varies widely based on geographic, demographic, socioeconomic, tumor, and treatment related factors [81].
Globally, the number of oral cavity (including lip) cancers in 2020 was more than twice as high for men than women with approximately 264,000 and 114,000 new cases, respectively; however, the age-standardized mortality rate in 2020 was lower in men than women (0.3 versus 0.5, respectively) [7]. When countries were grouped by the 4-tier human development index (HDI) – a statistical tool that measures a countries overall level of social and economic development – there was unsurprisingly an inverse correlation between development level and mortality rate [82]. The age-standardized mortality rate in 2020 was 2-3 times higher in countries with a low/medium HDI compared to countries with a high/very high HDI, reflecting the impact of socioeconomics on oral cavity cancer survival. In a retrospective, population-based national study in the United States, Yu et al. analyzed the relationship between race and 5-year mortality in 7630 patients with primary oral cavity SCC treated from 2010 to 2014. When compared to whites, black and Hispanic patients were more likely to present at later stages; however, black patients had a greater 5-year cause specific mortality than white patients, which was attributable to later stage of presentation and higher rate of uninsured patients among blacks [83]. This is consistent with SEER data, which from 2011-2017 found that relative 5-year survival for oral cavity and oropharyngeal cancers by race and ethnicity ranged from 50% in black patients to 69% in non-Hispanic white patients [80]. The effect of age is controversial, with some studies showing no relationship to prognosis while others demonstrate worse overall and disease specific survival in older patients [84][85][86][87][88][89][90][91]. Other demographic factors associated with worse prognosis include alcohol, tobacco, and betel nut use [84][88][89][92].
Tumor location within the oral cavity has a large effect on prognosis, with SEER data showing a relative 5-year survival for all stages of 72% for lip SCC compared to 49% for oral tongue, 41% for FOM, and 57% for gum and other oral cavity sites [80]. Presence and number of positive lymph nodes have a strong impact on survival [93][94]. In a study of 14,554 patients from the National Cancer Data base, number of positive lymph nodes was a continuous predictor of overall survival on multivariate analysis, with estimated 5-year overall survival of 65.3% for patients with no nodal disease compared to 49.9% with one positive node and as low as 9.7% for ten or more nodes [93]. A large metanalysis found similar results, with the highest hazard ratio for overall survival and disease specific survival at a cutoff of three positive nodes [94]. Depth of tumor invasion is another independent prognostic indicator. In a multicenter retrospective study of 3781 patients, greater depth of invasion was associated with decreased disease specific survival and was found to have predictive value independent from and complementary to tumor size [46]. Other negative tumor-related prognostic factors include adverse pathologic features such as ENE, PNI, and vascular or lymphatic invasion, as well as molecular features such as expression of Ki-67, homeobox gene B7 (HOXB7), collagen type IV (ColIV), RACK1, glucose transporter 1 (GLUT1) and cyclooxygenase-2 (COX-2) [34][35][36][38][95][96][97].
Treatment-related prognostic factors include time to diagnosis and initiation of treatment . In a retrospective study of 18672 patients from the national cancer data base with primary oral cavity SCC, time to surgery was an independent predictor of overall survival, with longer delays associated with worse outcomes [98][99]. Positive or close surgical margins on final pathology are also associated with increased disease recurrence and decreased survival, and as such National Comprehensive Cancer Network guidelines recommend re-resection to negative margins if feasible [49][87][100][101]. In oral cavity SCC patients undergoing neck dissection, greater lymph node yield is associated with increased overall survival, with two large studies identifying 18 nodes removed as the minimum threshold [93][102][103]. In patients with locally advanced but resectable oral SCC, a meta-analysis of 7 studies with 714 patients found increased overall and disease specific survival at three and five years in patients treated with surgery and adjuvant radiotherapy compared to definitive radiotherapy or chemoradiation [104]. In patients with locally advanced disease receiving adjuvant therapy after definitive surgery, a meta-analysis of 6 studies (5 randomized controlled trials) with 1155 patients found improved overall survival and disease free survival with adjuvant chemoradiation compared to adjuvant radiotherapy alone [105].
References
- Misztal CI, Green C, Mei C, et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers (Basel). 2021;13(23).
- Massey BT. Physiology of oral cavity, pharynx, and upper esophageal sphincter. GI Motility online. 2006. doi:10.1038/gimo2.
- Bakst RL, Glastonbury CM, Parvathaneni U, Katabi N, Hu KS, Yom SS. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2019;103(5):1109-1124.
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65(5):401-421.
- Chen YK, Huang HC, Lin LM, Lin CC. Primary oral squamous cell carcinoma: an analysis of 703 cases in southern Taiwan. Oral Oncol. 1999;35(2):173-179.
- American Cancer Society. Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
- Jiang X, Wu J, Wang J, Huang R. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tob Induc Dis. 2019;17:29.
- Wyss A, Hashibe M, Chuang SC, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol. 2013;178(5):679-690.
- Wang TW, Kenemer B, Tynan MA, Singh T, King B. Consumption of Combustible and Smokeless Tobacco - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2016;65:1357-1363.
- Goldstein BY, Chang SC, Hashibe M, La Vecchia C, Zhang ZF. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: an update. Eur J Cancer Prev. 2010;19(6):431-465.
- Mello FW, Melo G, Pasetto JJ, Silva CAB, Warnakulasuriya S, Rivero ERC. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and meta-analysis. Clin Oral Investig. 2019;23(7):2849-2859.
- Petti S, Masood M, Scully C. The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia. A meta-analysis of observational studies. PLoS One. 2013;8(11):e78999.
- Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319-1331.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550-4559.
- Rettig E, Kiess AP, Fakhry C. The role of sexual behavior in head and neck cancer: implications for prevention and therapy. Expert Rev Anticancer Ther. 2015;15(1):35-49.
- Tumban E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses. 2019;11(10).
- Gillison M. Chapter 8. HPV vaccines and potential prevention of HPV-positive head and neck cancer. In: IARC HPV Working Group. Primary End-points for Prophylactic HPV Vaccine Trials. Lyon, France: International Agency for Research on Cancer; 2014.
- Lai K, Killingsworth M, Matthews S, et al. Differences in survival outcome between oropharyngeal and oral cavity squamous cell carcinoma in relation to HPV status. J Oral Pathol Med. 2017;46(8):574-582.
- Prime S, Thakker N, Pring M, Guest P, Paterson I. A review of inherited cancer syndromes and their relevance to oral squamous cell carcinoma. Oral oncology. 2001;37(1):1-16.
- Pentenero M, Azzi L, Lodi G, Manfredi M, Varoni E. Chronic mechanical trauma/irritation and oral carcinoma: A systematic review showing low evidence to support an association. Oral Diseases. 2021.
- The American Cancer Society. What Are Oral Cavity and Oropharyngeal Cancers? https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/what-is-oral-cavity-cancer.html#references. Published 2021. Accessed September 30, 2021.
- PDQ Adult Treatment Editorial Board. Lip and Oral Cavity Cancer Treatment (Adult) (PDQ(R)): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda (MD)2002.
- The American Cancer Society Medical and Editorial Content Team. Signs and Symptoms of Oral Cavity and Oropharyngeal Cancer. https://www.cancer.org/cancer/oral-cavp. Published 2021. Accessed October 9, 2021.
- The American Cancer Society Medical and Editorial Content Team. Tests for Oral Cavity and Oropharyngeal Cancers. https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/detection-diagnosis-staging/how-diagnosed.html. Published 2021. Accessed.
- Sabharwal R, Mahendra A, Moon NJ, Gupta P, Jain A, Gupta S. Genetically altered fields in head and neck cancer and second field tumor. South Asian J Cancer. 2014;3(3):151-153.
- Maymone MBC, Greer RO, Burdine LK, et al. Benign oral mucosal lesions: Clinical and pathological findings. J Am Acad Dermatol. 2019;81(1):43-56.
- Gonsalves WC, Chi AC, Neville BW. Common Oral Lesions: Part I. Superficial Mucosal Lesions. Am Fam Physician. 2007;75(4):501-506.
- Pindborg JJ, Reichart P, Smith C, Van der Waal I. Histological Typing of Cancer and Precancer of the Oral Mucosa: In Collaboration with LH Sobin and Pathologists in 9 Countries. Springer Science & Business Media; 2012.
- Pereira MC, Oliveira DT, Landman G, Kowalski LP. Histologic subtypes of oral squamous cell carcinoma: prognostic relevance. Journal-Canadian Dental Association. 2007;73(4):339.
- Pisharodi LR. False-negative diagnosis in fine-needle aspirations of squamous-cell carcinoma of head and neck. Diagn Cytopathol. 1997;17(1):70-73.
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nature reviews cancer. 2011;11(1):9-22.
- Choi S, Myers J. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. Journal of dental research. 2008;87(1):14-32.
- Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma. Oncology letters. 2014;8(1):7-11.
- Wang Z, Zhang B, Jiang L, et al. RACK1, an excellent predictor for poor clinical outcome in oral squamous carcinoma, similar to Ki67. European Journal of Cancer. 2009;45(3):490-496.
- Wangsa D, Ryott M, Åvall-Lundqvist E, et al. Ki-67 expression predicts locoregional recurrence in stage I oral tongue carcinoma. British journal of cancer. 2008;99(7):1121-1128.
- Jones H, Sykes A, Bayman N, et al. The impact of lymphovascular invasion on survival in oral carcinoma. Oral oncology. 2009;45(1):10-15.
- Zhu J, Zhou R, Wang Y, Yu M. Perineural invasion as a prognostic factor in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Acta Otolaryngol. 2019;139(11):1038-1043.
- Ross G, Soutar D, MacDonald D, Shoaib T, Camilleri I, Robertson A. Improved staging of cervical metastases in clinically node-negative patients with head and neck squamous cell carcinoma. Annals of surgical oncology. 2004;11(2):213-218.
- Trotta BM, Pease CS, Rasamny JJ, Raghavan P, Mukherjee S. Oral cavity and oropharyngeal squamous cell cancer: key imaging findings for staging and treatment planning. Radiographics. 2011;31(2):339-354.
- Ong CK, Chong VF. Imaging of perineural spread in head and neck tumours. Cancer Imaging. 2010;10 Spec no A:S92-98.
- Lee H, Lazor JW, Assadsangabi R, Shah J. An Imager’s Guide to Perineural Tumor Spread in Head and Neck Cancers: Radiologic Footprints on 18F-FDG PET, with CT and MRI Correlates. Journal of Nuclear Medicine. 2019;60(3):304-311.
- Koo K, Harris R, Wiesenfeld D, Iseli TA. A role for panendoscopy? Second primary tumour in early stage squamous cell carcinoma of the oral tongue. J Laryngol Otol. 2015;129 Suppl 1:S27-31.
- Rodriguez-Bruno K, Ali MJ, Wang SJ. Role of panendoscopy to identify synchronous second primary malignancies in patients with oral cavity and oropharyngeal squamous cell carcinoma. Head Neck. 2011;33(7):949-953.
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Chicago, IL: American College of Surgeons; 2017.
- International Consortium for Outcome Research in H, Neck C, Ebrahimi A, et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1138-1148.
- Zanoni DK, Patel SG, Shah JP. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr Oncol Rep. 2019;21(6):52.
- Wreesmann VB, Katabi N, Palmer FL, et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1192-1199.
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 3.2021). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Published 2021. Accessed October 23rd, 2021.
- Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J Clin Oncol. 2015;33(29):3269-3276.
- Kuhnt T, Stang A, Wienke A, Vordermark D, Schweyen R, Hey J. Potential risk factors for jaw osteoradionecrosis after radiotherapy for head and neck cancer. Radiat Oncol. 2016;11:101.
- Fujiwara RJT, Burtness B, Husain ZA, et al. Treatment guidelines and patterns of care in oral cavity squamous cell carcinoma: Primary surgical resection vs. nonsurgical treatment. Oral Oncol. 2017;71:129-137.
- Koyfman SA, Ismaila N, Crook D, et al. Management of the Neck in Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37(20):1753-1774.
- Machiels JP, Rene Leemans C, Golusinski W, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462-1475.
- Shanti RM, O'Malley BW, Jr. Surgical Management of Oral Cancer. Dent Clin North Am. 2018;62(1):77-86.
- Rigby MH, Taylor SM. Soft tissue reconstruction of the oral cavity: a review of current options. Curr Opin Otolaryngol Head Neck Surg. 2013;21(4):311-317.
- Patel UA, Hartig GK, Hanasono MM, Lin DT, Richmon JD. Locoregional Flaps for Oral Cavity Reconstruction: A Review of Modern Options. Otolaryngol Head Neck Surg. 2017;157(2):201-209.
- Haughey BH, Fredrickson JM, Lerrick AJ, Sclaroff A, Gay WD. Fibular and iliac crest osteomuscular free flap reconstruction of the oral cavity. Laryngoscope. 1994;104(11 Pt 1):1305-1313.
- Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36(5):999-1004.
- Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843-850.
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944.
- Rana A, Rana P, Gupta M, Seam R, Gupta M. Conventional chemoradiation vs. induction chemotherapy followed by conventional chemoradiation for locally advanced head and neck cancer: A prospective, randomized study. World Acad Sci J. 2020;2(6):24.
- Zhang L, Jiang N, Shi Y, Li S, Wang P, Zhao Y. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Scientific Reports. 2015;5(1):10798.
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666-2672.
- Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824-832.
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578.
- Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171-2177.
- Bauml JM, Vinnakota R, Anna Park YH, et al. Cisplatin versus cetuximab with definitive concurrent radiotherapy for head and neck squamous cell carcinoma: An analysis of Veterans Health Affairs data. Cancer. 2019;125(3):406-415.
- Stokes WA, Sumner WA, Breggren KL, et al. A comparison of concurrent cisplatin versus cetuximab with radiotherapy in locally-advanced head and neck cancer: A bi-institutional analysis. Rep Pract Oncol Radiother. 2017;22(5):389-395.
- Kujan O, van Schaijik B, Farah CS. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Cancers. 2020;12(7):1937.
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928.
- Kitamura N, Sento S, Yoshizawa Y, Sasabe E, Kudo Y, Yamamoto T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int J Mol Sci. 2020;22(1).
- Knochelmann HM, Horton JD, Liu S, et al. Neoadjuvant presurgical PD-1 inhibition in oral cavity squamous cell carcinoma. Cell Rep Med. 2021;2(10):100426-100426.
- Philips R, Han C, Swendseid B, et al. Preoperative Immunotherapy in the Multidisciplinary Management of Oral Cavity Cancer. Front Oncol. 2021;11:682075-682075.
- Abdel-Rahman O, Fouad M. Correlation of cetuximab-induced skin rash and outcomes of solid tumor patients treated with cetuximab: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2015;93(2):127-135.
- Taberna M, Oliva M, Mesía R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front Oncol. 2019;9:383.
- Fasano M, Della Corte CM, Viscardi G, et al. Head and neck cancer: the role of anti-EGFR agents in the era of immunotherapy. Ther Adv Med Oncol. 2021;13:1758835920949418.
- Crosta S, Boldorini R, Bono F, et al. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers (Basel). 2021;13(2).
- Rasmussen JH, Lelkaitis G, Håkansson K, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer. 2019;120(10):1003-1006.
- Surveillance E, and End Results (SEER) Program. Cancer Stat Facts: Oral Cavity and Pharynx. National Cancer Institute. https://seer.cancer.gov/statfacts/html/oralcav.html. Published 2021. Accessed.
- Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontology. 2006;102(1):67-76.
- United Nations Development Programme (UNDP). Human Development Report 2019. Beyond Income, Beyond Averages, Beyond Today: Inequities in Human Development in the 21st Century. http://hdr.undp.org/en/content/human-development-report-2019. Published 2019. Accessed December 30th, 2021.
- Yu AJ, Choi JS, Swanson MS, et al. Association of Race/Ethnicity, Stage, and Survival in Oral Cavity Squamous Cell Carcinoma: A SEER Study. OTO Open. 2019;3(4):2473974X19891126.
- Lo W-L, Kao S-Y, Chi L-Y, Wong Y-K, Chang RC-S. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. Journal of oral and maxillofacial surgery. 2003;61(7):751-758.
- O-charoenrat P, Pillai G, Patel S, et al. Tumour thickness predicts cervical nodal metastases and survival in early oral tongue cancer. Oral oncology. 2003;39(4):386-390.
- Nguyen TV, Yueh B. Weight loss predicts mortality after recurrent oral cavity and oropharyngeal carcinomas. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2002;95(3):553-562.
- Al-Rajhi N, Khafaga Y, El-Husseiny J, et al. Early stage carcinoma of oral tongue: prognostic factors for local control and survival. Oral oncology. 2000;36(6):508-514.
- Leite I, Koifman S. Survival analysis in a sample of oral cancer patients at a reference hospital in Rio de Janeiro, Brazil. Oral oncology. 1998;34(5):347-352.
- Ribeiro KdCB, Kowalski LP, De Oliveira MDRD. Impact of comorbidity, symptoms, and patients' characteristics on the prognosis of oral carcinomas. Archives of otolaryngology–head & neck surgery. 2000;126(9):1079-1085.
- Luryi AL, Chen MM, Mehra S, Roman SA, Sosa JA, Judson BL. Treatment Factors Associated With Survival in Early-Stage Oral Cavity Cancer: Analysis of 6830 Cases From the National Cancer Data Base. JAMA Otolaryngology–Head & Neck Surgery. 2015;141(7):593-598.
- de Morais EF, Mafra RP, Gonzaga AKG, de Souza DLB, Pinto LP, da Silveira ÉJD. Prognostic Factors of Oral Squamous Cell Carcinoma in Young Patients: A Systematic Review. Journal of Oral and Maxillofacial Surgery. 2017;75(7):1555-1566.
- Ribeiro KdCB, Kowalski LP, De Oliveira MdRD. Perioperative complications, comorbidities, and survival in oral or oropharyngeal cancer. Archives of Otolaryngology–Head & Neck Surgery. 2003;129(2):219-228.
- Ho AS, Kim S, Tighiouart M, et al. Metastatic Lymph Node Burden and Survival in Oral Cavity Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(31):3601-3609.
- Tsai T-Y, Iandelli A, Marchi F, et al. The Prognostic Value of Lymph Node Burden in Oral Cavity Cancer: Systematic Review and Meta-Analysis. The Laryngoscope. 2022;132(1):88-95.
- Itoh S, Matsui K, Furuta I, Takano Y. Immunohistochemical study on overexpression of cyclooxygenase-2 in squamous cell carcinoma of the oral cavity: its importance as a prognostic predictor. Oral oncology. 2003;39(8):829-835.
- Greenberg JS, Fowler R, Gomez J, et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2003;97(6):1464-1470.
- Li CX, Sun JL, Gong ZC, Lin ZQ, Liu H. Prognostic value of GLUT-1 expression in oral squamous cell carcinoma: A prisma-compliant meta-analysis. Medicine (Baltimore). 2016;95(45):e5324.
- Rygalski CJ, Zhao S, Eskander A, et al. Time to Surgery and Survival in Head and Neck Cancer. Ann Surg Oncol. 2021;28(2):877-885.
- Schutte HW, Heutink F, Wellenstein DJ, et al. Impact of Time to Diagnosis and Treatment in Head and Neck Cancer: A Systematic Review. Otolaryngol Head Neck Surg. 2020;162(4):446-457.
- Woolgar J, Rogers S, Lowe D, Brown J, Vaughan E. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral oncology. 2003;39(2):130-137.
- Guerra MFM, Gı́as LN, Campo FRg, Pérez JS. Marginal and segmental mandibulectomy in patients with oral cancer: a statistical analysis of 106 cases. Journal of oral and maxillofacial surgery. 2003;61(11):1289-1296.
- Zenga J, Divi V, Stadler M, et al. Lymph node yield, depth of invasion, and survival in node-negative oral cavity cancer. Oral Oncol. 2019;98:125-131.
- Lemieux A, Kedarisetty S, Raju S, Orosco R, Coffey C. Lymph Node Yield as a Predictor of Survival in Pathologically Node Negative Oral Cavity Carcinoma. Otolaryngology–Head and Neck Surgery. 2015;154(3):465-472.
- Membreno PV, Luttrell JB, Mamidala MP, et al. Outcomes of primary radiotherapy with or without chemotherapy for advanced oral cavity squamous cell carcinoma: Systematic review. Head Neck. 2021;43(10):3165-3176.
- Shang J, Gu J, Han Q, Xu Y, Yu X, Wang K. Chemoradiotherapy is superior to radiotherapy alone after surgery in advanced squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Int J Clin Exp Med. 2014;7(9):2478-2487.




