Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khursheed Ahmad Shiekh | + 2696 word(s) | 2696 | 2021-12-30 03:30:45 | | | |
| 2 | Camila Xu | Meta information modification | 2696 | 2022-01-10 03:45:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shiekh, K. Polysaccharide-Based Active Coatings. Encyclopedia. Available online: https://encyclopedia.pub/entry/17893 (accessed on 08 February 2026).
Shiekh K. Polysaccharide-Based Active Coatings. Encyclopedia. Available at: https://encyclopedia.pub/entry/17893. Accessed February 08, 2026.
Shiekh, Khursheed. "Polysaccharide-Based Active Coatings" Encyclopedia, https://encyclopedia.pub/entry/17893 (accessed February 08, 2026).
Shiekh, K. (2022, January 07). Polysaccharide-Based Active Coatings. In Encyclopedia. https://encyclopedia.pub/entry/17893
Shiekh, Khursheed. "Polysaccharide-Based Active Coatings." Encyclopedia. Web. 07 January, 2022.
Copy Citation
Polysaccharide-based active coatings supplemented with plant extracts such as cashew leaves, pomegranate peel, red roselle, apple fiber, and green tea extracts rich in phenolic compounds and their derivatives have been reported to be excellent substituents to replace chemically formulated wax coatings.
coating
polysaccharide
bioactivity
1. Introduction
Fresh fruits containing essential nutrients, vitamins, and minerals are consumed worldwide in part because of their strong antioxidant potential against chronic diseases [1]. Fresh fruit packaging materials after single use are disposed of in the environment. The application of synthetic and non-biodegradable polymer-tailored packaging materials for fresh fruit has raised potentially alarming consequences for the environment [2]. Conventional packaging materials such as glass, wood, aluminum, tin, and paper have been employed as fresh fruit containers to prevent mechanical damage during bulk transportation [3]. The innovative designs of synthetic packaging materials have been of great convenience to customers in supermarkets [4]. Synthetic packaging materials used for fruits may lack the optimum oxygen and moisture barrier properties to maintain their postharvest quality in the markets [5]. Additionally, the production of synthetic packaging materials may directly have an impact on the sustainability of non-renewable petroleum-based resources [6].
Fresh fruits typically have a short postharvest shelf life due to ongoing physiological and biochemical changes occurring in the living tissues until consumption [7]. Mechanical damages and pathological changes during improper handling and transportation have been associated with heavy economic losses [8]. Conventional synthetic waxes and chemical fungicides have been used as postharvest treatments to minimize losses in fresh fruits. These materials have been reported to cause health and environmental concerns [9]. Chemical-based coatings fortified with synthetic antimicrobial additives have been associated with the antimicrobial resistance of food borne pathogenic strains. Taking all the research challenges into consideration, the novel idea of active food coatings composed of polysaccharides supplemented with natural essential oils, phenolics, and active nanoparticles has been an effective adjunct to conventional postharvest treatments of fresh fruits [10]. Because several polysaccharides have limited barrier and mechanical properties even after the addition of bioactive compounds, the inclusion of inorganic clays [11] or nanoparticles [12] has been proposed.
During the past decade, several findings reported applications of natural and biodegradable edible coatings that have proven to be sustainable alternatives with excellent barrier properties compared to synthetic plastic packaging commonly used in the market [13]. Edible coating materials employed consisted of a wide range of plant or crustacean-based polysaccharides [14]. Hydrocolloid-based coating forming solutions prepared from starches, pectin, alginate, carboxymethyl cellulose, and chitosan have been applied to delay ripening and prevent senescence or detachment of fruit skin during postharvest storage [15]. In addition to plasticizers, emulsifiers, surfactants, and hydrophobic materials, the use of inorganic clays [11] or nanoparticles [12] have also been proposed.
Essential oils (EOs) have been incorporated as active ingredients in polysaccharide-based coating materials against oxidation of vital nutrients and bacterial and fungal growth. EOs from different herbs such as clove, lemon, cinnamon, tea tree, lavender, oregano, and peppermint are a source of diverse bioactive compounds with higher antimicrobial efficacy for the preparation of active food coating materials employed in fresh fruits [16]. Bioactivity of EOs has been documented because of antioxidant and antimicrobial functional groups present such as monoterpenes, flavonoids, aldehydes, isoflavones, carotenoids, and phenolic compounds that exhibit numerous nutraceutical properties [17]. EOs incorporated in the polysaccharide coating materials to extend the shelf life of fresh fruits have generated tremendous interest and are generally recognized as safe food coating additives [18]. EOs incorporated in polysaccharide-based coating materials may result in a hydrophobic film on the coated fruits to reduce loss of weight and firmness [19]. Biodegradable and edible coatings (BECs) containing EOs may also suppress several hormonal and enzymatic reactions triggered by contact with atmospheric oxygen during postharvest storage of fruit [20]. In addition to physiochemical quality preservation, EOs have been investigated to provide protection against a broad spectrum of food-borne spoilage and pathogenic microorganisms [21].
2. Postharvest Quality Constraints of Fresh Fruits
Fruits are commonly harvested on the basis of conventional extrinsic factors such as firmness, color, size, and shape. More recently, intrinsic factors such as nutritional and functional attributes have been considered, including minerals, vitamins, dietary fibers, and other polyphenolic constituents that exhibit beneficial health properties [22]. During postharvest handling, transportation, and bulk storage, fruits may be highly susceptible to biological and/or mechanical hazards that can affect both intrinsic and extrinsic factors [23]. In addition to improper postharvest handling of fruits, mechanical vibrations may affect the fruit quality during transportation, triggering heavy losses during longer storage periods. The quality problems that emerge in metabolically active fruits during postharvest storage include physiological deterioration and microbial deterioration as evidenced by moisture loss, softening of flesh, ripening, and decay caused by pathogenic bacterial strains, molds, or yeast rots [22][24].
2.1 Microbial and Biochemical Causes of Deterioration in Fresh Fruits
Fruits after harvesting from the field may be contaminated with pathogenic microbes, insects, and pests. Fresh fruits in unprocessed and raw form contain infectious germs on the skin of fruits that can lead to food borne diseases [25]. The microbial population is an important factor in considering the quality of the food product [26]. The low pH fruits, like ripe tomatoes, in a pH range (3.9–4.5) could inhibit the human intestinal pathogens such as Shigella and Escherichia coli O157:H7. Melons and soft fruits with a pH of 4–6 can favor the growth and survival of Botrytis cinerea and Penicillium species [27]. Pathogenic organisms are transmitted from the environment mostly during fruit harvesting from plants, post-harvest displacements, processing, and transport movements [28]. Several types of microorganisms, such as bacteria, yeasts, and fungi that cause deterioration may be transmitted during postharvest storage. Approximately 80–90% of microbial contamination in fresh fruits is due to Pseudomonas and Enterobacteriaceae (Klebsiella, Enterobacter, Citrobacter, Salmonella, Escherichia coli, Shigella, Proteus, Serratia, and other species) referred as Gram-negative bacteria [27][29]. Additionally, lactic acid bacteria, which are a natural flora of fruits, are corrosive and develop unpleasant odors [27]. Moreover, fresh fruits contaminated with fungi (Rhizopus, Penicillium, Aspergillus, and Eurotiumand Wallemia) and the yeast (Debaryomyces, Pichia, Candida, Hanseniaspora, Zygo saccharomyces), also have major role in the spoilage of fresh fruits during postharvest handling and storage [27]. The use of chemical disinfectants such as organic acids, chlorine dioxide, hydrogen peroxide, hypochlorite, sodium bisulfite, sulfur dioxide, and ozone has been proposed for reducing the bacterial population during postharvest storage [30]. Such chemical-based disinfectants have limited applications due to ill effects on human health and degradation of sensory quality in fruits [31].
Biochemical quality deterioration may depend on the storage temperature and metabolic processes occurring during respiration of living tissues in postharvest storage of fruits. Temperature is an important factor responsible for controlling metabolism of carbohydrates, lipids, and amino acids in respiring fruits. Temperate fruit crops are commonly stored at temperatures (0–1 °C) compared to the tropical or subtropical fruits that must be stored at higher temperatures (7–15 °C) to avoid losses due to chilling injury (CI) [32]. CI may alter the ripening process by damaging the external peel, inducing internal flesh browning, pitting, loss of firmness, and discoloration evidenced after the removal of fruits from cold temperature storage [32].
Appropriate storage temperatures can extend storage life by approximately 2–4 weeks for crops such as apricots, sweet cherries, and peaches, and up to several months for apples, pears, and kiwifruits [32]. The general effect of low temperature storage upregulates stress-responsive genes, blocks signal transduction of ethylene production processes affecting metabolic changes in vital components of fruits [33][34]. Various commercially important fruits, such as apples, pears, kiwifruits, bananas, and nectarines, at physiological maturity are characterized by high starch content that is converted to sugars at low temperatures during postharvest storage [35]. Induction of chilling tolerance of nectarines stored at near freezing temperatures (−1.4 °C) was shown to reduced activities of sucrose metabolism-associated enzymes that resulted in higher sucrose contents [35]. Moreover, fatty acids are essential cell membrane components forming a selectively permeable barrier between the cells in a fruit matrix. Fruits are composed of different types of fatty acids that show active roles in the biochemical quality degradation during postharvest cold storage. Peaches containing plastidic glycerolipid and triacylglycerides (TAGs) are used as a source of energy during fruit senescence [36]. Phosphatidic acid (PA) is accumulated in pineapple fruit during blackheart development at 10 °C [37]. Increased levels of phospholipase D enzyme activities have been observed in cold stored pears [38][39]. Similarly, chilling injury of “Honeycrisp” apples with soggy flesh showed elevated contents of glycerol and TAGs [40]. During postharvest storage of fruits, proteins may be degraded into free amino acids due to the activation of proteolytic enzymes. Amino acids such as Glu, Gln, Asp, and Asn contents increased in tomatoes stored at 4 °C [41]. Similar results were also documented in kiwifruit that showed increased Thr, Ile, and Val contents [42].
Additionally, temperature fluctuations during turbulent transportation may lead to mechanical bruising of fruits without any postharvest coating, thereby accelerating their decay [43]. In this regard, it is of primary concern to apply different novel coating techniques to delay ripening and senescence in fruits [44]. The aim is to eradicate biochemical quality deterioration during defective cold chain management that may accelerate the rate of respiration in living tissues and induce undesirable ripening (the main cause of senescence), thereby shortening the shelf-life of fruits [45]. Fruit ripening increases the total soluble solids resulting in higher sugar content; it involves several metabolic processes that differ between ‘climacteric’ and ‘non-climacteric’ fruits [46]. During the ripening of climacteric fruit, respiration increases until it reaches a peak, which is accompanied by an increase in ethylene production. In contrast, respiration of non-climacteric fruit does not increase during ripening, and ethylene is not required in order to complete the ripening process [47]. Regardless of the type of ripening, this process, as well as other metabolic processes that lead to deterioration, are driven by respiration. After harvest, the fresh produce continues to respire, utilizing food reserves, taking in oxygen, and releasing carbon dioxide and heat from stored carbohydrates [32]. For that reason, postharvest active coating treatments are applied on the fruit surfaces through various methods to reduce respiration, delay deterioration processes, prolong shelf life, and help to maintain produce quality.
3. Application Methods of Polysaccharide-Based Active Edible Coatings in Fresh Fruits
BECs can be applied to fresh fruits after harvesting from the plants or trees using various methods as shown in Figure 1. The selection of BECs mainly depends on the fruit surface hydrophobicity and roughness and the physical properties of the BEC such as surface tension, viscosity, density, coating emulsion stability, cost, and drying conditions for industrial application [48]. The various methods of BEC application for fresh fruits explained in this reviewed work include conventional spraying, electrospraying, dipping, spreading, brushing, and layer by layer deposition techniques, respectively (Figure 1). Spraying is a conventional technique for applying low viscous BEC solutions on the fresh fruit surface [49]. A homogenous spray with fine droplets may form a uniform layer on the fruit surface at a high-pressure atomization in the range of 60–80 psi (4.1–5.5 bar) [50]. The desirable layer of coating thickness mainly relies on the lower hydrodynamic diameter of the droplet and atomizer features (spray gun type, operating pressure, and nozzle temperature) as well as the humidity and flow rate of air or liquid in the BEC solution [51]. Conventional spraying methods applied on the rough surfaces of strawberry fruit have shown lower transfer efficiency and coating evenness compared to the electrospraying method of coating [52].
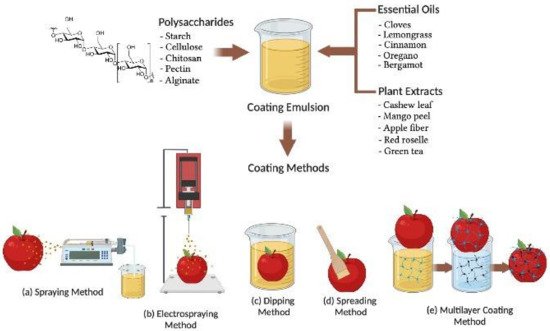
Figure 1. Schematic representation of biodegradable and edible active coating applications via (a) spraying; (b) electrospraying; (c) dipping; (d) spreading; and (e) multilayer coating methods, employed in the postharvest treatment of fruits.
Electrospraying is a novel method of coating in which a coating material is atomized in the presence of a high-intensity electric field, which enables the formation of micrometric and sub-micrometric charged droplets with an extremely narrow size distribution [53][54]. The tip of an emitter causes the formation of a Taylor cone of the nascent charged droplets and destabilizes the liquid surface to generate a cluster of charged droplets [55]. Electrospraying promotes the efficient adhesion to the surface of fresh fruit compared to conventional spraying because of electrostatic interactions of micrometric-sized charged droplets [56]. The droplet size, deposition rate, and coating thickness during electrospraying depend on the conductivity, flow rate, and viscosity of the coating solution [52]. The electrospraying coating method was employed to obtain even distribution of charged coating material droplets containing micro to nano size magnetic cellulose with special affinity to orient under an electric field, forming a compact coating film [57].
BECs applied by the dipping method undergo in three steps. The first step is immersing fresh fruits in the coating solution and holding for 2 to 3 min so the coating material can adhere on the fruit [58]. The last two steps are deposition and drainage of extra adhered BEC solution followed by evaporation and drying of coated fruit either at ambient temperature or flushed with hot air to accelerate drying [59]. Coating thickness and morphology of the coating’s material deposited by the dipping method on the surface of fruits depends on various factors such as immersion time, withdrawal speed, dip-coating cycles, density, viscosity, surface tension, and drying conditions [60][61][62]. Hydroxypropyl methylcellulose in a dip-coating solution was analyzed for viscosity, density, and surface tension during coating of Fuji apples, after which the internal oxygen and carbon dioxide levels were measured at room temperature for 4 days. Results indicated that coating thickness varied with viscosity, concentration, density, and draining time of the biopolymer solution. Coating thickness relates to the square root of viscosity and the inverse square root of draining time, which agrees with the theoretical approach for flat plate dip-coating in low-capillary-number Newtonian liquids. These results indicate the possibility of controlling coating thickness and internal gas composition based on coating solution properties [62]. The dipping compared to conventional spraying or electrospraying is more beneficial for coating fruits with complex and rough surfaces, resulting in excellent uniformity [63]. Dipping generally forms a thick coating layer on the fruit surface and may effectively reduce microbial load, contamination, respiration rate, and mechanical damage and prevent physiological changes of coated fruits [64][65].
The brushing method involves the use of a sterile brush for spreading high viscosity BECs on the fruit surface and depends on the wetting degree and the spreading rate parameters followed by a drying process [66]. Brushing of BECs is generally carried out manually by experienced operators and includes several factors to minimize manual error of BEC application and ingredient quality to achieve better coating layer uniformity [15]. The efficiency of BECs is also affected by the roughness of the fruit surface and geometry, viscosity, surface tension, density, drying temperature, and relative humidity [65]. The degree of spreading or wettability of BECs can be characterized on the surface of fruit by contact angle measurements that maintain mechanical equilibrium of the coating drops under the influence of mainly three surface tension forces—solid–liquid, liquid–vapor, and solid–vapor interfaces—to assess the adhesion properties of coating solutions on the fruit surfaces [65][67]. The ideal case of a contact angle value equal to 0° corresponds to a hydrophilic solid surface where total wetting conditions can be attained by an aqueous solution. A contact angle value between 0° and 180° suggests the occurrence of partial wetting, which is higher for a contact angle below 90°. The ideal case of a contact angle equal to 180° corresponds to a hydrophobic solid surface, where no wetting conditions occur when in contact with an aqueous medium. The contact angle can be measured directly on the food surface through the sessile drop method or atomic force microscopy to visualize the thickness and adherence of the coated surface [67][68].
BECs applied via the multilayer coating method include layer by layer deposition of coating solutions for better adhesion, especially on the surfaces of fresh-cut fruits [69]. Multilayer coating adhesion exhibits electrostatic interaction of the charged polyelectrolytes with that of the fruit surface [70][71]. The electrostatic interactions between the multilayer coatings of nano size dimensions may form chemical bonds, thereby providing effective control of physiological, mechanical, and functional properties on coated fruit [72]. In the multilayer coating method, coating materials containing oppositely charged polyelectrolytes are deposited through alternate dipping of the fruit in different coating solutions (Figure 1e). The dipping of fruit in many cycles creates a layer-by-layer deposition of a coating solution that mainly depends on the ionic strength, pH, and charge densities to form a bonded network via electrostatic forces of attraction [69]. Therefore, the application of the multilayer coating method has been reported in polysaccharides and charged polyelectrolytes capable of hydrogen and covalent bonding to increase compactness of the coating layers during postharvest storage of fruits [10][73].
References
- Jiang, H.; Zhang, W.; Li, X.; Xu, Y.; Cao, J.; Jiang, W. The anti-obesogenic effects of dietary berry fruits: A review. Food Res. Int. 2021, 147, 110539.
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772.
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428.
- Marsh, K.; Bugusu, B. Food packaging—roles, materials, and invironmental issues. J. Food Sci. 2007, 72, R39–R55.
- Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E.Y.X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3559.
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625.
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986.
- Iordachescu, G. Postharvest losses in transportation and storage for fresh fruits and vegetables sector. J. Int. Sci. Publ. 2019, 7, 244–251.
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095.
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813.
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676.
- Anugrah, D.S.; Alexander, H.; Pramitasari, R.; Hudiyanti, D.; Sagita, C.P. A review of polysaccharide-zinc oxide nanocomposites as safe coating for fruits preservation. Coatings 2020, 10, 988.
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674.
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs 2020, 18, 64.
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141.
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential oil-containing polysaccharide-based edible films and coatings for food security applications. Polymers 2021, 13, 575.
- Abifarin, T.O.; Otunola, G.A.; Afolayan, A.J. Chemical composition of essential oils obtained from Heteromorpha arborescens (Spreng.) cham. and schltdl leaves using two extraction methods. Sci. World J. 2020, 2020, 9232810.
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 343, 128403.
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. ‘Pedro Sato’ treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220.
- Jianglian, D.; Shaoying, Z. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol. 2013, 4, 227.
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 2017, 4, 58.
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133.
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469.
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681.
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750.
- Da Cunha, A.L.Q. Influence of gamma radiation treatment on the profile of phenolic compounds and on the quality parameters of strawberries cv. Albion during storage. Res. Soc. Dev. 2020, 9, e991975147.
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 2020, 103, 321–332.
- Paramithiotis, S.; Drosinos, E.H.; Skandamis, P.N. Quantitative Microbiology in Food Processing; Microbial Ecology of Fruits and Fruit-Based Products; John Wiley & Sons, Ltd.: Chichester, UK; Hoboken, NJ, USA, 2017; pp. 358–381.
- Serradilla, M.J.; Villalobos, M.D.C.; Hernández, A.; Martín, A.; Lozano, M.; Córdoba, M.D.G. Study of microbiological quality of controlled atmosphere packaged ‘Ambrunés’ sweet cherries and subsequent shelf-life. Int. J. Food Microbiol. 2013, 166, 85–92.
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.-D.A.; Bursać Kovačević, D. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419.
- Luesuwan, S.; Naradisorn, M.; Shiekh, K.A.; Rachtanapun, P.; Tongdeesoontorn, W. Effect of active packaging material fortified with clove essential oil on fungal growth and post-harvest quality changes in table grape during cold storage. Polymers 2021, 13, 3445.
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80.
- Yun, Z.; Jin, S.; Ding, Y.; Wang, Z.; Gao, H.; Pan, Z.; Xu, J.; Cheng, Y.; Deng, X. Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage. J. Exp. Bot. 2012, 63, 2873–2893.
- Lin, S.; Wu, T.; Lin, H.; Zhang, Y.; Xu, S.; Wang, J.; Wu, B.; Chen, Y.; Lin, S.; Lin, D.; et al. De Novo analysis reveals transcriptomic responses in Eriobotrya japonica fruits during postharvest cold storage. Genes 2018, 9, 639.
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435.
- Bustamante, C.A.; Brotman, Y.; Monti, L.L.; Gabilondo, J.; Budde, C.O.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Differential lipidome remodeling during postharvest of peach varieties with different susceptibility to chilling injury. Physiol. Plant. 2018, 163, 2–17.
- Zhou, Y.; Pan, X.; Qu, H.; Underhill, S.J. Low temperature alters plasma membrane lipid composition and ATPase activity of pineapple fruit during blackheart development. J. Bioenerg. Biomembr. 2014, 46, 59–69.
- Sheng, L.; Zhou, X.; Liu, Z.-Y.; Wang, J.-W.; Wang, L.; Zhang, Q.; Ji, S.-J. Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’ pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 2016, 117, 1–8.
- Shi, F.; Zhou, X.; Tan, Z.; Yao, M.-M.; Wei, B.-D.; Ji, S.-J. Membrane lipid metabolism changes and aroma ester loss in low-temperature stored Nanguo pears. Food Chem. 2017, 245, 446–453.
- Leisso, R.S.; Buchanan, D.A.; Lee, J.; Mattheis, J.P.; Sater, C.; Hanrahan, I.; Watkins, C.B.; Gapper, N.; Johnston, J.W.; Schaffer, R.J.; et al. Chilling-related cell damage of apple (Malus domestica Borkh.) fruit cortical tissue impacts antioxidant, lipid and phenolic metabolism. Physiol. Plant. 2015, 153, 204–220.
- Gonzalez, C.; Zanor, M.; Ré, M.; Otaiza, S.; Asis, R.; Valle, E.; Boggio, S. Chilling tolerance of Micro-Tom fruit involves changes in the primary metabolite levels and in the stress response. Postharvest Biol. Technol. 2019, 148, 58–67.
- Salzano, A.M.; Renzone, G.; Sobolev, A.P.; Carbone, V.; Petriccione, M.; Capitani, D.; Vitale, M.; Novi, G.; Zambrano, N.; Pasquariello, M.S.; et al. Unveiling kiwifruit metabolite and protein changes in the course of postharvest cold storage. Front. Plant Sci. 2019, 10, 71.
- Pathare, P.B.; Al-Dairi, M. Bruise damage and quality changes in impact-bruised, stored tomatoes. Horticulturae 2021, 7, 113.
- Zhang, W.; Zhao, H.; Zhang, J.; Sheng, Z.; Cao, J.; Jiang, W. Different molecular weights chitosan coatings delay the senescence of postharvest nectarine fruit in relation to changes of redox state and respiratory pathway metabolism. Food Chem. 2019, 289, 160–168.
- Amwoka, E.M.; Ambuko, J.L.; Jesang’, H.M.; Owino, W.O. Effectiveness of selected cold chain management practices to extend shelf life of mango fruit. Adv. Agric. 2021, 2021, 8859144.
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689.
- Brummell, D.A.; Atkinson, R.G.; Burdon, J.N.; Patterson, K.J.; Schaffer, R.J. Fruit growth, ripening and postharvest physiology. In Plant in Action; Plant and Food Research: Palmerston North, New Zealand; Auckland, New Zealand, 2016.
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An extensive review of natural polymers used as coatings for postharvest shelf-life extension: Trends and challenges. Polymers 2021, 13, 3271.
- Darmawati, E.; Nava, N.; Suyatma, N. Aloe vera as a coating material for tropical fruits using spray method. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 012011.
- Andrade, R.; Skurtys, O.; Osorio, F. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337.
- Pirozzi, A.; Ferrari, G.; Donsì, F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings 2021, 11, 990.
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.; Berrios, J.; Sambo, P.; McHugh, T. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2017, 10, 165–174.
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Electrospray coating of minimally processed strawberries and evaluation of the shelf-life quality properties. J. Food Process Eng. 2019, 42, e13082.
- Khan, M.; Schutyser, M.; Schroën, K.; Boom, R. Barrier properties and storage stability of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 23, 182–187.
- Lu, H.; Li, S.; Du, H.; Lu, Y.; Huang, X. Secondary breakup characteristics and mechanism of single electrified al/n-decane nanofluid fuel droplet in electrostatic field. Appl. Sci. 2020, 10, 5332.
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660.
- Dhar, P.; Kumar, A.; Katiyar, V. Magnetic cellulose nanocrystal based anisotropic polylactic acid nanocomposite films: Influence on electrical, magnetic, thermal, and mechanical properties. ACS Appl. Mater. Interfaces 2016, 8, 18393–18409.
- Mannozzi, C.; Glicerina, V.; Tylewicz, U.; Castagnini, J.M.; Canali, G.; Dalla Rosa, M.; Romani, S. Influence of two different coating application methods on the maintenance of the nutritional quality of fresh-cut melon during storage. Appl. Sci. 2021, 11, 8510.
- Senturk Parreidt, T.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The development of a uniform alginate-based coating for cantaloupe and strawberries and the characterization of water barrier properties. Foods 2019, 8, 203.
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404.
- Rahman, S.M.A.; Nassef, A.M.; Al-Dhaifallah, M.; Abdelkareem, M.A.; Rezk, H. The effect of a new coating on the drying performance of fruit and vegetables products: Experimental investigation and artificial neural network modeling. Foods 2020, 9, 308.
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 2003, 68, 503–510.
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of coating application methods on the postharvest quality of cassava. Int. J. Food Sci. 2019, 2019, 2148914.
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The effect of the application of edible coatings on or before ultraviolet treatment on postharvested longan fruits. J. Food Qual. 2017, 2017, 5454263.
- Pirozzi, A.; Pataro, G.; Donsì, F.; Ferrari, G. Edible coating and pulsed light to increase the shelf life of food products. Food Eng. Rev. 2021, 13, 544–569.
- Vaishali; Sharma, H.; Shami, V.; Samsher; Chaudhary, V.; Sunil, E.; Kumar, M. Importance of edible coating on fruits and vegetables: A review. J. Pharm. Phytochem. 2019, 8, 4104–4110.
- Osorio, F.; Valdés, G.; Skurtys, O.; Andrade, R.; Villalobos-Carvajal, R.; Silva-Weiss, A.; Silva-Vera, W.; Giménez, B.; Zamorano, M.; Lopez, J. Surface free energy utilization to evaluate wettability of hydrocolloid suspension on different vegetable epicarps. Coatings 2018, 8, 16.
- Sapper, M.; Bonet, M.; Chiralt, A. Wettability of starch-gellan coatings on fruits, as affected by the incorporation of essential oil and/or surfactants. LWT Food Sci. Technol. 2019, 116, 108574.
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014, 7, 1424–1432.
- McShane, M.; Lvov, Y. Electrostatic Self-Assembly: Layer-by-Layer. In Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; Schwarz, J.A., Lyshevski, S.E., Contescu, C.I., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2014; pp. 1342–1358.
- Adiletta, G.; Di Matteo, M.; Petriccione, M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int. J. Mol. Sci. 2021, 22, 2633.
- Zhang, L.; Huang, C.; Zhao, H. Application of pullulan and chitosan multilayer coatings in fresh papayas. Coatings 2019, 9, 745.
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Soleimani Aghdam, M.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol. Technol. 2019, 147, 29–38.
More
Information
Subjects:
Health Care Sciences & Services
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




