
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilavenil Soundharrajan | + 1994 word(s) | 1994 | 2021-09-06 07:45:23 | | | |
| 2 | Dean Liu | Meta information modification | 1994 | 2022-01-07 08:32:36 | | |
Video Upload Options
Lactic acid bacteria (LAB) are considered potent natural additives for animal feed production due to the efficient production of biological metabolites—notably, higher lactic acid content with marginal level acetic acid and other organic acids. Furthermore, LAB can utilize water-soluble carbohydrates and convert them into valuable organic acids, which increase the acidification of the surrounding environment. Rapid acidification (lower pH) could help to prevent undesirable microbial growth and toxic secondary metabolite secretion.
1. Introduction
Ensiling is a method to preserve raw plant materials based on spontaneous lactic acid production by controlling fermentation under anaerobic conditions. It has been used for many decades for the preservation of silages produced from various legumes, fodder, and residue crops ( Figure 1 ). Forage preservation by the ensiling method has attracted great attention, providing consistent, reliable, and predictable feed supply for ruminant production. Risk of digestible nutrient losses by plant oxidation, undesirable microbial population in plants, proteolytic activity, Clostridia fermentation, microbial deamination, and decarboxylation of amino acids could negatively affect conservation efficiency and increase energy and nutrient losses as well as the accumulation of anti-nutritional compounds in forages [1]. Epiphytic lactic acid bacteria (LAB) utilize water-soluble carbohydrates present in ensiled plants and metabolize them into lactic acid, with a lesser extent of acetic acid which can lower the pH of the silage and prevent undesirable microbial growth, allowing them to be stored for a long time. An abundance of epiphytic bacteria in ensiled plant materials is not sufficient to induce the production of sufficient amounts of lactic acid in silage samples. Populations of LAB in plants are often heterofermentative and low in numbers [2]. Heterofermentative bacteria can increase the converting ratio of lactic acid into other metabolites such as acetic acid and ethanol. However, homo-fermentative bacteria do not convert lactic acid to other organic acids. Thus, a high level of lactic acid is sustained in the silage. In general, lactic acid found at the highest concentration is an indicator of good silage [3]. To make high-quality silage with strong digestibility, stimulation of the ensiling process is required by adding different types of chemical and biological additives. Currently, the use of additives is recommended to ensile green folder with significant concentrations of mono-, di-, and oligo-saccharides and high protein content with high buffer capacity. Expected changes in silage production when ensiled with LAB include an increased ratio of lactic acid with marginal amounts of acetic acid, reduced proteolysis, and increased dry matter recovery [4].
2. Synthetic and Natural Additives for Silage Production
To maintain the quality of silage, several numbers of additives can be used to inhibit the growth of undesirable species. These inhibitors can be added during ensiling. For example, sodium nitrite and hexamine can prevent the growth of Clostridia . The growth of yeast can be restricted by sodium benzoate [5]. The use of chemical additives (calcium format, sodium nitrite, sodium benzoate) can improve the hygiene of corn silage and reduce concentrations of deoxynivalenol, zearalenone, fumonisins, and so on [6]. The addition of silage inoculants and organic acid can be used to synthesize some antimicrobial agents (ethanol, H2O2, exopolysaccharides, diacetyl) and antibacterial pesticides (bacteriocins).
The current practice of silage-making combines several LAB strains [7][8][9][10][11] to induce silage fermentation (alfalfa, legumes, maize, grains, meadow, etc.) through a synergistic action, thus enhancing the stability for more than one year of storage [12]. Lactic acid bacteria have been used for several centuries for the production of feed, silages, and food. LAB has a potential role in decreasing the pH, thus offering protection against harmful microorganisms. LABs have a good impact on humans and animals because they can act as probiotics. Feed companies are very interested in using LAB inoculums for silage making because LAB can fight against pathogens and enhance the quality of silage with suitable parameters. Insight on the current silage fermentation process has been gained due to advanced molecular techniques, metagenomic, and novel techniques that can target inoculants for silage production [13]. The significance of LAB in recent years has been comprehensively studied using novel strains to improve the silage-making process [14][15][16]. Selections of microbial inoculants are considered to be very precious.
3. Changes in Fermented Silage by LAB
LABs are mainly responsible for pH reduction and preservation of nutrients in ensiled silages for a long time [17]. Microbiological additives are frequently used in silage fermentation [4][18]. Microbial inoculant is a mixture of one or more species of microorganisms that should be viable at the time of use. Microbiota in the silages has positive impacts (Figure 4) by decreasing dry matter losses, increasing essential metabolites of interest, inhibiting undesirable microbial growth, and improving nutritional quality [1][4][18]. Selecting potent microbes is essential to achieve positive effects. When selecting novel microorganisms, we should focus on specific characteristics of target substrates and general conditions of the ensiling environment to ensure optimal effects [15][19][20]. The addition of LAB during the ensiling process improved fermentation quality (higher lactic acid) and maintained the crude protein (CP), acid detergent fiber (ADF), and neutral detergent fiber contents (NDF) [14][21]. Another report claimed that the addition of LAB significantly increased nutritive profiles of silage at different storage periods [22]. Increased acid detergent lignin [23] and dry matter content (DM) [24] were noted in silages treated with LAB, but the DM level was varied at different storage periods [7]. The organic matter (OM) and DM content were increased in the fresh and rain-treated ryegrass silages treated with LAB [25]. The water-soluble carbohydrate (WSC) level was reduced in the silage treated with LAB [23][24]; this may be due to LAB being able to utilize WSC and convert into organic acids. The plant metabolites, such as 3-hydroxydecanoic acid, 2-hydroxy-4-methylpentanoic acid, benzoic acid, catechol, hydrocinnamic acid, salicylic acid, 3-phenyllactic acid, 4-hydroxybenzoic acid, (trans, trans)-3,4-dihydroxycyclohexane- 1-carboxylic acid, p-hydrocoumaric acid, vanillic acid, azelaic acid, hydroferulic acid, p-coumaric acid, hydrocaffeic acid, ferulic acid, and caffeic acid were increased in grass silages inoculated with LAB [26]. LAB increased α -tocopherol levels in silages prepared from the mixture of birdsfoot trefoil and timothy, red clover and meadow fescue, or red clover and timothy; whereas, β-carotene levels were either the same or slightly reduced in same silage mixtures [27]; whereas, the silages from rye had higher β-carotene [28].
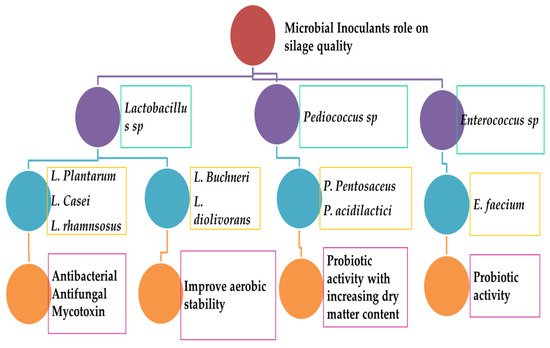
The current research practice focuses on homofermentative and heterofermentative lactic acid bacteria. Some non-LAB groups, chemicals, and enzymes are also involved in the silage-making process. Major factors that are important during silage fermentation include aerobic constancy, livestock consumption, and usage. The silage fermentation process involves two major microbes: homofermentative and heterofermentative species. Homofermentative inoculant groups include L actobacillus, Pediococcus, and Lactococcus species. These inoculants can lead to the high productivity of lactic acid, lower the pH, and reduce the breakdown of proteins and sugar molecules in crops. Heterofermentative inoculants include L. buchneri and L. brevis . Both species can produce a mixture of lactic acid and acetic acid that can prevent the growth of yeast and mold as contaminants. Recent updates on homofermentative lactic acid bacteria have revealed that they are highly dominant during the fermentation of silage and can lead to the high quality of products. These homofermentative inoculants include Lactiplantibacillus plantarum, L actobacillus acidophilus, P. acidilactici, P. pentacaceus , and Enterococcus faecium . The growth rate of bacterial inoculum on the dry matter content can increase very rapidly ( Enterococcus ≥ pediococcus ≥ Lactobacillus ). Most Pediococcus can tolerate higher DM content than Lactobacillus at a wide range of pH values and temperatures [29].
4. Insight on Homo- and Heterofermentative LAB
Homo- and hetero-fermentative LAB are widely used for the fermentation of silage. Recent updates on silage-making bacteria have been documented as facultative heterofermentative and relatively obligate homofermentative bacterial species [30]. Both species have diverged characteristics. Notably, homofermentative bacteria (ferment hexoses) and facultative heterofermentative bacteria (ferment pentoses) can both produce lactic acid. Facultative species include L. plantarum, L. casei, Enterococcus faecium, and Pediococcus sp . Higher lactic acid content is suitable for better recovery of the dry matter content of silages. A recent meta-analysis has revealed that the effects of various inoculants are different, depending on the specific kind of crop. Low pH and temperature can be maintained in legume plants, alfalfa, and grasses. However, they are not maintained in other plants (corn, sorghum, sugarcane). The reduction of acid using culture inoculum plays an important role in maximizing crop and dry matter content recovery, reaching ≥ 2.8% for most grass silages. However, it is linked to losses (≤ 2.4%) for untreated silages (corn, sugarcane, sorghum) [1]. Heterofermentative LAB usually can synthesize a high volume of acetic acid during silage making, which can prevent fungal growth and consequently store silages longer under exposure to air. The microbial inoculum rate is usually 10 5–10 6 cells per gram of crop [31]. Homo- and hetero-lactic acid bacteria (silage inoculants) are involved in alfalfa silage-making for the purpose of excellent animal feed production. A meta-analysis studied the effects of homo- and hetero- LAB on fermentation parameters, the value of nutrition, media composition, and aerobic stability of forages (alfalfa). Trending reports have suggested that both homo- and hetero-fermentative bacteria are good inoculants for enhancing the silage quality, reducing the contamination (yeast and molds), and increasing the forage conservation for livestock production [32].
The most commonly available starter culture contains homofermentative LABs, which are robust and efficient to produce lactic acid and thus improve the quality of silages. Lactiplantibacillus plantarum, Lactobacillus acidophilus, E. faecium, P. acidilactici and P. pentosaceus are the most popular LAB species [13]. Homofermentative bacterial groups are more powerful than heterofermentative ones. The significance of homofermentative bacteria is that they can catalyze each glucose molecule of lactic acid and yield high dry matter with less energy reduction for silages. Lactic acid is a more potent acid that can reduce silage pH more than other acids. The final pH is increased by a scale-up process of heterofermenters. Native bacterial populations are highly different across plant environment ecosystems. The addition of homofermentative bacterial inoculum can reduce the pH very fast while inhibiting other harmful bacterial contamination and storing plant proteins. The homofermentative inoculum can increase animal performance (3–5%). The most common microbial inoculants used for the production of silages are homofermentative LABs. The current scenario has shown that numerous bacteria are involved in the homo LAB fermentation process [33].
Both facultative and obligate heterofermentative inoculums are involved in the silage-making process (Lactobacillus, Oenococcus, Leuconostoc, Weissella ). The most dominant LABs are Lentilactobacillus buchneri, Limosilactobacillus reuteri, Lacticaseibacillus casei [34]. Other LAB groups include Levilactobacillus zymae, Apilactobacillus kunkeei, Levilactobacillus acidifarinae, Levilactobacillus namurensis, Levilactobacillus brevis, Levilactobacillus spicheri, Fructilactobacillus fructivorans, Fructilactobacillus fructivorans, and Levilactobacillus hammesii [35]. The obligate heterofermentative L. buchneri is considered the best silage additive. These bacteria can increase aerobic stability during heterofermentation. It can synthesize antifungal components [36]. A recent report has also suggested L. buchneri can strongly produce antimicrobial compounds (salicylic acid, 3-phenyl lactic acid, catechol, benzoic acid, hydrocinnamic acid, 4 hydroxybensoic acid) during grass silage fermentation [26]. These obligate heterofermentative bacteria (L. buchneri ) can be used as silage additives to induce aerobic stability during silage fermentation. They can lead to a medium level of acetic acid increase and reduce contamination of yeast [37]. Recent reports have revealed heterofermentative additives Lentilactobacillus diolivorans and L. reuteri of silage [38]. The most potential role of L. buchneri is that it can produce ferulic acid (esterase) in silage, leading to efficient fiber digestibility [39]. These heterofermentative bacteria can synthesize a sufficient amount of enzymes to produce suitable quantities of silages.
A combination of numerous strains has been used as microbial inoculum. The production also varies using potential groups of L. plantarum, P. acidilactici, and P. pentosaceus [40]. The paring of inoculants for 14 days of fermentation can give a successful production of corn silage [41]. A multi-inoculum preparation for alfalfa L. buchneri treated with P. pentosaceus for one week has resulted in a greater pH decline than untreated silage. A combination of L. buchneri, L. plantarum , and L. casei has been used for making barley silage on a lab scale [42]. The P. pentosaceus, P. freudenreichii, and L. buchneri are the most commercial inoculants used for bermudagrass silage production. The combination of more inoculants can enhance the initial stage of the fermentation process [43].
References
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Goncalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603.
- Ben-Dov, E.; Shapiro, O.H.; Siboni, N.; Kushmaro, A. Advantage of using inosine at the 3’ termini of 16S rRNA gene universal primers for the study of microbial diversity. Appl. Environ. Microbiol. 2006, 72, 6902–6906.
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033.
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000.
- Knický, M.; Lingvall, P. Possibilities to avoid growth of clostridia and/or fungi in wilted silage by use of organic and inorganic salts. In Proceedings of the XIX International Grassland Congress Brazil, São pedro, Brazil, 11–21 February 2001; pp. 788–789.
- Biro, D.; Juracek, M.; Kacaniova, M.; Simko, M.; Galik, B.; Michalkova, J.; Gyongyova, E. Occurrence of microscopic fungi and mycotoxins in conserved high moisture corn from Slovakia. Ann. Agric. Environ. Med. 2009, 16, 227–232.
- Nascimento Agarussi, M.C.; Gomes Pereira, O.; Paula, R.A.d.; Silva, V.P.d.; Santos Roseira, J.P.; Fonseca e Silva, F. Novel lactic acid bacteria strains as inoculants on alfalfa silage fermentation. Sci. Rep. 2019, 9, 8007.
- Guo, L.; Yao, D.; Li, D.; Lin, Y.; Bureenok, S.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria Isolated From Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and in vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2020, 10, 2998.
- Puntillo, M.; Gaggiotti, M.; Oteiza, J.M.; Binetti, A.; Massera, A.; Vinderola, G. Potential of Lactic Acid Bacteria Isolated From Different Forages as Silage Inoculants for Improving Fermentation Quality and Aerobic Stability. Front. Microbiol. 2020, 11, 3091.
- Kuppusamy, P.; Kim, D.; Soundharrajan, I.; Park, H.S.; Jung, J.S.; Yang, S.H.; Choi, K.C. Low-Carbohydrate Tolerant LAB Strains Identified from Rumen Fluid: Investigation of Probiotic Activity and Legume Silage Fermentation. Microorganisms 2020, 8, 1044.
- Ni, K.; Wang, Y.; Cai, Y.; Pang, H. Natural Lactic Acid Bacteria Population and Silage Fermentation of Whole-crop Wheat. Asian-Australas J. Anim. Sci. 2015, 28, 1123–1132.
- Zhang, T.; Li, L.; Wang, X.-f.; Zeng, Z.-h.; Hu, Y.-g.; Cui, Z.-j. Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation, aerobic stability, bacteria diversity and ruminal degradability of alfalfa silage. World J. Microbiol. Biotechnol. 2009, 25, 965–971.
- Fabiszewska, A.U.; Zielińska, K.J.; Wróbel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 76.
- Soundharrajan, I.; Kuppusamy, P.; Park, H.; Kim, J.; Kim, W.; Jung, J.; Choi, K. Lactic Acid Bacteria Mixture as Inoculants on Low Moisture Italian Ryegrass Silage Fermentation. J. Korean Soc. Grassl. Forage Sci. 2019, 39, 127–131.
- Amaral, R.C.; Carvalho, B.F.; Costa, D.M.; Morenz, M.J.F.; Schwan, R.F.; Ávila, C.L.d.S. Novel lactic acid bacteria strains enhance the conservation of elephant grass silage cv. BRS Capiaçu. Anim. Feed Sci. Technol. 2020, 264, 114472.
- Zhao, S.S.; Wang, Y.P.; Yang, F.Y.; Wang, Y.; Zhang, H. Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 2020, 129, 233–242.
- Ávila, C.L.S.; Carvalho, B.F. Silage fermentation—updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984.
- Wilkinson, J.M.; Rinne, M. Highlights of progress in silage conservation and future perspectives. Grass Forage Sci. 2018, 73, 40–52.
- Ávila, C.L.S.; Carvalho, B.F.; Pinto, J.C.; Duarte, W.F.; Schwan, R.F. The use of Lactobacillus species as starter cultures for enhancing the quality of sugar cane silage. J. Dairy Sci. 2014, 97, 940–951.
- Guan, H.; Shuai, Y.; Yan, Y.; Ran, Q.; Wang, X.; Li, D.; Cai, Y.; Zhang, X. Microbial Community and FermentationDynamics of Corn Silage Prepared withHeat-Resistant Lactic Acid Bacteria in a HotEnvironment. Microorganisms 2020, 8, 719.
- Kuppusamy, P.; Soundharrajan, I.; Park, H.; Kim, J.; Kim, W.; Jung, J.; Choi, K. Effects of Lactic Acid Bacteria Inoculants on Fermentation of Low Moisture Fresh Rice Straw Silage at Different Storage Periods. J. Korean Soc. Grassl. Forage Sci. 2019, 39, 165–170.
- Wang, Y.; Wang, C.; Zhou, W.; Yang, F.-y.; Chen, X.-y.; Zhang, Q. Effects of Wilting and Lactobacillus plantarum Addition on the Fermentation Quality and Microbial Community of Moringa oleifera Leaf Silage. Front. Microbiol. 2018, 9.
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring the microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, and developing the high-protein woody plant as ruminant feed. bioRxiv 2020, 275, 114766.
- Pereira, G.A.; Santos, E.M.; Araújo, G.G.L.; Oliveira, J.S.; Pinho, R.M.A.; Zanine, A.d.M.; Souza, A.F.N.; Macedo, A.J.S.; Neto, J.M.C.; Nascimento, T.V.C. Isolation and identification of lactic acid bacteria in fresh plants and in silage from Opuntia and their effects on the fermentation and aerobic stability of silage. J. Agric. Sci. 2020, 157, 684–692.
- Huyen, N.; Martinez, I.; Pellikaan, W. Using Lactic Acid Bacteria as Silage Inoculants or Direct-Fed Microbials to Improve In Vitro Degradability and Reduce Methane Emissions in Dairy Cows. Agronomy 2020, 10, 1482.
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552.
- Lindqvist, H.; Nadeau, E.; Jensen, S.K. Alpha-tocopherol and β-carotene in legume–grass mixtures as influenced by wilting, ensiling and type of silage additive. Grass Forage Sci. 2012, 67, 119–128.
- Zhao, G.Q.; Wei, S.N.; Liu, C.; Kim, H.J.; Kim, J.G. Effect of harvest dates on β-carotene content and forage quality of rye (Secale cereale L.) silage and hay. J. Anim. Sci. Technol. 2021, 63, 354–366.
- Kung, L., Jr.; Santos, M.; DerBedrosian, M. The effect of feeding cows corn silage with or without L. buchneri 40788 and supplemented with or without Levucell SC; Lallemand Animal Nutrition Internal Report; Lallemand Animal Nutrition: Milwaukee, WI, USA, 2010.
- Pahlow, G.; Muck, R.; Driehuis, F.; Oude Elferink, S.; Spoelstra, S.F. Microbiology of Ensiling. Silage Sci. Technol. 2003, 42, 31–93.
- Weinberg, Z.G.; Ashbell, G.; Hen, Y.; Azrieli, A.; Szakacs, G.; Filya, I. Ensiling whole-crop wheat and corn in large containers with Lactobacillus plantarum and Lactobacillus buchneri. J. Ind. Microbiol. Biotechnol. 2002, 28, 7–11.
- Blajman, J.; Vinderola, G.; Paez, R.; Signorini, M. The role of homofermentative and heterofermentative lactic acid bacteria for alfalfa silage: A meta-analysis. J. Agric. Sci. 2020, 158, 1–12.
- Muck, R.E. Recent advances in silage microbiology. ARS USDA Submiss. 2013, 22, 3–15.
- Hammes, W.P.; Hertel, C. Genus Lactobacillus beijerinck 1901, 212AL. The Firmicutes. In Bergey’s Manualof Systematic Bacteriology, 2nd ed.; De Vos, G.M.G., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009; pp. 465–490.
- Pot, B. Tsakalidou, Taxonomy and metabolism of Lactobacillus; Caister Acadedmic Press: Norfolk, UK, 2009; pp. 3–58.
- Ström, K.; Sjögren, J.; Broberg, A.; Schnürer, J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327.
- Oude Elferink, S.J.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132.
- Sriramulu, D.D.; Liang, M.; Hernandez-Romero, D.; Raux-Deery, E.; Lünsdorf, H.; Parsons, J.B.; Warren, M.J.; Prentice, M.B. Lactobacillus reuteri DSM 20016 produces cobalamin-dependent diol dehydratase in metabolosomes and metabolizes 1,2-propanediol by disproportionation. J. Bacteriol. 2008, 190, 4559–4567.
- Nsereko, V.L.; Smiley, B.K.; Rutherford, W.M.; Spielbauer, A.; Forrester, K.J.; Hettinger, G.H.; Harman, E.K.; Harman, B.R. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim. Feed Sci. Technol. 2008, 145, 122–135.
- Reich, L.J.; Kung, L. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109.
- Driehuis, F.; Oude Elferink, S.J.W.H.; Van Wikselaar, P.G. Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Sci. 2001, 56, 330–343.
- Addah, W.; Baah, J.; Okine, E.K.; McAllister, T.A. A third-generation esterase inoculant alters fermentation pattern and improves aerobic stability of barley silage and the efficiency of body weight gain of growing feedlot cattle1. J. Anim. Sci. 2012, 90, 1541–1552.
- Arriola, K.G.; Queiroz, O.C.M.; Romero, J.J.; Casper, D.; Muniz, E.; Hamie, J.; Adesogan, A.T. Effect of microbial inoculants on the quality and aerobic stability of bermudagrass round-bale haylage. J. Dairy Sci. 2015, 98, 478–485.




