Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chunsheng Wu | + 2365 word(s) | 2365 | 2021-12-03 02:20:59 | | | |
| 2 | Dean Liu | Meta information modification | 2365 | 2022-01-07 01:12:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, C. Field-Effect Sensors Using Biomaterials. Encyclopedia. Available online: https://encyclopedia.pub/entry/17822 (accessed on 07 February 2026).
Wu C. Field-Effect Sensors Using Biomaterials. Encyclopedia. Available at: https://encyclopedia.pub/entry/17822. Accessed February 07, 2026.
Wu, Chunsheng. "Field-Effect Sensors Using Biomaterials" Encyclopedia, https://encyclopedia.pub/entry/17822 (accessed February 07, 2026).
Wu, C. (2022, January 06). Field-Effect Sensors Using Biomaterials. In Encyclopedia. https://encyclopedia.pub/entry/17822
Wu, Chunsheng. "Field-Effect Sensors Using Biomaterials." Encyclopedia. Web. 06 January, 2022.
Copy Citation
Field-effect sensors using biomaterials that are able to detect specific target chemical substances with high sensitivity would have broad applications in many areas, ranging from biomedicine and environments to the food industry, but this has proved extremely challenging.
field-effect sensors
chemical sensors
biosensors
biomaterials
1. Introduction
In biological chemical sensing systems, the process of chemical signal detection is initialized by the special interactions between molecular detectors and specific chemical substances, which can trigger a cascade of intracellular biochemical reactions to convert the chemical signals into cellular responses such as cell membrane potential changes [1][2][3]. These cellular responses are transmitted to the central neural system for the further processing of chemical signals, which allows for the perception of specific chemical substances. Biological chemical sensing systems are the most powerful system for the detection of specific chemical substances with very high performances that cannot be matched by most existing artificial devices. Therefore, it is worthwhile to develop biosensors using biomaterials in order to obtain artificial chemical sensing devices with performances comparable to biological chemical sensing systems.
The main components of biosensors using biomaterials for chemical sensing include sensitive elements and transducers, which are combined to mimic the functions of biological chemical sensing systems to realize the conversion of chemical signals into measurable signals by existing devices such as electrical signals and optical signals. As shown in Figure 1, the basic idea of biosensors using biomaterials is to employ the extreme high capability of functional biomaterials originating from biological systems for the detection of specific chemical substances. The coupling of highly specialized biomaterials with a transducer could lead to the generation of potential devices and instruments with a performance comparable to that of biological chemical sensing systems for the detection of chemical signals in a trace level within complex environmental conditions.
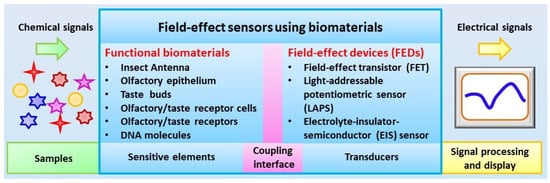
Figure 1. Schematic diagram of configurations of field-effect sensors using biomaterials.
2. Preparation of Functional Biomaterials
For the development of biosensors using biomaterials, it is required to obtain functional biomaterials, which maintain their unique capability of chemical sensing and are suitable to be used as sensitive elements to couple with transducers [4]. Because the activity of biomaterials has a direct influence on the performances of biosensors with regard to sensitivity, specificity, and stability, it is of great importance to obtain functional biomaterials for chemical sensing. In addition to maintaining the natural structures and native functions of biomaterials, it is also desirable to produce them in a cost-effective manner and store them in a convenient manner. At present, several methods have been applied in the preparation of functional biomaterials for chemical sensing, which can be divided into two main categories: one is direct isolation from natural biological chemical sensing systems, the other one is preparation based on biotechnology.
Direct isolation from natural biological chemical sensing systems is the most convenient approach to achieving functional biomaterials for the development of biosensors for chemical sensing. It is widely used in the early stage of biosensors, which has the advantages of maintaining the natural structure and functions of biomaterials allowing for the recognition of their natural ligands with high performances. In addition, the powerful capability of biological chemical sensing systems could be preserved to some extent, which helps to enhance the performance of biosensors. Different types of functional biomaterials have been isolated from biological chemical sensing systems and successfully utilized as sensitive elements for the development of biosensors. For instance, olfactory sensory neurons and olfactory receptors have been isolated from animals or insects and have served as sensitive elements in biosensors for odor detection [5][6][7][8][9]. Similarly, taste bud cells and taste receptors have also been isolated from animals and applied in the biosensors for taste substance detection [10]. However, this approach has some limitations that hamper further development. The main problem is related to the purification of desired biomaterials, which have crucial influences in the specificity of the biosensors. It is usually time-consuming and expensive to achieve sufficient functional biomaterials for biosensors. In addition, it is also challenging to maintain their native function during the preparation and measurement process of biosensors. All these limitations make it difficult to develop a practical applicable or commercially available biosensors, especially for those in-field applications.
Fast advances in biotechnology provide an alternative approach for the preparation of functional biomaterials for biosensors. This approach can be used to achieve functional biomaterials by the expression of desired type of olfactory or taste receptors either in a heterologous cell system or a cell-free protein synthesis system. This allows for the preparation of functional biomaterials with desired types of olfactory or taste receptors. In addition, this approach makes it easy to graft tags in the prepared receptors, which could greatly facilitate the purification and immobilization of functional biomaterials to improve the performance of biosensors. For example, desired types of olfactory receptors have been expressed in human embryonic kidney (HEK) cells [11][12] and yeast [13][14][15][16] and utilized as sensitive elements for biosensors towards odorant detection. Taste receptors have also been expressed based on biotechnology to prepare functional biomaterials for the development of biosensors for taste substance detection [17][18][19][20]. However, this approach still suffered from the labor-intensive and complex purification process of functional biomaterials. In addition, the expression of receptors in a heterologous cell system usually led to cellular toxic effects that are mainly induced by the membrane incorporation and incompatibility of heterologous expressed olfactory or taste receptors. This results in low expression efficiency, which makes it difficult to improve the preparation efficiency of functional biomaterials. Therefore, cell-free protein synthesis is introduced as an alternative method to prepare functional biomaterials to address this limitation. The synthesis system provides all the necessary components for receptor synthesis such as amino acids, nucleotides, salts and energy-generating factors [21][22]. This cell-free system can not only avoid the cell toxic effect induced by receptor expression, but also could make the preparation process faster, which could mean that the whole expression process could finish within a few hours. Recently, olfactory and taste receptors (Figure 2) have been prepared by a cell-free protein synthesis method and coupled with different transducers for the development of biosensors towards chemical sensing [23][24]. This method could also help the right receptor protein folding via the modification of synthesis reaction conditions. However, it is still a big challenge to produce olfactory receptors in a highly efficient and convenient manner due to their hydrophobicity and dependence on a lipid bilayer environment [5].
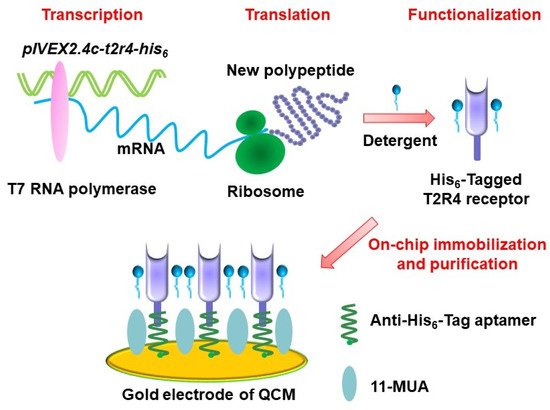
Figure 2. Schematics of preparation of a bitter receptor from cell-free protein expression system for chemical sensing. (Reprinted with permission from ref. [24]. Copyright 2020 Elsevier).
3. Fabrication of Field-Effect Devices
Another key component of biosensors is the transducers. Appropriate transducers are also highly essential in order to convert the chemical signals sensed by the functional biomaterials into the measurable signals. For the development of biosensors using biomaterials for chemical sensing, mass-sensitive devices (e.g., quartz crystal microbalances, QCM, and surface acoustic wave, SAW) and field-effect devices (FEDs) are the most commonly used transducers [25][26][27]. Both of them can record the responsive signals from functional biomaterials upon exposure of chemical substances. Basically, FEDs function as transducers to detect the chemical signals sensed by functional biomaterials and transmit the responsive signals to the peripheral circuits for further signal processing [28]. Therefore, it is crucial to achieve very good and stable coupling between field-effect devices and functional biomaterials in order to develop biosensors with high performances. These biosensors are usually configured with corresponding measurement setup and peripheral circuits in order to readout, collect, and process the detected chemical signals. In this review, we will focus on the biosensors using FEDs as transducers, which mainly include field-effect transistor (FET), light-addressable potentiometric sensor (LAPS), and capacitive electrolyte–insulator–semiconductor (EIS) sensors (Figure 3).
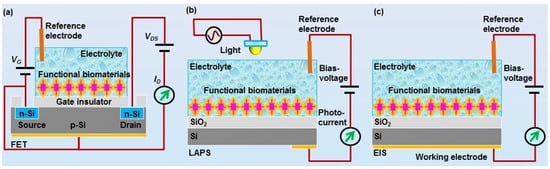
Figure 3. Schematics of field-effect devices utilkized for the development of field-effect sensors using biomaterials for chemical sensing, including (a) field-effect transistor (FET), (b) light-addressable potentiometric sensor (LAPS), and (c) electrolyte-insulator-semiconductor (EIS) sensor.
Fast advances in the micro-fabrication process have greatly facilitated the design and fabrication of various specialized field-effect devices, which could be used as transducers for the development of biosensors towards chemical sensing. For example, FET can be fabricated via a standard micro-fabrication process on silicon wafer [29][30]. The mechanisms and structure of FET are schematically shown in Figure 3a. Usually, an insulator layer is first grown on the surface of silicon wafer via thermal oxidation, which can be used as the gate of FET devices. In some cases, the insulator layer was further deposited with a Si3N4 layer to improve the performance of FET devices. By the following, polyimide is often utilized to form a passivation layer in order to fix with a printed circuit board. Then, the source and drain electrodes are usually fabricated based on photolithography process. Finally, epoxy resin could be used to encapsulate FET devices, which is then fixed with a detection chamber allowing for the exposure of gate surface to the measurement solution inside the detection chamber. With this configuration, the chemical signals sensed by functional biomaterials can be coupled to the gate electrodes of FET, which are then transmitted to the peripheral circuit via the source and drain electrodes of FET.
The LAPS devices and EIS devices are also silicon-based FEDs, as shown in Figure 3b,c [31][32][33]. Both of them have the same structure of electrolyte–insulator–semiconductor. The difference between them is the measurement configuration. LAPS usually require a moveable focused light to realize addressable measurement on the desired points, while EIS do not require any light illumination during measurement. The structures of LAPS and EIS devices are much simpler than that of FET devices, which greatly facilitated the fabrication process. They are often fabricated based on silicon wafer, which is first thermal oxide with a layer of SiO2 on its surface to service as insulator layer. In most cases, the insulation layer surface was further grown with a layer of Ta2O5 or Si3N4 to improve their performance. Then, the oxide layer was removed from the rear side of the wafer, which is then deposited with a metal layer (e.g., Al or Au) to be utilized as Ohmic contact. Finally, the wafer was cut into separate small chips and fixed with a detection chamber. They can thus be applied to the development of biosensors by the immobilization of functional biomaterials onto the gate surface of FEDs exposed to the detection chamber.
4. Coupling of Functional Biomaterials with Field-Effect Devices
The coupling of functional biomaterials with FEDs has a significant influence on the performance of biosensors. It is thus highly essential to achieve highly efficient coupling between functional biomaterials and FEDs [34][35]. Highly efficient coupling means not only maintaining the structure and functions of functional biomaterials to make them suitable to serve as the sensitive elements for chemical sensing, but also to transduce the responsive signals into the output signals via FEDs. The output signals will then be further processed by the peripheral circuits [36][37]. Therefore, biosensors usually require the related peripheral circuits and measurement setup to realize the detection of chemical signals.
Functional biomaterials used for the development of biosensors are mainly divided into two categories, i.e., cellular/tissue biomaterials [38] and biomolecules [5][37]. As shown in Figure 4, for cellular/tissue biomaterials, it is ideal to provide a surface that is similar to the cell culture dish, which can provide good surface hydrophilicity and proper surface charges for cell or tissue culture and attachment. However, the surface of FEDs usually consists of silicon dioxide or metal oxide, which shows poor biocompatibility and makes it unsuitable for direct cell or tissue attachment and culture. To improve the biocompatibility of FEDs, a surface modification process is usually required before cell or tissue attachment as reported in some cases [38]. For example, poly-l-ornithine and laminin mixture with a proper rate have been utilized to treat the surface of FEDs to achieve better coupling between cells and FEDs [32]. However, at present, it is still a huge challenge to obtain ideal coupling between cell membrane and the surface of FEDs for the development of biosensors towards chemical sensing.
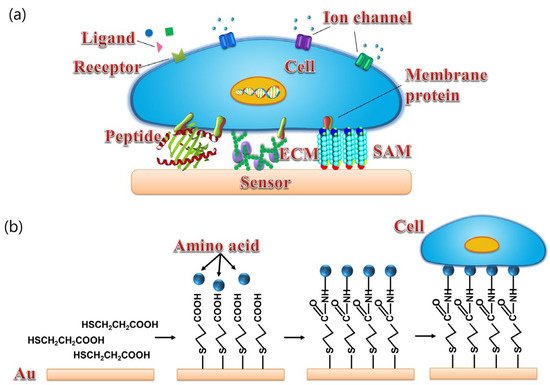
Figure 4. (a) Schematics of different surface modification of transducers for cell coupling with sensor including peptide, ECM, and SAM. (b) Schematic diagram of cells coupled with gold surface via SAM. (Reprinted with permission from ref. [38]. Copyright 2014 American Chemical Society).
For biomolecules, highly efficient coupling with FEDs usually requires capturing functional biomolecules and avoiding the non-specific adsorption of unrelated molecules to improve the specificity of the biosensors. Current available immobilization approaches mainly include physical adsorption, covalent attachment via chemical reactions, and specific binding via couple molecular pairs, such as a biotin–avidin system. It is crucial to choose the optimal approach to develop biosensors according to the properties of functional biomaterials and surface characters of transducers, since each approach has its intrinsic advantages and disadvantages. For example, physical adsorption has the advantage of being simple, label-free, and reproducible, but it often suffers from the instability of coupling since it can be easily disrupted by minor changes in the microenvironment such as salt density. On the other hand, covalent attachment is much more stable and robust than physical adsorption. In addition, it provides an approach to regulate the surface density of biomolecules, which is very important for achieving optical performances of biosensors [23]. However, the process of covalent attachment is complex and usually require the modification of biomaterials or sensitive surface of transducers, which hamper their applications to some extent. Similarly, the biotin–avidin system can provide strong and robust noncovalent binding between biomolecules and the gate surface of FEDs, which shows very high affinity due to the specific strong interactions between avidin and streptavidin. The biotin–(strept)avidin complex is very strong and robust even in complex environments, which contribute greatly to the repeatability and reproducibility of biosensors. However, the biotin–avidin system also suffers from the complex labelling and reaction process. In general, to obtain the best performances of biosensors, the key point is to specifically couple the functional biomaterials with transducers with high specificity and high stability, which could help to avoid the nonspecific adsorption and generate stable and highly sensitive responsive signals. In addition, it is also very important to maintain the natural sensing functions of biomolecules, especially for those membrane receptors such as olfactory and taste receptors. A hydrophobic environment often needs to be provided, which is crucial to maintaining the chemical sensing function of membrane receptors [26].
References
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans Can Discriminate More than 1 Trillion Olfactory Stimuli. Science 2014, 343, 1370–1372.
- Maffei, A.; Haley, M.; Fontanini, A. Neural processing of gustatory information in insular circuits. Curr. Opin. Neurobiol. 2012, 22, 709–716.
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391.
- Cheema, J.A.; Carraher, C.; Plank, N.O.V.; Travas-Sejdic, J.; Kralicek, A. Insect odorant receptor-based biosensors: Current status and prospects. Biotechnol. Adv. 2021, 53, 107840.
- Du, L.P.; Wu, C.S.; Liu, Q.J.; Huang, L.Q.; Wang, P. Recent advances in olfactory receptor-based biosensors. Biosens. Bioelectron. 2013, 42, 570–580.
- Wu, T.Z. A piezoelectric biosensor as an olfactory receptor for odour detection: Electronic nose. Biosens. Bioelectron. 1999, 14, 9–18.
- Huotari, M.J. Biosensing by insect olfactory receptor neurons. Sens. Actuators B Chem. 2000, 71, 212–222.
- Liu, Q.J.; Cai, H.; Xu, Y.; Li, Y.; Li, R.; Wang, P. Olfactory cell-based biosensor: A first step towards a neurochip of bioelectronic nose. Biosens. Bioelectron. 2006, 22, 318–322.
- Wu, C.S.; Chen, P.H.; Yu, H.; Liu, Q.J.; Zong, X.L.; Cai, H.; Wang, P. A novel biomimetic olfactory-based biosensor for single olfactory sensory neuron monitoring. Biosens. Bioelectron. 2009, 24, 1498–1502.
- Wu, C.S.; Du, L.P.; Zou, L.; Zhao, L.H.; Huang, L.Q.; Wang, P. Recent advances in taste cell- and receptor-based biosensors. Sens. Actuators B Chem. 2014, 201, 75–85.
- Kim, T.H.; Lee, S.H.; Lee, J.; Song, H.S.; Oh, E.H.; Park, T.H.; Hong, S. Single-Carbon-Atomic-Resolution Detection of Odorant Molecules using a Human Olfactory Receptor-based Bioelectronic Nose. Adv. Mater. 2009, 21, 91–94.
- Wu, C.S.; Du, L.P.; Wang, D.; Wang, L.; Zhao, L.H.; Wang, P. A novel surface acoustic wave-based biosensor for highly sensitive functional assays of olfactory receptors. Biochem. Bioph. Res. Commun. 2011, 407, 18–22.
- Benilova, I.V.; Vidic, J.M.; Pajot-Augy, E.; Soldatkin, A.P.; Martelet, C.; Jafftezic-Renault, N. Electrochemical study of human olfactory receptor OR17-40 stimulation by odorants in solution. Mat. Sci. Eng. C 2008, 28, 633–639.
- Hou, Y.X.; Jaffrezic-Renault, N.; Martelet, C.; Zhang, A.D.; Minic-Vidic, J.; Gorojankina, T.; Persuy, M.A.; Pajot-Augy, E.; Salesse, R.; Akimov, V.; et al. A novel detection strategy for odorant molecules based on controlled bioengineering of rat olfactory receptor 17. Biosens. Bioelectron. 2007, 22, 1550–1555.
- Marrakchi, M.; Vidic, J.; Jaffrezic-Renault, N.; Martelet, C.; Pajot-Augy, E. A new concept of olfactory biosensor based on interdigitated microelectrodes and immobilized yeasts expressing the human receptor OR17-40. Eur. Biophys. J. 2007, 36, 1015–1018.
- Vidic, J.; Pla-Roca, M.; Grosclaude, J.; Persuy, M.A.; Monnerie, R.; Caballero, D.; Errachid, A.; Hou, Y.X.; Jaffrezic-Renault, N.; Salesse, R.; et al. Gold surface functionalization and patterning for specific immobilization of olfactory receptors carried by nanosomes. Anal. Chem. 2007, 79, 3280–3290.
- Kim, T.H.; Song, H.S.; Jin, H.J.; Lee, S.H.; Namgung, S.; Kim, U.K.; Park, T.H.; Hong, S. “Bioelectronic super-taster” device based on taste receptor-carbon nanotube hybrid structures. Lab Chip 2011, 11, 2262–2267.
- Wu, C.S.; Du, L.P.; Zou, L.; Huang, L.Q.; Wang, P. A biomimetic bitter receptor-based biosensor with high efficiency immobilization and purification using self-assembled aptamers. Analyst 2013, 138, 5989–5994.
- Song, H.S.; Kwon, O.S.; Lee, S.H.; Park, S.J.; Kim, U.K.; Jang, J.; Park, T.H. Human Taste Receptor-Functionalized Field Effect Transistor as a Human-Like Nanobioelectronic Tongue. Nano Lett. 2013, 13, 172–178.
- Du, L.P.; Zou, L.; Zhao, L.H.; Huang, L.Q.; Wang, P.; Wu, C.S. Label-free functional assays of chemical receptors using a bioengineered cell-based biosensor with localized extracellular acidification measurement. Biosens. Bioelectron. 2014, 54, 623–627.
- Katzen, F.; Chang, G.; Kudlicki, W. The past, present and future of cell-free protein synthesis. Trends Biotechnol. 2005, 23, 150–156.
- Kaiser, L.; Graveland-Bikker, J.; Steuerwald, D.; Vanberghem, M.; Herlihy, K.; Zhang, S.G. Efficient cell-free production of olfactory receptors: Detergent optimization, structure, and ligand binding analyses. Proc. Natl. Acad. Sci. USA 2008, 105, 15726–15731.
- Chen, F.M.; Wang, J.; Du, L.P.; Zhang, X.; Zhang, F.; Chen, W.; Cai, W.; Wu, C.S.; Wang, P. Functional expression of olfactory receptors using cell-free expression system for biomimetic sensors towards odorant detection. Biosens. Bioelectron. 2019, 130, 382–388.
- Du, L.P.; Chen, W.; Tian, Y.L.; Zhu, P.; Wu, C.S.; Wang, P. A biomimetic taste biosensor based on bitter receptors synthesized and purified on chip from a cell-free expression system. Sens. Actuators B Chem. 2020, 312, 127949.
- Wu, C.S.; Wang, L.J.; Zhou, J.; Zhao, L.H.; Wang, P. The progress of olfactory transduction and biomimetic olfactory-based biosensors. Chin. Sci. Bull. 2007, 52, 1886–1896.
- Wu, C.S.; Lillehoj, P.B.; Wang, P. Bioanalytical and chemical sensors using living taste, olfactory, and neural cells and tissues: A short review. Analyst 2015, 140, 7048–7061.
- Wu, C.S.; Du, Y.W.; Huang, L.Q.; Galeczki, Y.B.; Dagan-Wiener, A.; Naim, M.; Niv, M.Y.; Wang, P. Biomimetic Sensors for the Senses: Towards Better Understanding of Taste and Odor Sensation. Sensors 2017, 17, 2881.
- Kwon, D.; Jung, G.; Shin, W.; Jeong, Y.; Hong, S.; Oh, S.; Kim, J.; Bae, J.H.; Park, B.G.; Lee, J.H. Efficient fusion of spiking neural networks and FET-type gas sensors for a fast and reliable artificial olfactory system. Sens. Actuators B Chem. 2021, 345, 130419.
- Schutz, S.; Schoning, M.J.; Schroth, P.; Malkoc, U.; Weissbecker, B.; Kordos, P.; Luth, H.; Hummel, H.E. An insect-based BioFET as a bioelectronic nose. Sens. Actuat. B Chem. 2000, 65, 291–295.
- Schoning, M.J.; Schroth, P.; Schutz, S. The use of insect chemoreceptors for the assembly of biosensors based on semiconductor field-effect transistors. Electroanalysis 2000, 12, 645–652.
- Hafeman, D.G.; Parce, J.W.; Mcconnell, H.M. Light-Addressable Potentiometric Sensor for Biochemical Systems. Science 1988, 240, 1182–1185.
- Ismail, A.B.M.; Yoshinobu, T.; Iwasaki, H.; Sugihara, H.; Yukimasa, T.; Hirata, I.; Iwata, H. Investigation on light-addressable potentiometric sensor as a possible cell-semiconductor hybrid. Biosens. Bioelectron. 2003, 18, 1509–1514.
- Bronder, T.S.; Poghossian, A.; Scheja, S.; Wu, C.S.; Keusgen, M.; Mewes, D.; Schoning, M.J. DNA Immobilization and Hybridization Detection by the Intrinsic Molecular Charge Using Capacitive Field-Effect Sensors Modified with a Charged Weak Polyelectrolyte Layer. ACS Appl. Mater. Inter. 2015, 7, 20068–20075.
- Son, M.; Kim, D.; Ko, H.J.; Hong, S.; Park, T.H. A portable and multiplexed bioelectronic sensor using human olfactory and taste receptors. Biosens. Bioelectron. 2017, 87, 901–907.
- Yang, H.; Lee, M.; Kim, D.; Hong, S.; Park, T.H. Bioelectronic nose using olfactory receptor-embedded nanodiscs. Methods Mol. Biol. 2018, 1820, 239–249.
- Full, J.; Baumgarten, Y.; Delbrück, L.; Sauer, A.; Miehe, R. Market perspectives and future fields of application of odor detection biosensors within the biological transformation—A systematic analysis. Biosensors 2021, 11, 93.
- Barbosa, A.J.M.; Oliveira, A.R.; Roque, A.C.A. Protein- and Peptide-Based Biosensors in Artificial Olfaction. Trends Biotechnol. 2018, 36, 1244–1258.
- Liu, Q.J.; Wu, C.S.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-Based Biosensors and Their Application in Biomedicine. Chem. Rev. 2014, 114, 6423–6461.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
928
Revisions:
2 times
(View History)
Update Date:
07 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




