
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elena-Emilia Tudoroiu | + 5353 word(s) | 5353 | 2021-12-09 08:44:27 |
Video Upload Options
Notwithstanding the progress regarding wound-healing management, the treatment of the majority of skin lesions still represents a serious challenge for biomedical and pharmaceutical industries. Thus, the attention of the researchers has turned to the development of novel materials based on cellulose derivatives. Cellulose derivatives are semi-synthetic biopolymers, which exhibit high solubility in water and represent an advantageous alternative to water-insoluble cellulose. These biopolymers possess excellent properties, such as biocompatibility, biodegradability, sustainability, non-toxicity, non-immunogenicity, thermo-gelling behavior, mechanical strength, abundance, low costs, antibacterial effect, and high hydrophilicity. They have an efficient ability to absorb and retain a large quantity of wound exudates in the interstitial sites of their networks and can maintain optimal local moisture. Cellulose derivatives also represent a proper scaffold to incorporate various bioactive agents with beneficial therapeutic effects on skin tissue restoration. Due to these suitable and versatile characteristics, cellulose derivatives are attractive and captivating materials for the development of multiple biomedical and pharmaceutical applications, such as wound dressings, drug delivery devices, and tissue engineering.
1. Introduction
2. Wound Dressings: Properties and Classification
In past years, due to the technology's noteworthy progress, various wound dressings were formulated worldwide to cure all types of tissue lesions. Dressings play a fundamental role in wound healing management, because these protect tissue lesions from external invasion (wound dressings are permeable for oxygen and moisture and function as physical barriers) [26], preventing the infection on the wound site [27]. Moreover, dressings contribute to the regeneration and restoration of epidermis and dermis layers [28][29].
2.1. Wound Dressing Properties
For the development of dressings, which allow rapid healing, with minimal scars on the body surface, it is necessary to develop new biopolymeric materials that accomplish some properties to create the ideal wound dressing that are reviewed in Figure 1.
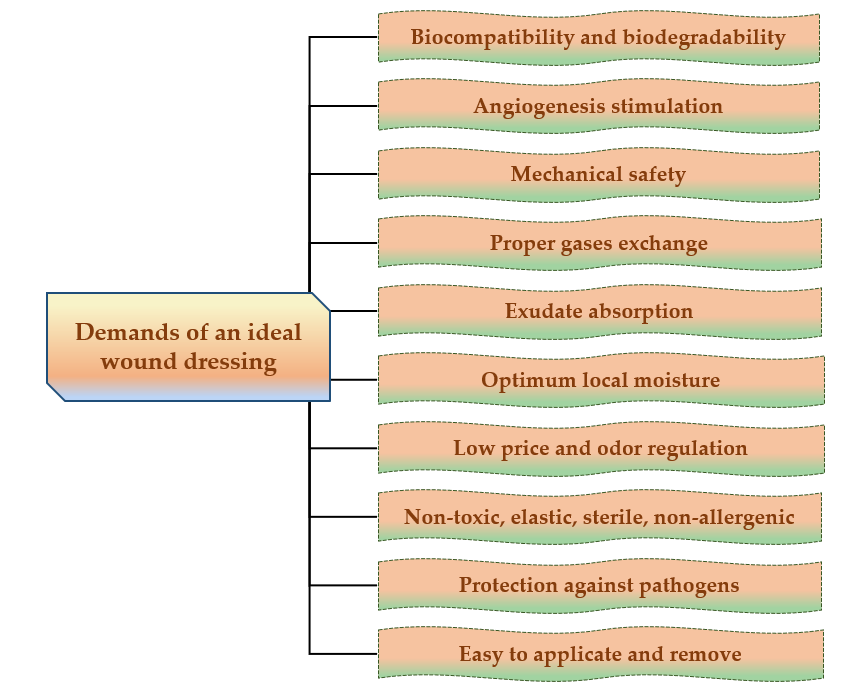
Figure 1. Major demands of an ideal wound dressing.
The ideal wound dressing preferably presents the following features: biocompatibility, biodegradability, non-toxicity, chemical inertness [30], to be applied effortlessly, to have the capacity to keep local moisture, to ensure a suitable exchange of gases (O2 and CO2), to absorb exudates that form on the lesion site [31], to stimulate the angiogenesis, to protect against extraneous pathogens, to clear the injured tissue, to eliminate nonviable tissues, to reduce the exposed area [32], to be able to be removed and replaced without difficulty [33], to adjust the odor, to sustain an adequate temperature to the lesion bed, to promote the blood circulation, and to stimulate cell expansion, to ensure mechanical safety [34]. Also, wound dressings materials must be elastic, sterile, non-adherent, non-allergenic [35], to have an acceptable price and to provide thermal insulation [36].
2.2. Wound Dressing Classification
A potential classification of wound dressings comprises passive dressings and active dressings, depending on the presence or absence of one or more pharmacologically active substances or natural substances [37], which can act to the site of the lesion, with local or systemic action, conditioned by the depth of the wound. Moreover, the progress of manufacturing led to the evolution of wound dressings from traditional dressings to modern (advanced) dressings [38].
Passive dressings can be considered dry traditional dressings, which are fundamental for a faster wound healing process. There is a wide simple range of passive dressings for several types of skin lesions: cotton wool, lint, gauze, natural and synthetic bandages – they work as primary dressing or secondary dressing [34][39]. Active dressings contain a large variety of pharmacologically active substances (antibiotics or other antimicrobials, non-steroidal anti-inflammatory, analgesic, antifungal, and local anesthetics drugs) or natural substances (plant extracts) with anti-inflammatory, astringent, emollient, epithelializing, antioxidant, demulcent, antimicrobial properties [28].
Modern or advanced dressings were designed to cover tissue lesions and in this category are included the hydrogels, hydrocolloids, semi-permeable films, semi-permeable foams, and alginate dressings [40][41]. The biggest difference between traditional and modern dressings is the local moisture maintenance. Thus, traditional dressings have a lower capacity to maintain the local moisture on the wound site [38], and modern dressings sustain excellent local moisture to enhance wound healing [42].The classification of wound dressings is illustrated in Figure 2.
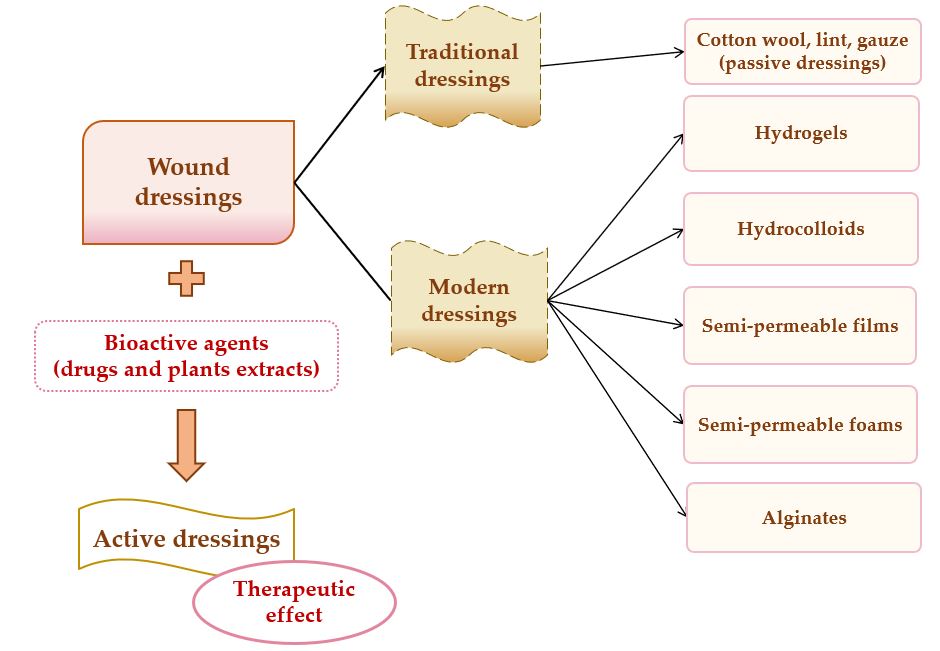
Figure 2. Wound dressings classification.
The main materials underlying the modern wound dressings are polymers, which can be natural (collagen, gelatin, cellulose, hemicellulose, chitin, chitosan, pectins and gums, chondroitin sulfate, alginic acid and alginates, agar, dextran, carrageenan, elastin, hyaluronic acid, silk fibroin, fibrinogen, and fibrin) [43][44], semi-synthetic (cellulose derivatives) [45] or synthetic (poly(α-ester)s, polyanhydrides, polycarbonates, poly(amide), poly(esteramide)s, polyphosphazenes, polyurethanes, pseudo poly(amino acids), polyacetals) [46][47][48].
3. Cellulose Derivatives as Wound Dressings
3.1. Cellulose Derivatives Classification
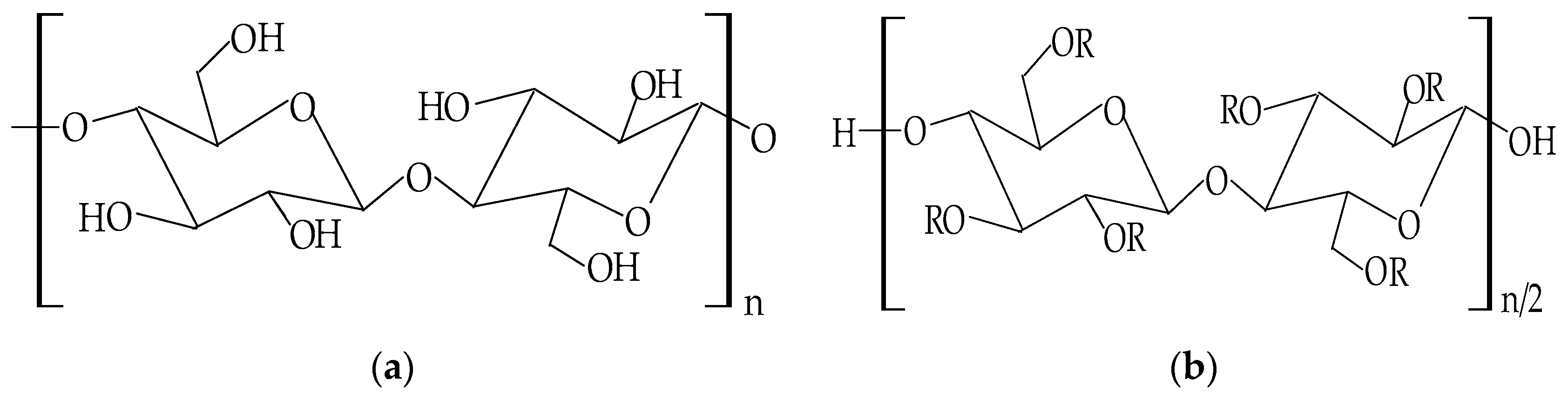
| Cellulose Ethers | R Groups |
|---|---|
| Methylcellulose | H, CH3 |
| Ethylcellulose | H, CH2CH3 |
| Benzylcellulose | H, C6H5CH2 |
| Sodium carboxymethylcellulose | H, CH2COONa |
| Hydroxyethylcellulose | H, [CH2CH2O]nH |
| Hydroxypropylcellulose | H, [CH2CH(CH3)O]nH |
| Hydroxyethylmethylcellulose | H, CH3, [CH2CH2O]nH |
| Hydroxypropylmethylcellulose | H, CH3, [CH2CH(CH3)O]nH |
| Cellulose Esters | R Groups |
|---|---|
| Acetate | H, I |
| Acetate trimelliate | H, I, II |
| Acetate phthalate | I, III |
| Hydroxypropylmthylphthalate | H, CH3, CH2CH(OH)CH3, III, IV |
| Hydroxypropylmthylphthalate acetate succinate |
H, CH3, CH2CH(OH)CH3, III, V |
Due to the general properties of wound dressings presented in Section 2.1., but also the particular properties, such as hydrophilicity, mechanical toughness, pH stability, and rheological characteristics, cellulose and cellulose derivatives have multiple applications in many fields [67]. Areas of the applicability of all these biopolymers involve: biomedical and pharmaceutical industries, where they can act as drug-delivery devices, wound dressings, muco- and bioadhesive drugs, excipients for drug formulations, and support for tissue engineering [68]; also, they can be used for cosmetic and hygienic products, in the textile area, in the food industry and agriculture [64][69]. The representation of cellulose derivatives-based wound dressing on an open wound is illustrated in Figure 4.
Figure 4. The representation of cellulose derivatives-based wound dressing on an open wound. This illustration has been created with BioRender.com, Inkscape, and PowerPoint.
3.2. Sodium Carboxymethylcellulose-Based Wound Dressings
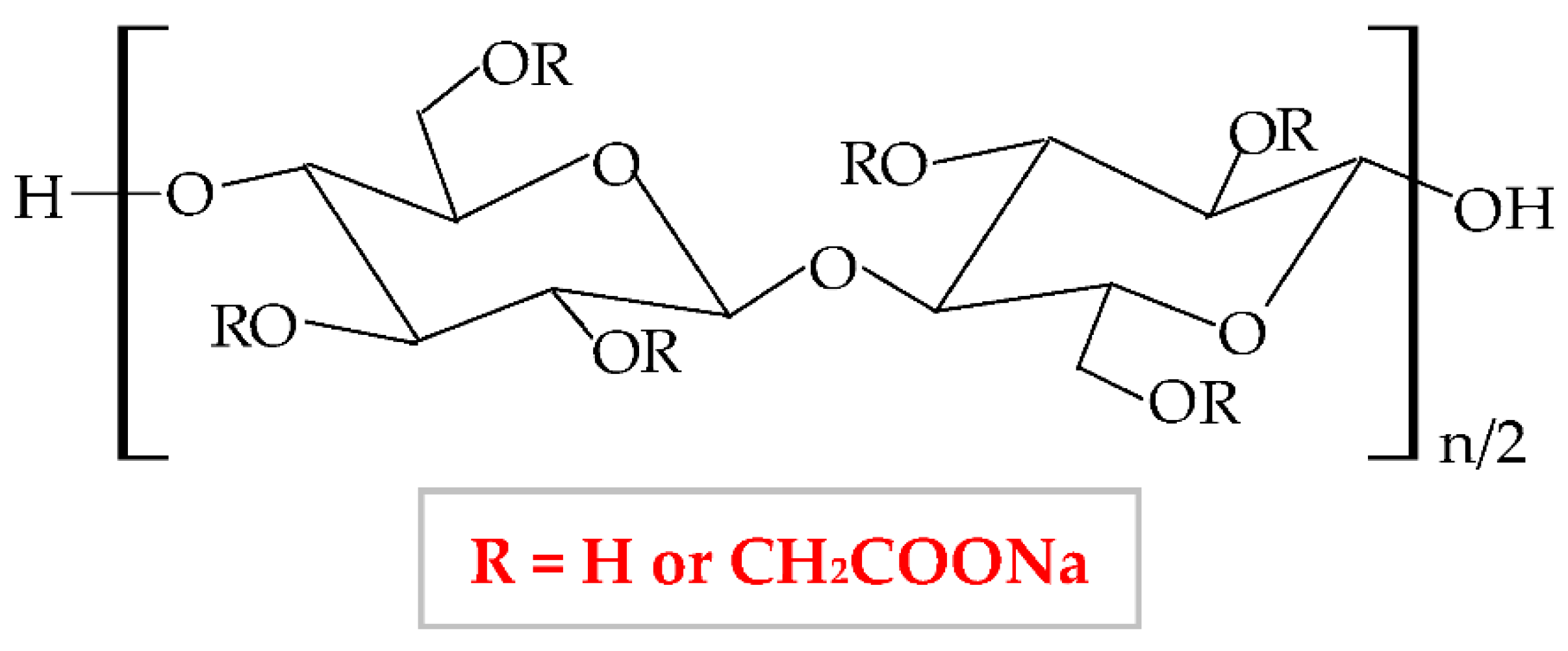
Figure 5. Chemical structure of sodium carboxymethylcellulose (NaCMC).
The NaCMC network illustrates a thixotropic behavior to generate 3D structures through intermolecular attraction. Its thixotropy is influenced by concentration and degree of substitution [76]. NaCMC presents excellent physicochemical and mechanical properties [77], optimal biocompatibility and biodegradability, proper capacity to absorb the water and to swell, high gelation behavior, non-toxicity, and low-immunogenicity [78]. It is the most used cellulose derivative in the pharmaceutical industry, mainly for the development of new wound dressings because it has the capacity to absorb heavy exudates [79][80], to ensure excellent moisture at the lesion site, and to avoid skin tissues water loss and tissues necrosis. Moreover, an optimal local humidity can impede dehydration, facilitate the synergy between target cells and growth factors, promote angiogenesis advancement, the mitigation of the ache, and the disruption of the fibrin network [81]. NaCMC is also used as a drug-delivery device and excipient for drug formulations (used as an emulsifier, thickener, stabilizer, and film-maker) [82]. Besides its applicability in the pharmaceutical area, this biopolymer possesses different usefulness in the food (E466 food additive) industry [83], in paper, textile and cosmetics domains [73][84], for tissue culture and dental medicine field [85].NaCMC can be combined with other polymers to enhance its properties and to develop its applicability. Thus, it is more advantageous to blend two or more polymers for the development of a new material comparative to the chemical industrial development of that material. Moreover, the new material obtained by mixing other well-known polymers presents all the properties or is more favorable than the component polymers [30]. Furthermore, the blend of polymers can be realized to compensate for their drawbacks. Hence, Liu et al., combined NaCMC with HEC by electrostatic complexing and obtained a sponge and a membrane with a porous network, enhanced viscoelastic properties, and high swelling behavior [86]. Hu et al., mixed NaCMC with PVA and quaternized chitosan and designed a new composite with enhanced flexibility, water absorption rate, mechanical strength, swelling ratio, and humidity permeability [87]. A novel NaCMC/PVA-based composite was formulated, with higher properties than two polymers: improved swelling capacity, elasticity, water solubility, porosity, water vapor transmission rate, bioavailability, and biodegradability for the tissue repair process; this formulation also presented an extension of its applicability, such as agriculture, biomedical field as drug delivery systems and food packaging [88][89]. NaCMC was blended with PEG through a photo-click reaction based on thiol-norbornene. It formed a pH-sensitive hydrogel with an augmented swelling ratio [85]. Zhang et al., designed a novel hydrogel based on NaCMC and sodium alginate. In a ratio of 1:4, the hydrogel exhibited high biocompatibility, mechanical characteristics, degradation rate, and local humidity [90]. Shin et al., blended NaCMC with PVA and PEG 400 through cyclic freezing/thawing method and obtained a hydrogel with improved properties: the swelling rate, the compressive strength, and cytocompatibility [91].
3.3. Hydroxypropylmethylcellulose-Based Wound Dressings
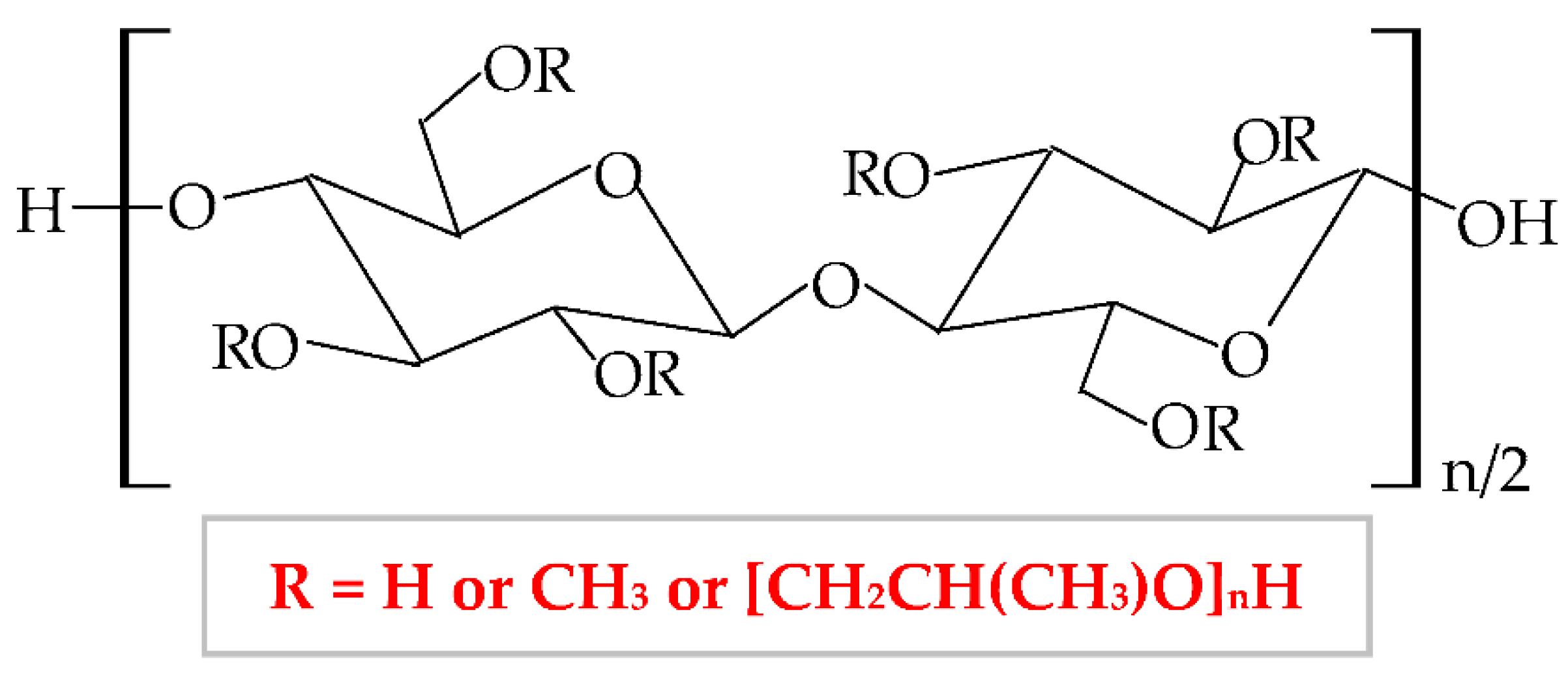
Therefore, HPMC presents many degrees of substitution, that give to this biopolymer different molecular weight and physicochemical features (rheological properties and crystalline nature) [95][96]. The hydrophilic or hydrophobic nature is related to the values of the degree of substitution (DS) and the molar substitution (MS). Thus, the HPMC molecule with decreased values of DS and MS is more hydrophilic and the HPMC molecule with increased values of DS and MS is more hydrophobic [97]. Following this chemical substitution, HPMC gets both polar (hydroxypropyl) and non-polar (methyl) character; consequently, it can form hydrophobic, intermolecular, and intramolecular linkages with many other materials [95]. The non-ionic character leads to a limited adhesive capacity [98]. At high temperature, the biopolymer can suffer a thermoreversible phase transition from sol to gel, with a temperature of gelation over 60°C, superior to the temperature of the body (37°C) [99]. HPMC-based hydrogels are temperature-responsive [100].
According to United States Pharmacopeia (USP), there are four distinct forms of HPMC, which are categorized by the content of methoxy, respectively hydroxypropoxy groups in: HPMC 1828, HPMC 2208, HPMC 2906, and HPMC 2910 [101]. This biopolymer has been approved as a food additive, E464 [102], by the American Institute, Food and Drug Administration (FDA), by the European Institution, European Parliament, and Council Directive, and by the Joint Expert Committee on Food Additives [103].
HPMC has a proper solubility in water, and it is one of the most used cellulose derivatives in many industries. It is widely used in the biotechnological field (construction, food, cosmetics, biomedical, and pharmaceutical industry), due to its excellent characteristics, such as biocompatibility, biodegradability, superior stability, large availability, excellent swelling, high surface activity, and mechanical properties [104], remarkable ability to form films and poor toxicity [105]. Regarding the applicability of HPMC in biomedical and pharmaceutical domains, it is used as a drug-delivery device, with a large practice for wound dressings development and it can also have remarkable applicability in tissue engineering [106]. HPMC can also be used as an excipient because it possesses proper abilities of emulsification, stabilization, suspension, and thickening [107][108].
HPMC can be combined with other polymers to enhance its properties and to develop its applicability [109]. To improve the physicochemical properties of a new composite, HPMC has been blended with several natural, semi-synthetic, or synthetic polymers [110]. In this way, to improve the thermal stability, HPMC has been blended with collagen [111][112], gelatin [109], chitosan [113], chitosan, and xanthan gum [114]; to improve the mechanical properties (tensile strength and ultimate elongation), HPMC has been mixed with chitosan [115], collagen [112], poloxamer 407 [116], silk fibroin [117], PVA and PVP [118], chitosan and xanthan gum [114]; to increase the swelling rate, HPMC has been combined with methylcellulose [119], κ-carrageenan [120], chitosan and hyaluronic acid [121], chitosan and xanthan gum [114].3.4. Methylcellulose-Based Wound Dressings
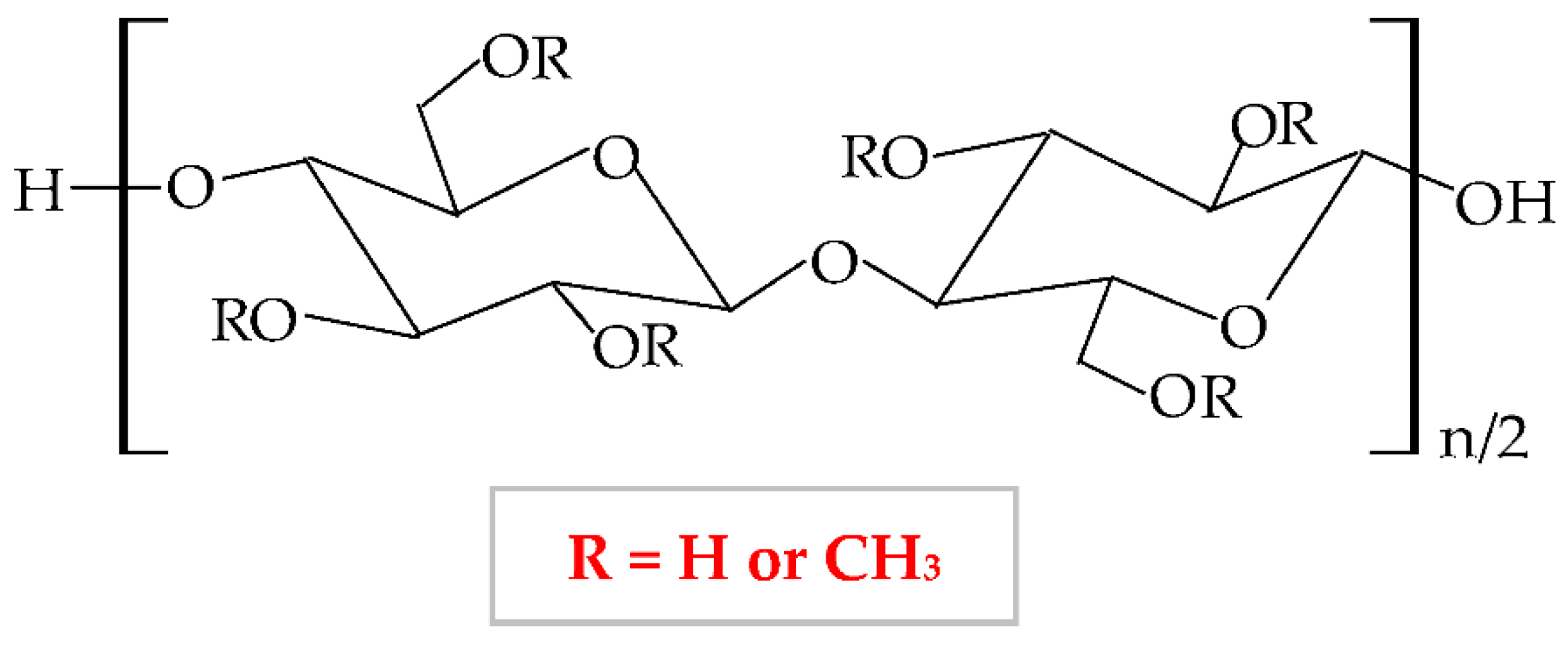
At a variation of temperature, MC has a thermo-sensitive behavior with a reversible sol-gel transition in an aqueous solution [125]. At a lower temperature than lower critical solution temperature, it realizes the hydration of the MC network in solution, with the formation of hydrogen bonds. At a higher temperature than lower critical solution temperature, the MC aqueous solution takes in the heat, with the disintegration of hydrogen bonds [100]. Thus, MC presents increased viscosity at higher temperatures, and at lower temperatures it exhibits a reduced viscosity [126].
The degree of substitution for commercial MC varies from 1.7 to 2.2 when it results in a semiflexible biopolymer because the inter-and intra- hydrogen bonds from cellulose molecule break off [127]. There are many substances, which influence the gelation behavior of MC, such as inorganics salts, ethanol, propylene glycol, polyethylene glycol 400, sucrose, glycerin, sorbitol, and different surfactants (sodium dodecyl sulfate and cetyltrimethylammonium bromide) [128]. MC is extensively used in biomedical, pharmaceutical, cosmetic, and food industries as a thickening, binding, and film-forming agent because it possesses excellent biocompatibility, biodegradability, and reduced toxicity [129][130][131].
To improve the characteristics of MC, it can be blended with other polymers in different ratios to enhance the physicochemical, morphological, and structural properties of both polymers and of the resulting composite [131]. Abu et al., illustrated that a higher concentration of MC led to increased hydrophilicity and porosity of the MC-chitosan scaffold due to the hydroxyl groups from the MC molecule, which can attract water molecules. The higher wettability has been described by the suitable results of the water uptake capacity [132]. Another combination of MC and chitosan was studied by Tan et al., They illustrated that an augmented concentration of MC led to improved tensile strength, moisture content, whitish index, and elongation at break [133]. El-Naggar et al., mixed MC with PVA and doxycycline hyclate (drug model) to develop a new drug delivery device, which showed a proper swelling capacity and a high drug release at basic medium [129]. The combination between MC and poly(acrylic acid) presented optimal mechanical properties and thermal stability [134]. The novel composite resulting by blending MC and tragacanth gum exhibited a higher capacity to form a gel and adequate mechanical and rheological properties [135].3.5. Hydroxyethylcellulose-Based Wound Dressings
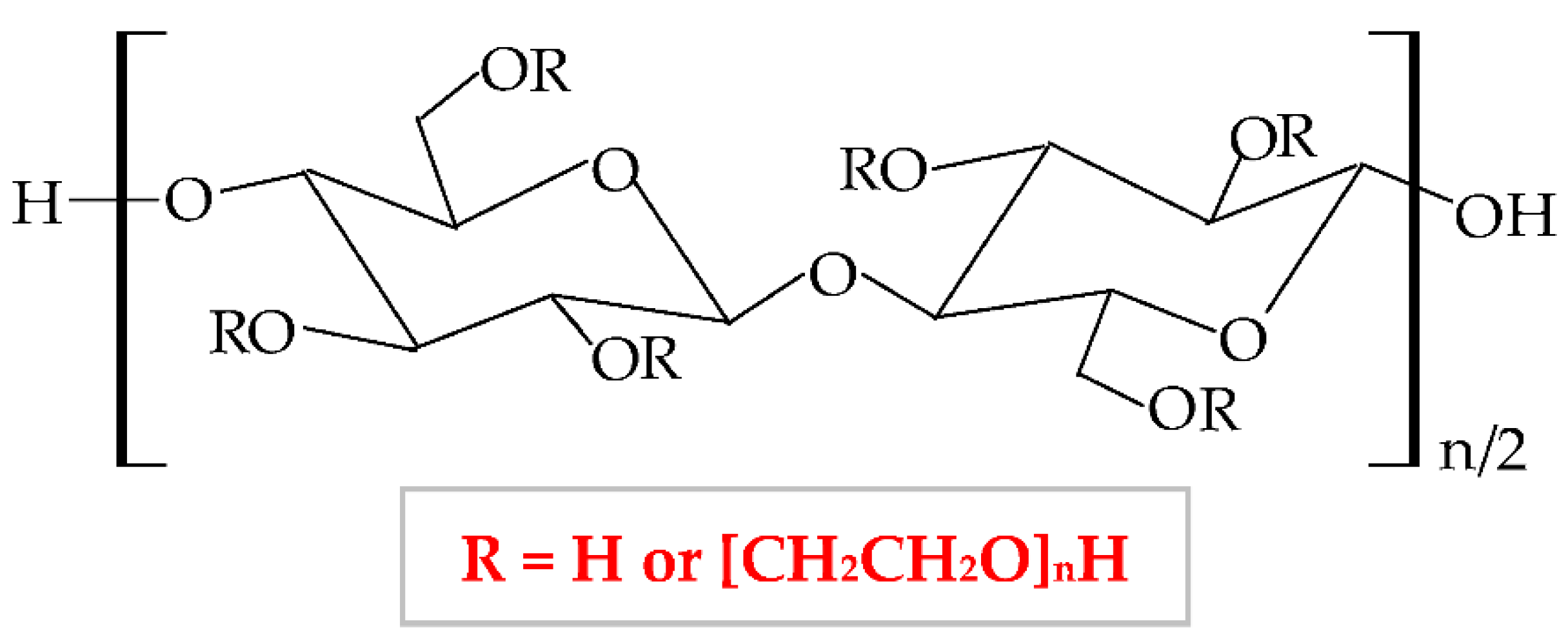
It has a low price, without taste and smell, with no color to light yellowish [138]; presents optimal stability at pH values between 2 and 12 [139]. HEC exhibits a proper capacity to scavenge free radicals and to form hydrogen and electrostatic bonds [140]. HEC is regarded as a hydrogel-like material, with two important characteristics: liquid-like and solid-like. Due to its polysaccharide structure, this hydrophilic biopolymer exhibits a high capacity to absorb and hold a large quantity of water or wound exudates. The elastic strength of its structure leads to an expansion of the molecule dimensions, without the modification of the structural stability and the gel form [141]. HEC possesses excellent physicochemical properties: rheological, hydrodynamic, and thermodynamic [142]. HEC also presents adequate biocompatibility, biodegradability, insignificant toxicity, immunogenicity, and cementing properties [143]. Due to its nonionic behavior, HEC exhibits the ability to coexist with a large field of other polymers, which have an appropriate solubility in water, salts, or surfactants. Therefore, HEC presents optimal toughness in a dielectric solution with a large concentration [144]. This biopolymer presents the largest commercial availability from all cellulose derivatives [145]; therefore, HEC is a noticeable biopolymer, which can be used successfully as an emulsifier, film-coating, stabilizer, suspender, and thickener agent in biomedical, pharmaceutical (wound dressing development) [146], cosmetic, food, adhesive, and textile industries [138][147][148]. The most predictive method for hydrogels synthesis is the crosslinking of free radicals generated by irradiation (electron beam and gamma-radiation) [149].
To enhance its properties, HEC can be blended with other polymers. For example, Zia et al., mixed HEC with poly(lactic acid) and polyurethane. They obtained a new composite with higher thermal stability and mechanical (tensile strength and elongation) properties compared to other polymers [150][151]. Moreover, HEC has been blended with polyvinyl alcohol (PVA), resulting in suitable electrical conductibility, viscoelasticity, stretchability, and thermosensitivity [152]. Guo et al., combined HEC with poly(caprolactone) by trimethylsilyl group technology and the result was the formation of a new copolymer with enhanced thermal properties [153]. HEC was also blended with chitosan to obtain a copolymer with improved physicochemical and mechanical characteristics [154], with gelatin to obtain a superparamagnetic composite [155], with sodium alginate to form a copolymer with enhanced swelling efficacy and drug delivery profile.3.6. Ethylcellulose-Based Wound Dressings
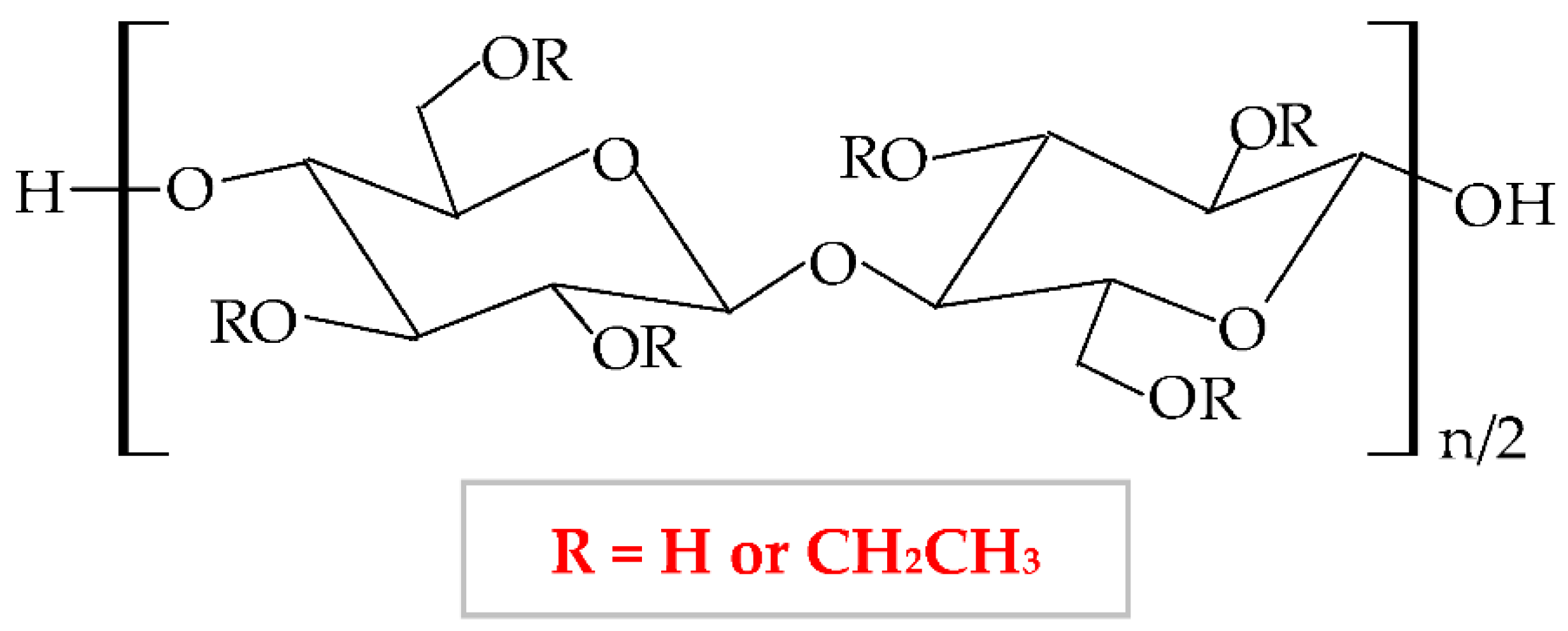
This biopolymer presents numerous advantageous characteristics, such as mechanical properties, biodegradability, flexibility, lowcity, hydrophobicity, gelling capacity [157], light, moisture, oxygen resistance, thermoplasticity [158], and low price, which make EC an excellent material for use in different industries (pharmaceutical, cosmetic and food) [159]. Moreover, this biopolymer has several particular features in addition to the other cellulose derivatives: high film-forming capacity, suitable chemical strength, and optimal mechanical properties [160]. EC represents the most extensively analyzed biopolymer due to its capacity to form film for coating solid pharmaceutical forms (tablets, microcapsules, and microspheres) and formulation of new topical forms [161]. EC is a promising material to be used for encapsulation due to its optimal optical transparency, processing temperature, and electronic insulation [162]. It also presents a good capacity to bind, preserve and dissolve [163], and possesses a proper control of drug delivery [164]. Films based on EC are brittle because of the stiffness of hydrogen linkages from its molecule. This biopolymer has high stability to chemical substances and can be associated with different plasticizers to design heavy and impermeable films [165].
EC can be mixed with various polymers to enhance the physicochemical and mechanical properties and thus, its applicability. To develop a novel drug-delivery device, Li et al., blended EC by electrospinning method with poly(di(ethylene glycol) methyl ether methacrylate), a thermosensitive polymer. The new formulation showed normal morphology, a large porosity, and an increased wettability at a higher temperature, which led to more hydrophobic behavior, causing an extended release of the drug [166]. EC was mixed with poly (ethylene-co-vinyl acetate) and resulted in a new composite with higher mechanical properties[167]. Chen et al., mixed EC and poly(β-hydroxybutyrate) when EC acted as a thickening agent because it increased the viscosity of the new composite. In a concentration of 1%, EC augmented the tensile strength [168]. Li et al., blended EC with konjac glucomannan to formulate a novel composite with higher mechanical properties, moisture resistance, permeability of oxygen, and stability at a high temperature [169]. EC was also associated with another cellulose derivative, HPC, and obtained a scaffold with enhanced mechanical properties and 3D printing capacity [170].3.7. Hydroxypropylcellulose-Based Wound Dressings
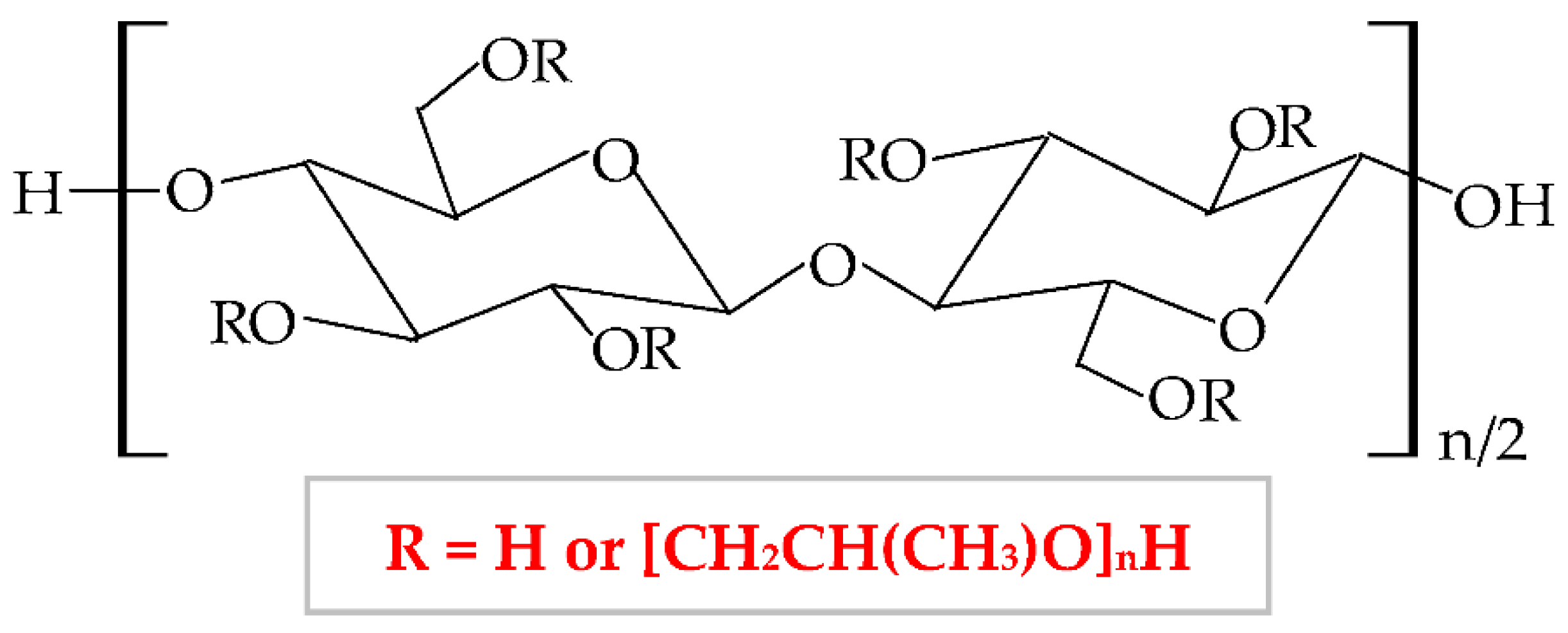
It has numerous advantageous properties, such as amphiphilicity, low price, electrical neutrality, biocompatibility, biodegradability, non-toxicity, high power of swelling the wounds exudate [173][174], adequate chemical strength, and film-forming efficiency [175]. At a high temperature and in a concentrated aqueous solution, HPC generates a cholesteric liquid crystalline network, depending on its concentration [176]. HPC exhibits a thermoplastic behavior and develops temperature-responsive hydrogels [100][177]. Regarding the HPC-based films, these are defined by high flexibility, good impermeability for oil and fat, and a low value of Tg (glass transition temperature) at excessive humidity. The LCST (lower critical solution temperature) water value is about 41°C. At a slightly higher temperature than LCST, HPC presents a phase change because the water solution of this biopolymer generates metastable nanosphere aggregates [178]. Moreover, the solubility of HPC is influenced by LCST values. At a lower temperature than LCST, HPC dissolves easily in water and at a higher temperature than LCST, HPC does not dissolve [179]. Thus, this cellulose derivative is an optimal material to be used in biomedical and pharmaceutical fields as a binding, disintegrating, emulsifying, thickening, filler, and coating agent [180][181] and in the construction domain [182]. It can also be used in the food industry because the United States Food and Drug Administration (FDA) authorized HPC as a safe food additive [183].
HPC can be blended with other polymers to improve the physicochemical and mechanical properties and thus, to extend its applicability. For instance, Veerapur et al., combined HPC and chitosan, and the new formulated composite presented higher hydrophilicity, swelling capacity, and permeation rate [184]. By mixing HPC with cellulose acetate phthalate resulted a composite with higher properties than compounds: increased pseudoplasticity and viscoelastic behavior [185]. Gan et al., prepared a high-performance hydrogel with enhanced tensile strength, toughness, biocompatibility, wear resistance, and low friction coefficient from HPC, sodium alginate, and poly(vinyl alcohol); these excellent characteristics extend the area of use to biosensors and nerve replacement [186]. Lu et al., blended HPC with poly(vinyl alcohol) to obtain a new scaffold with augmented toughness, elasticity, conductivity, and mechanical strength that is a promising material for the development of biosensors and interaction between humans and machines [187].3.8. Combinations of Cellulose Derivatives-Based Wound Dressings
| Biopolymer/-s | Active Pharmaceutical Ingredient (Natural or Synthetic Substances) |
Type of Wound Dressing | Main Findings | References |
|---|---|---|---|---|
| EC/HPMC | Paromomycin and Gentamicin | Film | Optimum drugs release and inhibition of Leishmania tropica growth. | [188] |
| Aloe vera | Nanofibers | Nanofibers with 10% Aloe vera showed suitable mechanical properties, biocompatibility, bioadhesion, and suitable antibacterial activity. | [189] | |
| NaCMC/HPMC | Grapefruit seed extract | Film | Suitable elongation at break, stability in water, and proper antibacterial action. | [190] |
| CuO | Film | Good biocompatibility and antibacterial effect. | [191] | |
| Tetracycline/Methylene blue | Film | Nanoporous network, increased Tg and elongation at break, sustained drug release for 72 h and high antibacterial effect. | [192] | |
| ZnO NPs | Film | Biocompatibility and optimum antibacterial action. | [193] | |
| NaCMC/HPMC/ CAB |
Resveratrol | Membrane | Excellent adhesive capacity, hydration efficiency, and higher porous structure; in vivo studies showed accelerated wound healing. | [194] |
| NaCMC/MC | Simvastatin | Membrane | In a ratio of 2:1, the membrane exhibited appropriate flexibility, viscosity, stability, and sponginess; optimal drug delivery for suppurating injuries. | [195] |
4. Conclusions and Future Perspectives
References
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W.; 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv Healthc Mater 2019, 8, e1801019, 10.1002/adhm.201801019.
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N.; Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int J Biol Macromol 2020, 147, 1239-1247, 10.1016/j.ijbiomac.2019.09.251.
- Zimmerman, A.; Bai, L.; Ginty, D.D.; The gentle touch receptors of mammalian skin. Science 2014, 346, 950-954, 10.1126/science.1254229.
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C.; et al. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379-391, 10.1016/j.biomaterials.2015.02.112.
- Kilic Bektas, C.; Kimiz, I.; Sendemir, A.; Hasirci, V.; Hasirci, N.; A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications. J Biomater Sci Polym Ed 2018, 29, 1764-1784, 10.1080/09205063.2018.1498718.
- Kolarsick, P.A.; Kolarsick, M.A.; Goodwin, C.; Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203-213, 10.1097/JDN.0b013e3182274a98.
- McLafferty, E.; Hendry, C.; Alistair, F.; The integumentary system: anatomy, physiology and function of skin.. Nurs. Stand. R. Coll. Nurs. G. B. 2012, 27, 35-42, 10.7748/ns2012.09.27.3.35.c9299.
- Joodaki, H.; Panzer, M.B.; Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2018, 232, 323-343, 10.1177/0954411918759801.
- Lai-Cheong, J.E.; McGrath, J.A.; Structure and function of skin, hair and nails. Medicine 2021, 49, 337-342, 10.1016/j.mpmed.2021.03.001.
- Brown, T.M.; Krishnamurthy, K. . StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020; pp. -.
- Hashmi, S.; Marinkovich, M.P.; Molecular organization of the basement membrane zone. Clin Dermatol 2011, 29, 398-411, 10.1016/j.clindermatol.2011.01.009.
- Zimoch, J.; Zielinska, D.; Michalak-Micka, K.; Rütsche, D.; Böni, R.; Biedermann, T.; Klar, A.S.; Bioengineering a prevascularized human tri-layered skin substitute containing a hypodermis. Acta Biomater 2021, 134, 215-227, 10.1016/j.actbio.2021.07.033.
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K.; The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92-98, 10.1111/exd.12832.
- Bragazzi, N.L.; Sellami, M.; Salem, I.; Conic, R.; Kimak, M.; Pigatto, P.D.M.; Damiani, G.; Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients 2019, 11, 249, 10.3390/nu11020249.
- Rodrigues, C.; de Assis, A.M.; Moura, D.J.; Halmenschlager, G.; Saffi, J.; Xavier, L.L.; Fernandes Mda, C.; Wink, M.R.; New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS One 2014, 9, e96241, 10.1371/journal.pone.0096241.
- Okoye, E.; Okolie, T.; Development and in vitro characterization of ciprofloxacin loaded polymeric films for wound dressing. Int. J. Health Allied Sci. 2015, 4, 234, 10.4103/2278-344x.167660.
- Varshosaz, J.; Taymouri, S.; Minaiyan, M.; Rastegarnasab, F.; Baradaran, A.; Development and in vitro/in vivo evaluation of HPMC/chitosan gel containing simvastatin loaded self-assembled nanomicelles as a potent wound healing agent. Drug Dev. Ind. Pharm. 2018, 44, 276-288, 10.1080/03639045.2017.1391832.
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Jarbrink, K.; Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8-15, 10.1016/j.annepidem.2018.10.005.
- Sen, C.K.; Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281-292, 10.1089/wound.2021.0026.
- Walker, J.; Cullen, M.; Chambers, H.; Mitchell, E.; Steers, N.; Khalil, H.; Identifying wound prevalence using the Mobile Wound Care program. Int. Wound J. 2014, 11, 319-325, 10.1111/iwj.12118.
- Kapp, S.; Miller, C.; Santamaria, N.; The quality of life of people who have chronic wounds and who self-treat. J. Clin. Nurs. 2018, 27, 182-192, 10.1111/jocn.13870.
- Moreira, M.E.; Markovchick, V.J.; Wound Management. Crit. Care Nurs. Clin. N. Am. 2012, 24, 215-237, 10.1016/j.ccell.2012.03.008.
- Ghica, M.V.; Kaya, M.G.A.; Dinu-Pirvu, C.E.; Lupuleasa, D.; Udeanu, D.I.; Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552, 10.3390/molecules22091552.
- Clark, M.. Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer Series in Biomaterials Science and Engineering; Springer-Verlag Berlin: Berlin, 2018; pp. 213-222.
- Aramwit, P.. Wound Healing Biomaterials; Agren, M.S., Eds.; Woodhead Publ Ltd: Cambridge, 2016; pp. 3-38.
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C.; Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater 2013, 9, 7093-7114, 10.1016/j.actbio.2013.03.033.
- Kus, K.J.B.; Ruiz, E.S.; Wound Dressings - A Practical Review. Curr. Dermatol. Rep. 2020, 9, 298-308, 10.1007/s13671-020-00319-w.
- Mogoşanu, G.D.; Grumezescu, A.M.; Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127-136, 10.1016/j.ijpharm.2013.12.015.
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S.; Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J, Biol. Macromol. 2016, 85, 327-334, 10.1016/j.ijbiomac.2015.12.076.
- Trevisol, T.C.; Fritz, A.R.M.; de Souza, S.M.A.G.U.; Bierhalz, A.C.K.; Valle, J.A.B.; Alginate and carboxymethyl cellulose in monolayer and bilayer films as wound dressings: Effect of the polymer ratio. J. Appl. Polym. Sci. 2019, 136, 46941, 10.1002/app.46941.
- Mishra, S.K.; Mary, D.S.; Kannan, S.; Copper incorporated microporous chitosan-polyethylene glycol hydrogels loaded with naproxen for effective drug release and anti-infection wound dressing. Int. J. Biol. Macromol. 2017, 95, 928-937, 10.1016/j.ijbiomac.2016.10.080.
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S.; Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1-14, 10.1016/j.arabjc.2014.07.005.
- Patil, P.P.; Reagan, M.R.; Bohara, R.A.; Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613-4627, 10.1016/j.ijbiomac.2020.08.041.
- Dhivya, S.; Padma, V.V.; Santhini, E.; Wound dressings - a review. Biomedicine 2015, 5, 22, 10.7603/s40681-015-0022-9.
- Marin, S.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pirvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M.; Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224, 10.3390/pharmaceutics10040224.
- Akrami-Hasan-Kohal, M.; Tayebi, L.; Ghorbani, M.; Curcumin-loaded naturally-based nanofibers as active wound dressing mats: morphology, drug release, cell proliferation, and cell adhesion studies. New J. Chem. 2020, 44, 10343-10351, 10.1039/d0nj01594f.
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D.; Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148, 10.1016/j.apmt.2021.101148.
- Farahani, M.; Shafiee, A.; Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477, 10.1002/adhm.202100477.
- Sharma, G.; Lee, S.W.; Atanacio, O.; Parvizi, J.; Kim, T.K.; In search of the optimal wound dressing material following total hip and knee arthroplasty: a systematic review and meta-analysis. Int. Orthop. 2017, 41, 1295-1305, 10.1007/s00264-017-3484-4.
- Pawar, H.V.; Tetteh, J.; Boateng, J.S.; Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloid Surf. B Biointerfaces 2013, 102, 102–110, 10.1016/j.colsurfb.2012.08.014.
- Kong, D.; Zhang, Q.; You, J.; Cheng, Y.; Hong, C.; Chen, Z.; Jiang, T.; Hao, T.; Adhesion loss mechanism based on carboxymethyl cellulose-filled hydrocolloid dressings in physiological wounds environment. Carbohydr. Polym. 2020, 235, 115953, 10.1016/j.carbpol.2020.115953.
- Yadav, V.; Mittal, A.; Bansal, P.; Singh, S.K.; Regulatory approval process for advanced dressings in India: an overview of rules. J. Wound Care 2019, 28, S32-S42, 10.12968/jowc.2019.28.Sup8.S32.
- Olatunji, O.. Natural Polymers; Olatunji, O., Eds.; Springer, Cham: Switzerland, 2016; pp. 1-17.
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S.; Natural polymers–A comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597-1613.
- Alves, T.F.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P.J.C.; Applications of natural, semi-synthetic, and synthetic polymers in cosmetic formulations. Cosmetics 2020, 7, 75, 10.3390/cosmetics7040075.
- Bhatia, S. . Natural Polymer Drug Delivery Systems; Springer, Cham: Switzerland, 2016; pp. 95-118.
- Maitz, M.F.; Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161-176, 10.1016/j.bsbt.2015.08.002.
- Gunatillake, P.; Mayadunne, R.; Adhikari, R.; Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301-347.
- Crabbe-Mann, M.; Tsaoulidis, D.; Parhizkar, M.; Edirisinghe, M.; Ethyl cellulose, cellulose acetate and carboxymethyl cellulose microstructures prepared using electrohydrodynamics and green solvents. Cellulose 2018, 25, 1687-1703, 10.1007/s10570-018-1673-y.
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al.et al. Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021, 259, 117740, 10.1016/j.carbpol.2021.117740.
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D.. Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Eds.; IntechOpen: 5 Princes Gate Court London, United Kindom, 2016; pp. 64.
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, A.H.P.; Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557, 10.3390/nano10030557.
- Heinze, T. . Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Eds.; Springer-Verlag Berlin: Berlin, 2016; pp. 1-52.
- Miao, J.J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S.; Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557-9567, 10.1016/j.biomaterials.2011.08.080.
- Wang, Y.G.; Wang, X.J.; Xie, Y.J.; Zhang, K.; Functional nanomaterials through esterification of cellulose: a review of chemistry and application. Cellulose 2018, 25, 3703-3731, 10.1007/s10570-018-1830-3.
- Dmour, I.; Taha, M.O.. Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Eds.; William Andrew Publishing: Amsterdam, Netherlands, 2018; pp. 35-100.
- Haldar, D.; Purkait, M.K.; Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937, 10.1016/j.carbpol.2020.116937.
- Raucci, M.; Alvarez‐Perez, M.; Demitri, C.; Giugliano, D.; De Benedictis, V.; Sannino, A.; Ambrosio, L.J.; Effect of citric acid crosslinking cellulose‐based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 2045-2056.
- Yu, J.; Wang, C.P.; Wang, J.F.; Chu, F.X. Synthesis and Characterization of Ethyl Cellulose Based Acrylate. In Proceedings of the 4th International Conference on Manufacturing Science and Engineering (ICMSE 2013), Dalian, China, 30-31 March 2013; pp. 124-130.
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L.J.C.; Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157-4165.
- Shaghaleh, H.; Xu, X.; Wang, S.F.; Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825-842, 10.1039/c7ra11157f.
- Abdelhak, M.J.; A Review: Application of Biopolymers in the Pharmaceutical Formulation. J. Adv. Bio-Pharm. Pharmacovigil. 2019, 1, 15-25, 10.5281/zenodo.2577643.
- Goncalves, C.; Favre, C.; Feuardant, P.; Klein, S.; Vaca-Garcia, C.; Cecutti, C.; Thiebaud-Roux, S.; Vedrenne, E.; Synthesis of new cellulose ethers using Suzuki-Miyaura reactionsSynthesis of new cellulose ethers using Suzuki-Miyaura reactions. Carbohydr. Polym. 2015, 116, 51-59, 10.1016/j.carbpol.2014.03.089.
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N.; Cellulose-based hydrogel materials: chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153-174, 10.1007/s40204-018-0095-0.
- Gao, C.Z.; Liu, S.; Edgar, K.J.; Regioselective chlorination of cellulose esters by methanesulfonyl chloride. Carbohydr. Polym. 2018, 193, 108-118, 10.1016/j.carbpol.2018.03.093.
- Shokri, J.; Adibki, K.. Cellulose - Medical, Pharmaceutical and Electronic Applications; van de Ven, T.; Godbout, L., Eds.; IntechOpen: 5 Princes Gate Court London, United Kindom, 2013; pp. -.
- Mao, J.; Li, S.; Huang, J.; Meng, K.; Chen, G.; Lai, Y.. Cellulose-Based Superabsorbent Hydrogels ; Mondal, M., Eds.; Springer, Cham: Switzerland, 2018; pp. 37-64.
- Oprea, M.; Voicu, S.I.; Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683, 10.1016/j.carbpol.2020.116683.
- Kamel, S.; Khattab, T.A.; Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67, 10.3390/bios10060067.
- Burduşel, A.-C.; Stancu, I.; Marin, I.B.M.Ş.; Chelaru, C.; Serafim, A.; Drăguşin, D.; Kaya, M.G.A.; Coară, G. Development and characterization of collagen-carboxymethylcellulose materials for lenses. In Proceedings of the International Conference on Advanced Materials and Systems (ICAMS), Qingdao, China, 26-27 March 2016; pp. 215-220.
- Vinklarkova, L.; Masteikova, R.; Vetchy, D.; Dolezel, P.; Bernatoniene, J.; Formulation of Novel Layered Sodium Carboxymethylcellulose Film Wound Dressings with Ibuprofen for Alleviating Wound Pain. Biomed Res. Int. 2015, 2015, 892671, 10.1155/2015/892671.
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D.; Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561, 10.1016/j.carbpol.2020.117561.
- Ramli, N.A.; Wong, T.W.; Sodium carboxymethylcellulose scaffolds and their physicochemical effects on partial thickness wound healing. Int. J. Pharm. 2011, 403, 73-82, 10.1016/j.ijpharm.2010.10.023.
- Fan, L.H.; Peng, M.; Zhou, X.Y.; Wu, H.; Hu, J.; Xie, W.G.; Liu, S.H.; Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohydr. Polym. 2014, 112, 32-38, 10.1016/j.carbpol.2014.05.056.
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R.; Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963-975, 10.1016/j.ijbiomac.2020.07.160.
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B.; Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345, 10.3390/polym13081345.
- Mallakpour, S.; Tukhani, M.; Hussain, C.M.; Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415, 10.1016/j.cis.2021.102415.
- Udeanu, D.I.; Kaya, M.G.A.; Ghica, M.V.; Marin, S.; Marin, M.M.; Kaya, D.A.; Popa, L.; Dinu-Pirvu, C.; Anti-inflammatory drug-loaded biopolymeric spongious matrices with therapeutic perspectives in burns treatment. Farmacia 2018, 66, 783-790, 10.31925/farmacia.2018.5.7.
- Miraftab, M.; Qiao, Q.; Kennedy, J.F.; Knill, C.J.; Groocock, M.R.; Advanced Wound-care Materials: Ultra High Absorbing Fibres made from Alginates Containing Branan Ferulate and Carboxymethyl Cellulose. J. Text. Inst. 2010, 95, 341-348, 10.1533/joti.2003.0063.
- Alavi, M.; Nokhodchi, A.; Antimicrobial and Wound Treatment Aspects of Micro- and Nanoformulations of Carboxymethyl, Dialdehyde, and TEMPO-Oxidized Derivatives of Cellulose: Recent Advances. Macromol. Biosci. 2020, 20, 1900362, 10.1002/mabi.201900362.
- Wong, T.W.; Ramli, N.A.; Carboxymethylcellulose film for bacterial wound infection control and healing. Carbohydr. Polym. 2014, 112, 367-375, 10.1016/j.carbpol.2014.06.002.
- Ali, M.; Khan, N.R.; Basit, H.M.; Mahmood, S.; Physico-chemical based mechanistic insight into surfactant modulated sodium Carboxymethylcellulose film for skin tissue regeneration applications. J. Polym. Res. 2019, 27, 20, 10.1007/s10965-019-1987-y.
- Krizova, H.; Wiener, J.; Development of carboxymethyl cellulose/polyphenols gels for textile applications. Autex Res. J. 2013, 13, 33-36, 10.2478/v10304-012-0021-9.
- Fukuyama, Y.; Maruo, T.; Nishiyama, Y.; Nemoto, Y.; Murayama, K.; Kayanuma, H.; Kawarai, S.; Application of a novel carboxymethyl cellulose-based Mohs sol-gel on malignant wounds in three dogs. J. Vet. Med. Sci. 2021, 83, 385-389, 10.1292/jvms.20-0670.
- Lee, S.; Park, Y.H.; Ki, C.S.; Fabrication of PEG-carboxymethylcellulose hydrogel by thiol-norbornene photo-click chemistry. Int. J. Biol. Macromol. 2016, 83, 1-8, 10.1016/j.ijbiomac.2015.11.050.
- Liu, Y.; Chen, Y.; Zhao, Y.; Tong, Z.R.; Chen, S.S.; Superabsorbent Sponge and Membrane Prepared by Polyelectrolyte Complexation of Carboxymethyl Cellulose/Hydroxyethyl Cellulose-Al3+. BioResources 2015, 10, 6479-6495.
- Hu, D.; Qiang, T.; Wang, L.; Quaternized chitosan/polyvinyl alcohol/sodium carboxymethylcellulose blend film for potential wound dressing application. Wound Med. 2017, 16, 15-21, 10.1016/j.wndm.2016.12.003.
- Nargesi khoramabadi, H.; Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A.; A review of Polyvinyl alcohol / Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69-76, 10.29252/jcc.2.2.2.
- Lim, S.J.; Lee, J.H.; Piao, M.G.; Lee, M.K.; Oh, D.H.; Hwang, D.H.; Quan, Q.Z.; Yong, C.S.; Choi, H.G.; Effect of sodium carboxymethylcellulose and fucidic acid on the gel characterization of polyvinylalcohol-based wound dressing. Arch. Pharm. Res. 2010, 33, 1073-1081, 10.1007/s12272-010-0714-3.
- Zhang, K.; Wang, Y.N.; Wei, Q.H.; Li, X.P.; Guo, Y.; Zhang, S.; Design and Fabrication of Sodium Alginate/Carboxymethyl Cellulose Sodium Blend Hydrogel for Artificial Skin. Gels 2021, 7, 115, 10.3390/gels7030115.
- Shin, J.Y.; Lee, D.Y.; Kim, B.Y.; Yoon, J.I.; Effect of polyethylene glycol molecular weight on cell growth behavior of polyvinyl alcohol/carboxymethyl cellulose/polyethylene glycol hydrogel. J. Appl. Polym. Sci. 2020, 137, 49568, 10.1002/app.49568.
- Birsan, M.; Bibire, N.; Vieriu, M.; Panainte, A.D.; Cojocaru, I.; Influence of Hydroxypropyl Methylcellulose on Flowing and Swelling Parameters in Biomucoadhesive Tablets with Miconazole Nitrate. Rev. Chim. 2017, 68, 2346-2349.
- Zhang, L.; Lu, Y.Q.; Qian, J.Y.; Yue, L.N.; Li, Q.; Xiao, L.X.; Ding, X.L.; Guan, C.R.; Microstructures, physical and sustained antioxidant properties of hydroxypropyl methylcellulose based microporous photophobic films. Int. J. Biol. Macromol. 2020, 152, 1002-1009, 10.1016/j.ijbiomac.2019.10.187.
- Rao, B.L.; Shivananda, C.S.; Shetty, G.R.; Harish, K.V.; Madhukumar, R.; Sangappa, Y. Influence of UV Irradiation on Hydroxypropyl Methylcellulose Polymer Films. In Proceedings of the 2nd International Conference on Condensed Matter and Applied Physics (ICC), Bikaner, India, 24-25 November 2017
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sanchez, V.; Amalraj, J.; Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302, 10.1016/j.carbpol.2020.117302.
- Akinosho, H.; Hawkins, S.; Wicker, L.; Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276-281, 10.1016/j.carbpol.2013.05.081.
- Tundisi, L.L.; Mostaco, G.B.; Carricondo, P.C.; Petri, D.F.S.; Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736, 10.1016/j.ejps.2021.105736.
- Kamel, R.; El-batanony, R.; Salama, A.; Pioglitazone-loaded three-dimensional composite polymeric scaffolds: A proof of concept study in wounded diabetic rats. Int. J. Pharm. 2019, 570, 118667, 10.1016/j.ijpharm.2019.118667.
- Wang, T.; Chen, L.M.; Shen, T.T.; Wu, D.Y.; Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775-782, 10.1016/j.ijbiomac.2016.09.038.
- Bonetti, L.; De Nardo, L.; Fare, S.; Thermo-Responsive Methylcellulose Hydrogels: From Design to Applications as Smart Biomaterials. Tissue Eng. Part B Rev. 2020, 27, 486-513, 10.1089/ten.TEB.2020.0202.
- Siepmann, J.; Peppas, N.A.; Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163-174, 10.1016/j.addr.2012.09.028.
- Kumari, S.; Harjai, K.; Chhibber, S.; Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. J. Infect. Dev. Ctries. 2010, 4, 367-377, 10.3855/jidc.312.
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Asl, A.K.; Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose-A review. Food Sci. Nutr. 2019, 7, 3363-3377, 10.1002/fsn3.1206.
- Liu, H.W.; Chaw, J.R.; Shih, Y.C.; Huang, C.C.; Designed hydrocolloid interpenetrating polymeric networks for clinical applications of novel drug-carrying matrix systems using Tris (6-isocyanatohexyl) isocyanurate and hydroxypropylmethylcellulose. Bio-Med. Mater. Eng. 2014, 24, 2065-2072, 10.3233/bme-141016.
- Song, J.; Feng, H.; Wu, M.; Chen, L.; Xia, W.; Zhang, W.; Preparation and characterization of arginine-modified chitosan/hydroxypropyl methylcellose antibacterial film. Int. J. Biol. Macromol. 2020, 145, 750-758, 10.1016/j.ijbiomac.2019.12.141.
- Yin, J.; Fang, Y.; Xu, L.; Ahmed, A.; High-throughput fabrication of silk fibroin/hydroxypropyl methylcellulose (SF/HPMC) nanofibrous scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1210-1221, 10.1016/j.ijbiomac.2021.05.026.
- Uslu, I.; Aytimur, A.; Serincay, H.; Preparation of PVA/PAA/PEG/PVP Nanofibers with HPMC and Aloe Vera. Curr. Nanosci. 2013, 9, 489-493.
- Zhang, L.; Yue, L.N.; Qian, J.Y.; Ding, X.L.; Effect of Curdlan on the Rheological Properties of Hydroxypropyl Methylcellulose. Foods 2021, 10, 34, 10.3390/foods10010034.
- Liu, X.X.; Ji, Z.L.; Peng, W.W.; Chen, M.; Yu, L.; Zhu, F.; Chemical mapping analysis of compatibility in gelatin and hydroxypropyl methylcellulose blend films. Food Hydrocoll. 2020, 104, 105734, 10.1016/j.foodhyd.2020.105734.
- Wang, Y.F.; Yu, L.; Xie, F.W.; Li, S.; Sun, Q.J.; Liu, H.S.; Chen, L.; On the investigation of thermal/cooling-gel biphasic systems based on hydroxypropyl methylcellulose and hydroxypropyl starch. Ind. Crop. Prod. 2018, 124, 418-428, 10.1016/j.indcrop.2018.08.010.
- Ding, C.C.; Zhang, M.; Li, G.Y.; Rheological Properties of Collagen/Hydroxypropyl Methylcellulose (COL/HPMC) Blended Solutions. J. Appl. Polym. Sci. 2014, 131, 40042, 10.1002/app.40042.
- Shao, X.R.; Sun, H.T.; Zhou, R.; Zhao, B.B.; Shi, J.F.; Jiang, R.P.; Dong, Y.; Effect of bovine bone collagen and nano-TiO2 on the properties of hydroxypropyl methylcellulose films. Int. J. Biol. Macromol. 2020, 158, 937-944, 10.1016/j.ijbiomac.2020.04.107.
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Costa, C.M.; Lanceros-Mendez, S.; Maciavello, M.N.T.; Ribelles, J.L.G.; Sentanin, F.; Pawlicka, A.; Silva, M.M.; et al. Thermal-mechanical behaviour of chitosan-cellulose derivative thermoreversible hydrogel films. Cellulose 2015, 22, 1911-1929, 10.1007/s10570-015-0603-5.
- Ngwabebhoh, F.A.; Zandraa, O.; Patwa, R.; Saha, N.; Capakova, Z.; Saha, P.; Self-crosslinked chitosan/dialdehyde xanthan gum blended hypromellose hydrogel for the controlled delivery of ampicillin, minocycline and rifampicin. Int. J. Biol. Macromol. 2021, 167, 1468-1478, 10.1016/j.ijbiomac.2020.11.100.
- da Silva, M.N.; Fonseca, J.D.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Campos, C.E.M.; Di Luccio, M.; Monteiro, A.R.; Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217, 10.1016/j.foodhyd.2019.105217.
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L.; Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268, 10.1016/j.carbpol.2020.116268.
- Dong, T.; Mi, R.X.; Wu, M.; Zhong, N.P.; Zhao, X.; Chen, X.; Shao, Z.Z.; The regenerated silk fibroin hydrogel with designed architecture bioprinted by its microhydrogel. J. Mat. Chem. B 2019, 7, 4328-4337, 10.1039/c9tb00783k.
- Tekko, I.A.; Chen, G.Y.; Dominguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larraneta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al.et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580, 10.1016/j.ijpharm.2020.119580.
- Chen, Y.C.; Chen, Y.H.; Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2019, 655, 958-967, 10.1016/j.scitotenv.2018.11.264.
- Sun, G.H.; Liang, T.Q.; Tan, W.Y.; Wang, L.J.; Rheological behaviors and physical properties of plasticized hydrogel films developed from kappa-carrageenan incorporating hydroxypropyl methylcellulose. Food Hydrocoll. 2018, 85, 61-68, 10.1016/j.foodhyd.2018.07.002.
- Hu, M.; Yang, J.L.; Xu, J.H.; Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Drug Deliv. 2021, 28, 607-619, 10.1080/10717544.2021.1895906.
- Pišlová, M.; Kolářová, K.; Vokatá, B.; Brož, A.; Ulbrich, P.; Bačáková, L.; Kolská, Z.; Švorčík, V.; A new way to prepare gold nanoparticles by sputtering – Sterilization, stability and other properties. Mater. Sci. Eng. C 2020, 115, 111087, 10.1016/j.msec.2020.111087.
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.F.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J.; Cellulose and its derivatives: towards biomedical applications. Cellulose 2021, 28, 1893-1931, 10.1007/s10570-020-03674-w.
- Schuhladen, K.; Mukoo, P.; Liverani, L.; Nescakova, Z.; Boccaccini, A.R.; Manuka honey and bioactive glass impart methylcellulose foams with antibacterial effects for wound-healing applications. Biomed. Mater. 2020, 15, 065002, 10.1088/1748-605X/ab87e5.
- Fattahpour, S.; Shamanian, M.; Tavakoli, N.; Fathi, M.; Sadeghi-Aliabadi, H.; Sheykhi, S.R.; Fesharaki, M.; Fattahpour, S.; An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int. J. Biol. Macromol. 2020, 151, 220-229, 10.1016/j.ijbiomac.2020.02.002.
- Kar, A.K.; Shil, A.; Kar, B.; Dey, S.; Formulation development and statistical optimization of zingiberol incorporated sodium alginate-methyl cellulose blend microspheres. Int. J. Biol. Macromol. 2020, 162, 1578-1586, 10.1016/j.ijbiomac.2020.07.233.
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P.; Methyl cellulose solutions and gels: fibril formation and gelation properties. Prog. Polym. Sci. 2021, 112, 101324, 10.1016/j.progpolymsci.2020.101324.
- Sangfai, T.; Tantishaiyakul, V.; Hirun, N.; Li, L.; Microphase Separation and Gelation of Methylcellulose in the Presence of Gallic Acid and NaCl as an In Situ Gel-Forming Drug Delivery System. AAPS Pharm. Sci. Tech. 2017, 18, 605-616, 10.1208/s12249-016-0546-7.
- El-Naggar, A.W.M.; Senna, M.M.; Mostafa, T.A.; Helal, R.H.; Radiation synthesis and drug delivery properties of interpenetrating networks (IPNs) based on poly(vinyl alcohol)/methylcellulose blend hydrogels. Int. J. Biol. Macromol. 2017, 102, 1045-1051, 10.1016/j.ijbiomac.2017.04.084.
- Sharma, K.; Bullock, A.; Ralston, D.; MacNeil, S.; Development of a one-step approach for the reconstruction of full thickness skin defects using minced split thickness skin grafts and biodegradable synthetic scaffolds as a dermal substitute. Burns 2014, 40, 957-965, 10.1016/j.burns.2013.09.026.
- Synytsya, A.; Grafova, M.; Slepicka, P.; Gedeon, O.; Synytsya, A.; Modification of chitosan-methylcellulose composite films with meso-tetrakis(4-sulfonatophenyl)porphyrin. Biomacromolecules 2012, 13, 489-498, 10.1021/bm2015366.
- Abu, N.; Kasim, S.H.; Hisham, S.F.; Shamsudin, S.; Noorsal, K.; Mastor, A.; Effect of Methylcellulose on the Hydrophilicity of Chitosan 3D-Porous Scaffold. Advanced Materials Research 2016, 1133, 55-59, 10.4028/www.scientific.net/AMR.1133.55.
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z.; Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2020, 146, 53-61, 10.1016/j.ijbiomac.2019.12.044.
- Negim, E.S.M.; Nurpeissova, Z.A.; Mangazbayeva, R.A.; Khatib, J.M.; Williams, C.; Mun, G.A.; Effect of pH on the physico-mechanical properties and miscibility of methyl cellulose/poly(acrylic acid) blends. Carbohydr. Polym. 2014, 101, 415-422, 10.1016/j.carbpol.2013.09.047.
- Varshosaz, J.; Sajadi-Javan, Z.S.; Kouhi, M.; Mirian, M.; Effect of bassorin (derived from gum tragacanth) and halloysite nanotubes on physicochemical properties and the osteoconductivity of methylcellulose-based injectable hydrogels. Int. J. Biol. Macromol. 2021, 192, 869-882, 10.1016/j.ijbiomac.2021.10.009.
- Sun, N.; Wang, T.; Yan, X.; Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49-59, 10.1016/j.carbpol.2017.05.026.
- Lin, P.J.; Liu, L.L.; He, G.H.; Zhang, T.; Yang, M.; Cai, J.Z.; Fan, L.H.; Tao, S.X.; Preparation and properties of carboxymethyl chitosan/oxidized hydroxyethyl cellulose hydrogel. Int. J. Biol. Macromol. 2020, 162, 1692-1698, 10.1016/j.ijbiomac.2020.07.282.
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R.; A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289-303, 10.1016/j.ijbiomac.2020.01.265.
- Yamane, L.T.; de Paula, E.; Jorge, M.P.; de Freitas-Blanco, V.S.; Montanari, I.; Figueira, G.M.; Anholeto, L.A.; de Oliveira, P.R.; Rodrigues, R.A.; Acmella oleracea and Achyrocline satureioides as Sources of Natural Products in Topical Wound Care. Evid.-based Complement. Altern. Med. 2016, 2016, 3606820, 10.1155/2016/3606820.
- Rosic, R.; Kocbek, P.; Baumgartner, S.; Kristl, J.; Electro-spun hydroxyethyl cellulose nanofibers: the relationship between structure and process. J. Drug Deliv. Sci. Technol. 2011, 21, 229-236, 10.1016/s1773-2247(11)50031-0.
- Raafat, A.I.; Ali, A.E.H.; A novel Lawsonia inermis (Henna)/(hydroxyethylcellulose/polyvinylpyrrolidone) wound dressing hydrogel: radiation synthesis, characterization and biological evaluation. Polym. Bull. 2019, 76, 4069-4086, 10.1007/s00289-018-2587-4.
- Luo, P.F.; Liu, L.L.; Xu, W.Y.; Fan, L.H.; Nie, M.; Preparation and characterization of aminated hyaluronic acid/oxidized hydroxyethyl cellulose hydrogel. Carbohydr. Polym. 2018, 199, 170-177, 10.1016/j.carbpol.2018.06.065.
- Ma, Y.L.; Zhou, G.; Ding, J.F.; Li, S.L.; Wang, G.; Preparation and characterization of an agglomeration-cementing agent for dust suppression in open pit coal mining. Cellulose 2018, 25, 4011-4029, 10.1007/s10570-018-1826-z.
- Ning, F.; Zhang, J.; Kang, M.X.; Ma, C.P.; Li, H.; Qiu, Z.M.; Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2021, 138, 49880, 10.1002/app.49880.
- Kang, M.; Oderinde, O.; Han, X.; Fu, G.; Zhang, Z.; Development of oxidized hydroxyethyl cellulose-based hydrogel enabling unique mechanical, transparent and photochromic properties for contact lenses. Int. J. Biol. Macromol. 2021, 183, 1162-1173, 10.1016/j.ijbiomac.2021.05.029.
- Zulkifli, F.H.; Shahitha, F.; Yusuff, M.M.; Hamidon, N.N.; Chahal, S.; Cross-Linking Effect on Electrospun Hydroxyethyl Cellulose/Poly(Vinyl Alcohol) Nanofibrous Scaffolds. Procedia Eng. 2013, 53, 689-695, 10.1016/j.proeng.2013.02.089.
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S.; Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649-659, 10.1016/j.ijbiomac.2018.01.040.
- Taheri, H.; Hietala, M.; Oksman, K.; One-step twin-screw extrusion process of cellulose fibers and hydroxyethyl cellulose to produce fibrillated cellulose biocomposite. Cellulose 2020, 27, 8105-8119, 10.1007/s10570-020-03287-3.
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L.; Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300-308, 10.1016/j.carbpol.2017.02.108.
- Zia, F.; Zia, K.M.; Aftab, W.; Tabasum, S.; Nazli, Z.-i.-H.; Mohammadi, M.; Zuber, M.; Synthesis and characterization of graphene nanoplatelets-hydroxyethyl cellulose copolymer-based polyurethane bionanocomposite system. Int. J. Biol. Macromol. 2020, 165, 1889-1899, 10.1016/j.ijbiomac.2020.10.069.
- Zia, F.; Zia, K.M.; Nazli, Z.-i.-H.; Tabasum, S.; Khosa, M.K.; Zuber, M.; Preparation of hydroxyethyl cellulose/halloysite nanotubes graft polylactic acid-based polyurethane bionanocomposites. Int. J. Biol. Macromol. 2020, 153, 591-599, 10.1016/j.ijbiomac.2020.03.038.
- Huang, S.; Shuyi, S.; Gan, H.; Linjun, W.; Lin, C.; Danyuan, X.; Zhou, H.; Lin, X.; Qin, Y.; Facile fabrication and characterization of highly stretchable lignin-based hydroxyethyl cellulose self-healing hydrogel. Carbohydr. Polym. 2019, 223, 115080, 10.1016/j.carbpol.2019.115080.
- Guo, M.; Yin, X.X.; Wang, P.; C-13 NMR Characteristics and Thermal Properties of Hydroxyethyl Cellulose Grafting Poly(caprolactone) Copolymer. Asian J. Chem. 2013, 25, 4501-4505, 10.14233/ajchem.2013.14033.
- Demina, T.S.; Birdibekova, A.V.; Svidchenko, E.A.; Ivanov, P.L.; Kuryanova, A.S.; Kurkin, T.S.; Khaibullin, Z.I.; Goncharuk, G.P.; Zharikova, T.M.; Bhuniya, S.; et al.et al. Solid-State Synthesis of Water-Soluble Chitosan-g-Hydroxyethyl Cellulose Copolymers. Polymers 2020, 12, 611, 10.3390/polym12030611.
- Balaita, L.; Peptu, C.A.; Postolache, P.; Lisa, G.; Popa, M.; Gelatin-hydroxyethyl cellulose magnetic microparticles as drug carriers: preparation and characterization. J. Optoelectron. Adv. Mater. 2012, 14, 1023-1033.
- Li, H.Y.; Zhang, Z.W.; Godakanda, V.U.; Chiu, Y.J.; Angkawinitwong, U.; Patel, K.; Stapleton, P.G.; de Silva, R.M.; de Silva, K.M.N.; Zhu, L.M.; et al.et al. The effect of collection substrate on electrospun ciprofloxacin-loaded poly (vinylpyrrolidone) and ethyl cellulose nanofibers as potential wound dressing materials. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 109917, 10.1016/j.msec.2019.109917.
- Zhang, Y.; Sadgrove, M.P.; Mumper, R.J.; Jay, M.; Transdermal Prodrug Delivery for Radionuclide Decorporation: Nonaqueous Gel Formulation Development and In Vitro and In Vivo Assessment. Drug Dev. Res. 2013, 74, 322-331, 10.1002/ddr.21082.
- Lin, Y.M.; Li, M.; Xia, J.L.; Ding, H.Y.; Xu, L.N.; Yang, X.H.; Li, S.H.; Synthesis of plant oil derived polyols and their effects on the properties of prepared ethyl cellulose composite films. Cellulose 2021, 28, 4211-4222, 10.1007/s10570-021-03811-z.
- Ghorbani, M.; Ramezani, S.; Rashidi, M.R.; Fabrication of honey-loaded ethylcellulose/gum tragacanth nanofibers as an effective antibacterial wound dressing. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 621, 126615, 10.1016/j.colsurfa.2021.126615.
- Xu, J.Y.; Jia, H.G.; Yang, N.; Wang, Q.J.; Yang, G.X.; Zhang, M.Y.; Xu, S.P.; Zang, Y.; Ma, L.Q.; Jiang, P.F.; et al.et al. High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids. Polymers 2019, 11, 1900, 10.3390/polym11111900.
- Mahnaj, T.; Ahmed, S.U.; Plakogiannis, F.M.; Characterization of ethyl cellulose polymer. Pharm. Dev. Technol. 2013, 18, 982-989, 10.3109/10837450.2011.604781.
- Zhao, X.Y.; Yan, G.H.; Sun, Y.; Tang, X.; Zeng, X.H.; Lin, L.; Lei, T.Z.; Preparation of Ethyl Cellulose Composite Film with Down Conversion Luminescence Properties by Doping Perovskite Quantum Dots. ChemistrySelect 2019, 4, 6516-6523, 10.1002/slct.201900822.
- Djerafi, R.; Masmoudi, Y.; Crampon, C.; Meniai, A.; Badens, E.; Supercritical anti-solvent precipitation of ethyl cellulose. J. Supercrit. Fluids 2015, 105, 92-98, 10.1016/j.supflu.2015.02.033.
- Suksaeree, J.; Thuengernthong, A.; Pongpichayasiri, K.; Maneewattanapinyo, P.; Settharaksa, S.; Pichayakorn, W.; Formulation and Evaluation of Matrix Type Transdermal Patch Containing Silver Nanoparticles. J. Polym. Environ. 2018, 26, 4369-4375, 10.1007/s10924-018-1305-5.
- Su, X.C.; Yang, Z.; Tan, K.B.; Chen, J.F.; Huang, J.L.; Li, Q.B.; Preparation and characterization of ethyl cellulose film modified with capsaicin. Carbohydr. Polym. 2020, 241, 116259.
- Li, H.Y.; Liu, K.L.; Sang, Q.Q.; Williams, G.R.; Wu, J.Z.; Wang, H.J.; Wu, J.R.; Zhu, L.M.; A thermosensitive drug delivery system prepared by blend electrospinning. Colloid Surf. B Biointerfaces 2017, 159, 277-283, 10.1016/j.colsurfb.2017.07.058.
- Girija, B.G.; Sailaja, R.R.N.; Biswas, S.; Deepthi, M.V.; Mechanical and Thermal Properties of Eva Blended with Biodegradable Ethyl Cellulose. J. Appl. Polym. Sci. 2010, 116, 1044-1056, 10.1002/app.31660.
- Chen, J.X.; Wu, D.F.; Pan, K.R.; Effects of ethyl cellulose on the crystallization and mechanical properties of poly(beta-hydroxybutyrate). Int. J. Biol. Macromol. 2016, 88, 120-129, 10.1016/j.ijbiomac.2016.03.048.
- Li, X.; Jiang, F.T.; Ni, X.W.; Yan, W.L.; Fang, Y.P.; Corke, H.; Xiao, M.; Preparation and characterization of konjac glucomannan and ethyl cellulose blend films. Food Hydrocolloids 2015, 44, 229-236, 10.1016/j.foodhyd.2014.09.027.
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A.; Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573-1589, 10.1007/s10570-019-02881-4.
- Ogawa, A.; Nakayama, S.; Uehara, M.; Mori, Y.; Takahashi, M.; Aiba, T.; Kurosaki, Y.; Pharmaceutical properties of a low-substituted hydroxypropyl cellulose (L-HPC) hydrogel as a novel external dressing. Int. J. Pharm. 2014, 477, 546-552, 10.1016/j.ijpharm.2014.10.043.
- Bhatt, N.; Gupta, P.M.; Naithani, S.; Hydroxypropyl cellulose from alpha-cellulose isolated from Lantana camara with respect to DS and rheological behavior. Carbohydr. Polym. 2011, 86, 1519-1524, 10.1016/j.carbpol.2011.06.054.
- Lin, C.M.; Chang, Y.C.; Cheng, L.C.; Liu, C.H.; Chang, S.C.; Hsien, T.Y.; Wang, D.M.; Hsieh, H.J.; Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose 2020, 27, 2651-2667, 10.1007/s10570-019-02940-w.
- Miao, L.; Hu, J.W.; Lu, M.G.; Tu, Y.Y.; Chen, X.; Li, Y.W.; Lin, S.D.; Li, F.; Hu, S.Y.; Alkynyl-functionalization of hydroxypropyl cellulose and thermoresponsive hydrogel thereof prepared with P(NIPAAm-co-HEMAPCL). Carbohydr. Polym. 2016, 137, 433-440, 10.1016/j.carbpol.2015.11.001.
- Prabu, D.; Majdalawieh, A.F.; Abu-Yousef, I.A.; Inbasekaran, K.; Balasubramaniam, T.; Nallaperumal, N.; Gunasekar, C.J.; Preparation and characterization of gatifloxacin-loaded sodium alginate hydrogel membranes supplemented with hydroxypropyl methylcellulose and hydroxypropyl cellulose polymers for wound dressing. Int. J. Pharm. Investig. 2016, 6, 86-95, 10.4103/2230-973x.177810.
- Szabo, P.; Kallai-Szabo, B.; Kallai-Szabo, N.; Sebe, I.; Zelko, R.; Preparation of hydroxypropyl cellulose microfibers by high-speed rotary spinning and prediction of the fiber-forming properties of hydroxypropyl cellulose gels by texture analysis. Cellulose 2014, 21, 4419-4427, 10.1007/s10570-014-0391-3.
- Ishii, D.; Ueda, K.; Stroeve, P.; Nakaoki, T.; Hayashi, H.; Transport properties of chemically crosslinked hydroxypropyl cellulose in solvated state. Cell Chem. Technol. 2016, 50, 755-760.
- Ma, L.; Wang, L.L.; Wu, L.X.; Zhuo, D.X.; Weng, Z.X.; Ren, R.R.; Cellulosic nanocomposite membranes from hydroxypropyl cellulose reinforced by cellulose nanocrystals. Cellulose 2014, 21, 4443-4454, 10.1007/s10570-014-0405-1.
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S.; Green Electrospining of Hydroxypropyl Cellulose Nanofibres for Drug Delivery Applications. J. Nanosci. Nanotechnol. 2018, 18, 805-814, 10.1166/jnn.2018.13852.
- Saraiva, D.V.; Chagas, R.; de Abreu, B.M.; Gouveia, C.N.; Silva, P.E.S.; Godinho, M.H.; Fernandes, S.N.; Flexible and Structural Coloured Composite Films from Cellulose Nanocrystals/Hydroxypropyl Cellulose Lyotropic Suspensions. Crystals 2020, 10, 122, 10.3390/cryst10020122.
- Bielska, D.; Karewicz, A.; Kaminski, K.; Kielkowicz, I.; Lachowicz, T.; Szczubialka, K.; Nowakowska, M.; Self-organized thermo-responsive hydroxypropyl cellulose nanoparticles for curcumin delivery. Eur. Polym. J. 2013, 49, 2485-2494, 10.1016/j.eurpolymj.2013.02.012.
- El-Wakil, N.A.; Kassem, N.F.; Hassan, M.L.; Hydroxypropyl cellulose/rice straw oxidized cellulose nanocrystals nanocomposites and their use in paper coating. Ind. Crop. Prod. 2016, 93, 186-192, 10.1016/j.indcrop.2016.02.026.
- Sudo, S.; Dielectric Properties of the Free Water in Hydroxypropyl Cellulose. J. Phys. Chem. B 2011, 115, 2-6, 10.1021/jp104950q.
- Veerapur, R.S.; Gudasi, K.B.; Aminabhavi, T.M.; Pervaporation dehydration of isopropanol using blend membranes of chitosan and hydroxypropyl cellulose. J. Membr. Sci. 2007, 304, 102-111, 10.1016/j.memsci.2007.07.006.
- Onofrei, M.D.; Dobos, A.M.; Stoica, I.; Olaru, N.; Olaru, L.; Ioan, S.; Lyotropic Liquid Crystal Phases in Cellulose Acetate Phthalate/Hydroxypropyl Cellulose Blends. J. Polym. Environ. 2014, 22, 99-111, 10.1007/s10924-013-0618-7.
- Gan, S.C.; Bai, S.H.; Chen, C.; Zou, Y.L.; Sun, Y.J.; Zhao, J.H.; Rong, J.H.; Hydroxypropyl cellulose enhanced ionic conductive double-network hydrogels. Int. J. Biol. Macromol. 2021, 181, 418-425, 10.1016/j.ijbiomac.2021.03.068.
- Lu, C.W.; Wang, C.P.; Zhang, D.H.; Wang, J.F.; Yong, Q.; Chu, F.X.; Ultra-strong hydroxypropyl cellulose/polyvinyl alcohol composite hydrogel by combination of triple-network and mechanical training. Int. J. Biol. Macromol. 2021, 184, 200-208, 10.1016/j.ijbiomac.2021.06.054.
- Tolouei, S.; Hasheminia, S.J.; Narimani, M.; Khamesipour, A.; Shatalebi, M.A.; Hejazi, S.H.; Leishmanicidal Activity of Films Containing Paromomycin and Gentamicin Sulfate both In Vitro and In Vivo. Iran. J. Parasitol. 2011, 6, 60-65.
- Mohebian, Z.; Tajmohammadi, I.; Maroufi, L.Y.; Ramezani, S.; Ghorbani, M.; A Novel Aloe Vera-Loaded Ethylcellulose/Hydroxypropyl Methylcellulose Nanofibrous Mat Designed for Wound Healing Application. J. Polym. Environ. 2021, -, 11, 10.1007/s10924-021-02240-0.
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R.; Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose-grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833-842, 10.1016/j.ijbiomac.2020.01.018.
- Dharmalingam, K.; Bordoloi, D.; Kunnumakkara, A.B.; Anandalakshmi, R.; Preparation and characterization of cellulose-based nanocomposite hydrogel films containing CuO/Cu2O/Cu with antibacterial activity. J. Appl. Polym. Sci. 2020, 137, 49216, 10.1002/app.49216.
- Dharmalingam, K.; Anandalakshmi, R.; Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int. J. Biol. Macromol. 2019, 134, 815-829, 10.1016/j.ijbiomac.2019.05.027.
- Dharmalingam, K.; Bordoloi, D.; Kunnumakkara, A.B.; Anandalakshmi, R.; Formation and characterization of zinc oxide complexes in composite hydrogel films for potential wound healing applications. Polym. Compos. 2020, 41, 2274-2287, 10.1002/pc.25538.
- Amanat, S.; Taymouri, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A.; Carboxymethyl cellulose-based wafer enriched with resveratrol-loaded nanoparticles for enhanced wound healing. Drug Deliv. Transl. Res. 2020, 10, 1241-1254, 10.1007/s13346-020-00711-w.
- Rezvanian, M.; Tan, C.K.; Ng, S.F.; imvastatin-loaded lyophilized wafers as a potential dressing for chronic wounds. Drug Dev. Ind. Pharm. 2016, 42, 2055-2062, 10.1080/03639045.2016.1195400.




