
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Dobrut | + 4063 word(s) | 4063 | 2022-01-04 10:58:25 | | | |
| 2 | Vivi Li | Meta information modification | 4063 | 2022-01-05 02:10:42 | | |
Video Upload Options
Streptococcus agalactiae (Group B Streptococcus, GBS) is an opportunistic pathogen, which asymptomatically colonizes the gastrointestinal and genitourinary tract of up to one third of healthy adults. Nevertheless, GBS carriage in pregnant women may lead to several health issues in newborns causing life threatening infection, such as sepsis, pneumonia or meningitis. Recommended GBS screening in pregnant women significantly reduced morbidity and mortality in infants. Nevertheless, intrapartum antibiotic prophylaxis, recommended following the detection of carriage or in case of lack of a carriage test result for pregnant women who demonstrate certain risk factors, led to the expansion of the adverse phenomenon of bacterial resistance to antibiotics.
1. Introduction
2. Alpha-like Protein
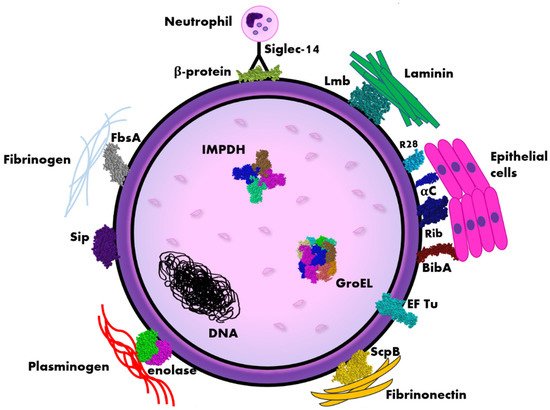
3. β Protein
| Proteins’ Name | Molecular Mass | Function/Characteristic | Immunoreactivity | Localization in Cell |
|---|---|---|---|---|
| Alpha-like proteins: -αC, -Alp 1 (epsilon) -Alp 2 -Alp 3 (R28), -Alp 4, -Rib |
65 kDa–165 kDa |
|
|
Surface anchored |
| β-protein (Bac) | 130 kDa |
|
|
Cell surface |
| Laminin binding protein (Lmb, LmbP) | 43 kDa |
|
|
Cell surface |
| Surface immunogenic protein (Sip) | 53 kDa |
|
|
Cell surface |
| Group B Streptococcus immunogenic bacterial adhesin (BibA) | 80 kDa |
|
|
Cell surface |
| Fibrinogen-binding protein (FsbA) | ap. 26 kDa |
|
|
Cell surface |
| Streptococcal peptidase C5a (ScpB) | 126 kDa |
|
|
Cell surface |
| Enolase | 47 kDa |
|
|
Cell wall |
| Elongation factor thermo unstable (EF Tu) | 44 kDa |
|
|
Part of membrane cytoskeleton, cell surface |
| Inosine 5′-monophosphate dehydrogenase (IMPDH) | 53 kDa |
|
|
Intracellular |
| GroEL | 57 kDa |
|
|
Cytosol, cell surface, secretome |
4. Lmb Protein
5. Sip Protein
6. BibA Protein
2.6. FsbA Protein
References
- Rodriguez-Granger, J.; Alvargonzalez, J.C.; Berardi, A.; Berner, R.; Kunze, M.; Hufnagel, M.; Melin, P.; Decheva, A.; Orefici, G.; Poyart, C.; et al. Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2097–2104.
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Maternal Colonization with Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S100–S111.
- Dermer, P.; Lee, C.; Eggert, J.; Few, B. A history of neonatal group B streptococcus with its related morbidity and mortality rates in the United States. J. Pediatr. Nurs. 2004, 19, 357–363.
- Filkins, L.; Hauser, J.; Robinson-Dunn, B.; Tibbetts, R.; Boyanton, B.; Revell, P.; on behalf of the American Society for Microbiology Clinical and Public Health Microbiology Committee, Subcommittee on Laboratory Practices. Guidelines for Detection and Identification of Group B Streptococcus; American Society for Microbiology: Washington, DC, USA, 2020; Available online: https://asm.org/Guideline/Guidelines-for-the-Detection-and-Identification-of (accessed on 16 July 2021).
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of Perinatal Group B Streptococcal Disease. Revised Guidelines from CDC, 2010. Recomm. Rep. 2010, 19, 59.
- Schrag, S.J.; Verani, J.R. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: Experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 2013, 31 (Suppl. S4), D20–D26.
- Shabayek, S.; Spellerberg, B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol. 2018, 9, 437.
- Berardi, A.; Lugli, L.; Baronciani, D.; Rossi, C.; Ciccia, M.; Creti, R.; Gambini, L.; Mariani, S.; Papa, I.; Tridapalli, E.; et al. Group B Streptococcus early-onset disease in Emilia-romagna: Review after introduction of a screening-based approach. Pediatr. Infect. Dis. J. 2010, 29, 115–121.
- Maeland, J.A.; Afset, J.E.; Lyng, R.V.; Radtke, A. Survey of immunological features of the alpha-like proteins of Streptococcus agalactiae. Clin. Vaccine Immunol. 2015, 22, 153–159.
- Baron, M.J.; Bolduc, G.R.; Goldberg, M.B.; Aupérin, T.C.; Madoff, L.C. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 2004, 279, 24714–24723.
- Navarre, W.W.; Schneewind, O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229.
- Lindahl, G.; Stålhammar-Carlemalm, M.; Areschoug, T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin. Microbiol. Rev. 2005, 18, 102–127.
- Bevanger, L.; Naess, A.L. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta. Pathol. Microbiol. Immunol. Scand. B 1985, 93, 121–124.
- Lachenauer, C.S.; Creti, R.; Michel, J.L.; Madoff, L.C. Mosaicism in the alpha-like protein genes of group B streptococci. Proc. Natl. Acad. Sci. USA 2000, 97, 9630–9635.
- Lachenauer, C.S.; Madoff, L.C. A protective surface protein from type V group B streptococci shares N-terminal sequence homology with the alpha C protein. Infect. Immun. 1996, 64, 4255–4260.
- Li, J.; Kasper, D.L.; Ausubel, F.M.; Rosner, B.; Michel, J.L. Inactivation of the alpha C protein antigen gene, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B Streptococcus. Proc. Natl. Acad. Sci. USA 1997, 94, 13251–13256.
- Michel, J.L.; Madoff, L.C.; Kling, D.E.; Kasper, D.L.; Ausubel, F.M. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect. Immun. 1991, 59, 2023–2028.
- Hedén, L.O.; Frithz, E.; Lindahl, G. Molecular characterization of an IgA receptor from group B streptococci: Sequence of the gene, identification of a proline-rich region with unique structure and isolation of N-terminal fragments with IgA binding capacity. Eur. J. Immunol. 1991, 21, 1481–1490.
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369.
- Stålhammar-Carlemalm, M.; Stenberg, L.; Lindahl, G. Protein rib: A novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 1993, 177, 1593–1603.
- Heath, P.T. Status of vaccine research and development of vaccines for GBS. Vaccine 2016, 34, 2876–2879.
- Fischer, P.; Pawlowski, A.; Cao, D.; Bell, D.; Kitson, G.; Darsley, M.; Johansson-Lindbom, B. Safety and immunogenicity of a prototype recombinant alpha-like protein subunit vaccine (GBS-NN) against Group B Streptococcus in a randomised placebo-controlled double-blind phase 1 trial in healthy adult women. Vaccine 2021, 39, 4489–4499.
- Lancefield, R.C.; Perlmann, G.E. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J. Exp. Med. 1952, 96, 83–97.
- Stålhammar-Carlemalm, M.; Areschoug, T.; Larsson, C.; Lindahl, G. Cross-protection between group A and group B streptococci due to cross-reacting surface proteins. J. Infect. Dis. 2000, 182, 142–149.
- Stålhammar-Carlemalm, M.; Areschoug, T.; Larsson, C.; Lindahl, G. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 1999, 33, 208–219.
- Brzychczy-Włoch, M.; Gosiewski, T.; Bodaszewska, M.; Talaga, K.; Natkaniec, J.; Adamski, J.; Heczko, P.B. Occurrence of surface protein genes from alpha-like protein (Alp) family in Streptococcus agalactiae isolates. Med. Dosw. Mikrobiol. 2011, 63, 5–14.
- Dmitriev, A.; Hu, Y.Y.; Shen, A.D.; Suvorov, A.; Yang, Y.H. Chromosomal analysis of group B streptococcal clinical strains; bac gene-positive strains are genetically homogenous. FEMS Microbiol. Lett. 2002, 208, 93–98.
- Vasilyeva, A.; Santos Sanches, I.; Florindo, C.; Dmitriev, A. Natural Mutations in Streptococcus agalactiae Resulting in Abrogation of β Antigen Production. PLoS ONE 2015, 10, e0128426.
- Berner, R.; Ruess, M.; Bereswill, S.; Brandis, M. Polymorphisms in the cell wall spanning domain of the C protein beta-antigen in clinical Streptococcus agalactiae isolates are caused by genetic instability of repeating DNA sequences. Pediatr. Res. 2002, 51, 106–111.
- Nagano, N.; Nagano, Y.; Taguchi, F. High expression of a C protein beta antigen gene among invasive strains from certain clonally related groups of type Ia and Ib group B streptococci. Infect. Immun. 2002, 70, 4643–4649.
- Carlin, A.F.; Chang, Y.C.; Areschoug, T.; Lindahl, G.; Hurtado-Ziola, N.; King, C.C.; Varki, A.; Nizetet, V. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J. Exp. Med. 2009, 206, 1691–1699.
- Ali, S.R.; Fong, J.J.; Carlin, A.F.; Busch, T.D.; Linden, R.; Angata, T.; Areschoug, T.; Parast, M.; Varki, N.; Murray, J.; et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 2014, 211, 1231–1242.
- Madoff, L.C.; Michel, J.L.; Gong, E.W.; Rodewald, A.K.; Kasper, D.L. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect. Immun. 1992, 60, 4989–4994.
- Flores, A.E.; Nelson, J.A.; Wu, X.Y.; Ferrieri, P. Antibody profiles to the group B streptococcal β antigen in maternal and infant paired sera. APMIS 2003, 101, 41–49.
- Pannaraj, P.S.; Kelly, J.K.; Madoff, L.C.; Rench, M.A.; Lachenauer, C.S.; Edwards, M.S.; Baker, C.J. Group B Streptococcus bacteremia elicits beta C protein-specific IgM and IgG in humans. J. Infect. Dis. 2007, 195, 353–356.
- Jenkinson, H.F. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 1994, 121, 133–140.
- Spellerberg, B.; Rozdzinski, E.; Martin, S.; Weber-Heynemann, J.; Schnitzler, N.; Lütticken, R.; Podbielski, A. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 1999, 67, 871–878.
- Franken, C.; Haase, G.; Brandt, C.; Weber-Heynemann, J.; Martin, S.; Lämmler, C.; Podbielski, A.; Lütticken, R.; Spellerberg, B. Horizontal gene transfer and host specificity of beta-haemolytic streptococci: The role of a putative composite transposon containing scpB and lmb. Mol. Microbiol. 2001, 41, 925–935.
- Terao, Y.; Kawabata, S.; Kunitomo, E.; Nakagawa, I.; Hamada, S. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect. Immun. 2002, 70, 993–997.
- Elsner, A.; Kreikemeyer, B.; Braun-Kiewnick, A.; Spellerberg, B.; Buttaro, B.A.; Podbielski, A. Involvement of Lsp, a member of the LraI-lipoprotein family in Streptococcus pyogenes, in eukaryotic cell adhesion and internalization. Infect. Immun. 2002, 70, 4859–4869.
- Stevens, D.L. Invasive group A streptococcal disease. Infect. Agents. Dis. 1996, 5, 157–166.
- Cartwright, K. Group A streptococcal infections in humans. Soc. Appl. Bacteriol. Symp. Ser. 1997, 26, 52S–61S.
- Wahid, R.M.; Yoshinaga, M.; Nishi, J.; Maeno, N.; Sarantuya, J.; Ohkawa, T.; Jalil, A.M.; Kobayashi, K.; Miyata, K. Immune response to a laminin-binding protein (Lmb) in group A streptococcal infection. Pediatr. Int. 2005, 47, 196–202.
- Brodeur, B.R.; Boyer, M.; Charlebois, I.; Hamel, J.; Couture, F.; Rioux, C.R.; Martin, D. Identification of Group B Streptococcal Sip Protein, Which Elicits Cross-Protective Immunity. Infect. Immun. 2000, 68, 5610–5618.
- Yao, Y.Y.; Chen, D.D.; Cui, Z.W.; Zhang, X.Y.; Zhou, Y.Y.; Guo, X.; Li, A.H.; Zhang, Y.A. Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing Sip. Fish Shellfish Immunol. 2019, 86, 999–1008.
- Li, W.; Li, Y.; Hu, Y.Z.; Mo, X.B.; Xu, G.H.; Xie, L.W.; Li, A.X. GroEL, a novel vaccine candidate of piscine Streptococcus agalactiae identified by immunoproteome. Fish Shellfish Immunol. 2019, 84, 377–383.
- Diaz-Dinamarca, D.A.; Soto, D.A.; Leyton, Y.Y.; Altamirano-Lagos, M.J.; Avendaño, M.J.; Kalergis, A.M.; Vasquez, A.E. Oral vaccine based on a surface immunogenic protein mixed with alum promotes a decrease in Streptococcus agalactiae vaginal colonization in a mouse model. Mol. Immunol. 2018, 103, 63–70.
- Bu, R.E.; Wang, J.L.; DebRoy, C.; Wu, J.H.; Xi, L.G.; Liu, Y.; Shen, Z.Q. Development of an indirect ELISA for bovine mastitis using Sip protein of Streptococcus agalactiae. Iran. J. Vet. Res. 2015, 16, 283–287.
- Bu, R.E.; Wang, J.L.; Wu, J.H.; Xilin, G.W.; Chen, J.L.; Wang, H. Indirect enzyme-linked immunosorbent assay method based on Streptococcus agalactiae rSip-Pgk-FbsA fusion protein for detection of bovine mastitis. Pol. J. Vet. Sci. 2017, 20, 355–362.
- Cheng, S.; Han, J.; Huang, Y.; Yan, Q.; Lu, G.; Yuan, Z.; Huang, G.; Zheng, J.; Liu, T. The correlation between expression of sip protein in different serotypes of group b streptococcus and diagnosis. Heliyon 2019, 5, e01899.
- Takayama, Y.; Matsui, H.; Adachi, Y.; Nihonyanagi, S.; Wada, T.; Mochizuki, J.; Unno, N.; Hanaki, H. Detection of Streptococcus agalactiae by immunochromatography with group B streptococcus-specific surface immunogenic protein in pregnant women. J. Infect. Chemother. 2017, 23, 678–682.
- Manne, K.; Chattopadhyay, D.; Agarwal, V.; Blom, A.M.; Khare, B.; Chakravarthy, S.; Chang, C.; Ton-That, H.; Narayana, S.V.L. Novel structure of the N-terminal helical domain of BibA, a group B streptococcus immunogenic bacterial adhesin. Acta. Crystallogr. D Struct. Biol. 2020, 76, 759–770.
- Santi, I.; Scarselli, M.; Mariani, M.; Pezzicoli, A.; Masignani, V.; Taddei, A.; Grandi, G.; Telford, J.L.; Soriani, M. BibA: A novel immunogenic bacterial adhesin contributing to group B Streptococcus survival in human blood. Mol. Microbiol. 2007, 63, 754–767.
- Santi, I.; Grifantini, R.; Jiang, S.M.; Brettoni, C.; Grandi, G.; Wessels, M.R.; Soriani, M. CsrRS regulates group B Streptococcus virulence gene expression in response to environmental pH: A new perspective on vaccine development. J. Bacteriol. 2009, 191, 5387–5397.
- Santi, I.; Maione, D.; Galeotti, C.L.; Grandi, G.; Telford, J.L.; Soriani, M. BibA induces opsonizing antibodies conferring in vivo protection against group B Streptococcus. J. Infect. Dis. 2009, 200, 564–570.
- Lamy, M.C.; Dramsi, S.; Billoët, A.; Réglier-Poupet, H.; Tazi, A.; Raymond, J.; Guérin, F.; Couvé, E.; Kunst, F.; Glaser, P.; et al. Rapid detection of the “highly virulent” group B Streptococcus ST-17 clone. Microbes. Infect. 2006, 8, 1714–1722.
- Dos Santos, N.F.B.; da Silva, L.R.; Costa, F.J.M.D.; de Mattos, D.M.; de Carvalho, E.; de Souza Ferreira, L.C.; Rita de Cássia Café Ferreira, R. Immunization with a recombinant BibA surface protein confers immunity and protects mice against group B Streptococcus (GBS) vaginal colonization. Vaccine 2020, 38, 5286–5296.
- Dzanibe, S.; Kwatra, G.; Adrian, P.V.; Kimaro-Mlacha, S.Z.; Cutland, C.L.; Madhi, S.A. Association between antibodies against group B Streptococcus surface proteins and recto-vaginal colonisation during pregnancy. Sci. Rep. 2017, 7, 16454.
- Pietrocola, G.; Schubert, A.; Visai, L.; Torti, M.; Fitzgerald, J.R.; Foster, T.J.; Reinscheid, D.J.; Speziale, P. FbsA, a fibrinogen-binding protein from Streptococcus agalactiae, mediates platelet aggregation. Blood 2005, 105, 1052–1059.
- Gutekunst, H.; Eikmanns, B.J.; Reinscheid, D.J. The Novel Fibrinogen-Binding Protein FbsB Promotes Streptococcus agalactiae Invasion into Epithelial Cells. Infect. Immun. 2004, 72, 3495–3504.
- Jonsson, I.M.; Pietrocola, G.; Speziale, P.; Verdrengh, M.; Tarkowski, A. Role of fibrinogen-binding adhesin expression in septic arthritis and septicemia caused by Streptococcus agalactiae. J. Infect. Dis. 2005, 192, 1456–1464.
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Eisen, J.A.; Peterson, S.; Wessels, M.R.; Paulsen, I.T.; Nelson, K.E.; Margarit, I.; Read, T.D.; et al. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 2002, 99, 12391–12396.
- Papasergi, S.; Cariccio, V.L.; Pietrocola, G.; Domina, M.; D’Aliberti, D.; Trunfio, M.G.; Signorino, G.; Peppoloni, S.; Biondo, C.; Mancuso, G.; et al. Immunogenic properties of Streptococcus agalactiae FbsA fragments. PLoS ONE 2013, 8, e75266.
- Yi, T.; Li, Y.W.; Liu, L.; Xiao, X.X.; Li, A.X. Protection of Nile tilapia (Oreochromis niloticus L.) against Streptococcus agalactiae following immunization with recombinant FbsA and α-enolase. Aquaculture 2014, 428, 35–40.
- Zhang, Z. Research Advances on Tilapia Streptococcosis. Pathogens 2021, 10, 558.




