
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriele Meroni | + 2736 word(s) | 2736 | 2021-12-17 04:19:08 | | | |
| 2 | Vicky Zhou | Meta information modification | 2736 | 2022-01-05 02:12:01 | | |
Video Upload Options
Bacterial populations inhabiting a variety of natural and human-associated niches have the ability to grow in the form of biofilms. A large part of pathological chronic conditions, and essentially all the bacterial infections associated with implanted medical devices or prosthetics, are caused by microorganisms embedded in a matrix made of polysaccharides, proteins, and nucleic acids. Biofilm infections are generally characterized by a slow onset, mild symptoms, tendency to chronicity, and refractory response to antibiotic therapy. Even though the molecular mechanisms responsible for resistance to antimicrobial agents and host defenses have been deeply clarified, effective means to fight biofilms are still required. Lactic acid bacteria (LAB), used as probiotics, are emerging as powerful weapons to prevent adhesion, biofilm formation, and control overgrowth of pathogens. Hence, using probiotics or their metabolites to quench and interrupt bacterial communication and aggregation, and to interfere with biofilm formation and stability, might represent a new frontier in clinical microbiology and a valid alternative to antibiotic therapies.
1. Introduction
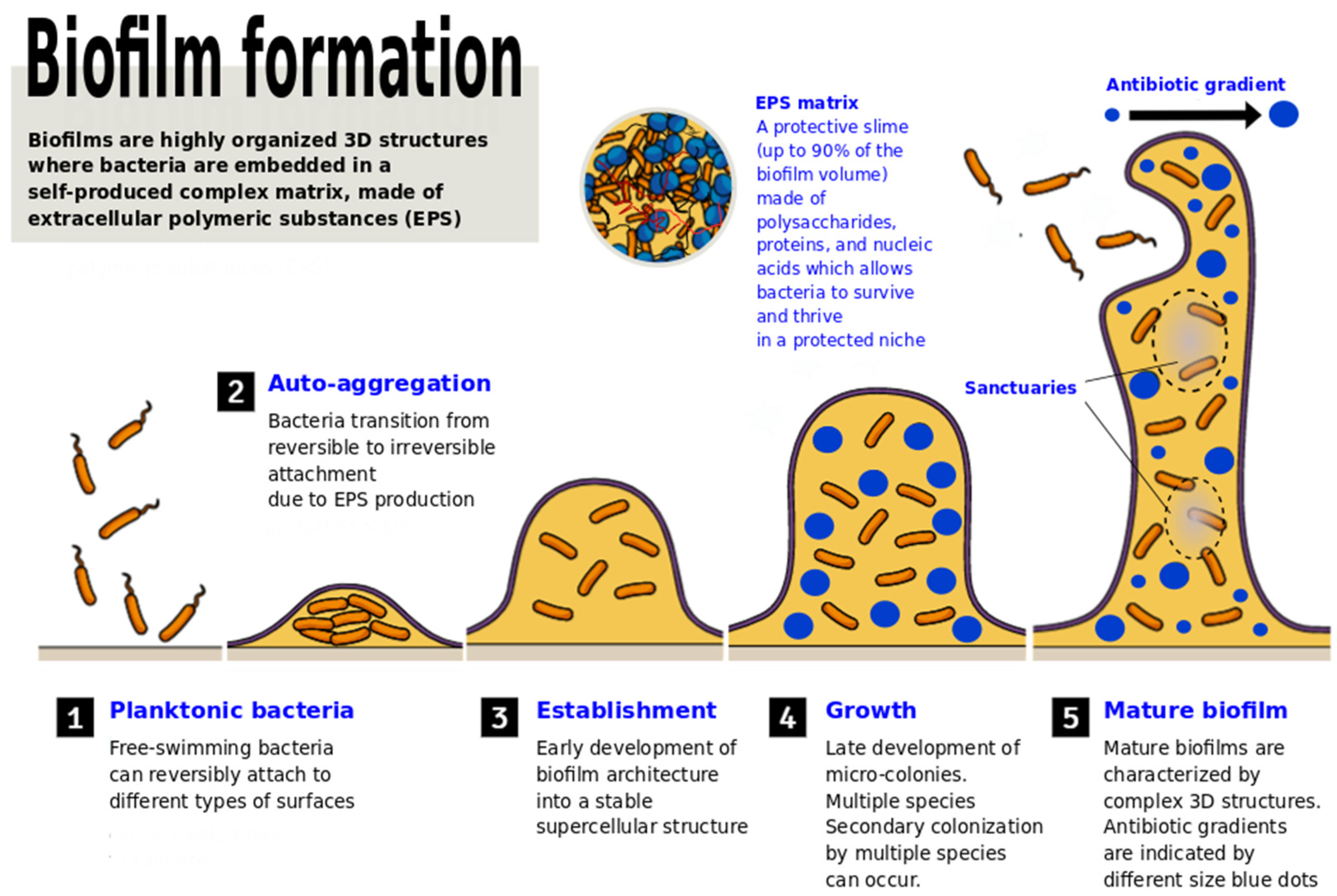
2. The Battle of LAB against Pathogenic Biofilms
2.1. How Lactobacillus May Contrast Biofilm Formation and Stability
2.2. How Bifodobacteria May Contrast Pathogenic Biofilms
3. Conclusions
References
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Kim, Y.-M. Antibiotics Application Strategies to Control Biofilm Formation in Pathogenic Bacteria. Curr. Pharm. Biotechnol. 2020, 21, 270–286.
- Mann, R.; Holmes, A.; McNeilly, O.; Cavaliere, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Evolution of biofilm-forming pathogenic bacteria in the presence of nanoparticles and antibiotic: Adaptation phenomena and cross-resistance. J. Nanobiotechnol. 2021, 19, 1–17.
- Moons, P.; Michiels, C.; Aertsen, A. Bacterial interactions in biofilms. Crit. Rev. Microbiol. 2009, 35, 157–168.
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681.
- Bakhtiari, N.M.; Gooraninezhad, S.; Karami, M. Biofilm-Producing Ability of Bovine Extraintestinal Pathogenic Escherichia coli and Its Correlation with Attachment Factors. Jundishapur J. Heal. Sci. 2018, 10.
- Cergole-Novella, M.C.; Pignatari, A.C.; Guth, B.E. Adhesion, biofilm and genotypic characteristics of antimicrobial resistant Escherichia coli isolates. Braz. J. Microbiol. 2015, 46, 167–171.
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886.
- Fakruddin, M.D.; Bin Mannan, K.S.; Mazumdar, R.M. Correlation Between in vitro Biofilm Formation and Virulence Properties of Extra-Intestinal Pathogenic Escherichia Coli (Expec). Online J. Biol. Sci. 2014, 14, 261–270.
- Wood, T.K.; Barrios, A.F.G.; Herzberg, M.; Lee, J. Motility influences biofilm architecture in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 361–367.
- Prüß, B.M.; Besemann, C.; Denton, A.; Wolfe, A.J. A Complex Transcription Network Controls the Early Stages of Biofilm Development by Escherichia coli. J. Bacteriol. 2006, 188, 3731–3739.
- Cai, P.; Sun, X.; Wu, Y.; Gao, C.; Mortimer, M.; Holden, P.A.; Redmile-Gordon, M.; Huang, Q. Soil biofilms: Microbial interactions, challenges, and advanced techniques for ex-situ characterization. Soil Ecol. Lett. 2019, 1, 85–93.
- Yi, L.; Li, J.; Liu, B.; Wang, Y. Advances in research on signal molecules regulating biofilms. World J. Microbiol. Biotechnol. 2019, 35, 130.
- Campoccia, D.; Mirzaei, R.; Montanaro, L.; Arciola, C.R. Hijacking of immune defences by biofilms: A multifront strategy. Biofouling 2019, 35, 1055–1074.
- Esanchez-Vizuete, P.; Eorgaz, B.; Eaymerich, S.; Le Coq, D.; Ebriandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Front. Microbiol. 2015, 6, 705.
- González, J.F.; Hahn, M.; Gunn, J.S. Chronic biofilm-based infections: Skewing of the immune response. Pathog. Dis. 2018, 76.
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76.
- CDC Antibiotic Resistance Threats in the United State; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019; pp. 1–113.
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795.
- Marquardt, R.R.; Li, S. Antimicrobial resistance in livestock: Advances and alternatives to antibiotics. Anim. Front. 2018, 8, 30–37.
- Bjorkman, I.; Berg, J.; Roing, M.; Erntell, M.; Lundborg, C.S. Perceptions among Swedish hospital physicians on prescribing of antibiotics and antibiotic resistance. BMJ Qual. Saf. 2010, 19, e8.
- Kumar, S.; Little, P.; Britten, N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ 2003, 326, 138.
- Machowska, A.; Lundborg, C.S. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27.
- Reynolds, L.; McKee, M. Factors influencing antibiotic prescribing in China: An exploratory analysis. Health Policy 2009, 90, 32–36.
- Sirota, M.; Round, T.; Samaranayaka, S.; Kostopoulou, O. Expectations for antibiotics increase their prescribing: Causal evidence about localized impact. Health Psychol. 2017, 36, 402–409.
- Ouwehand, A.C.; Tiihonen, K.; Saarinen, M.; Putaala, H.; Rautonen, N. Influence of a combination ofLactobacillus acidophilusNCFM and lactitol on healthy elderly: Intestinal and immune parameters. Br. J. Nutr. 2008, 101, 367–375.
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Collins, S.; Reid, G. Distant Site Effects of Ingested Prebiotics. Nutrients 2016, 8, 523.
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; Younes, J.A.T.H.; Heschl, A.; Turk, D.M.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. BioMed Res. Int. 2019, 2019, 7585486.
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218.
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2018, 111, 537–547.
- Lerner, A.; Shoenfeld, Y.; Matthias, T. Probiotics: If It Does Not Help It Does Not Do Any Harm. Really? Microorganisms 2019, 7, 104.
- Barzegari, A.; Kheyrolahzadeh, K.; Khatibi, S.M.H.; Sharifi, S.; Memar, M.Y.; Vahed, S.Z. The Battle of Probiotics and Their Derivatives against Biofilms. Infect. Drug Resist. 2020, 13, 659–672.
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.; Eichenberger, E.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Genet. 2019, 17, 203–218.
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481.
- Melo, T.A.; Dos Santos, T.F.; De Almeida, M.E.; Junior, L.A.G.F.; Andrade, E.F.; Rezende, R.P.; Marques, L.M.; Romano, C.C. Inhibition of Staphylococcus aureus biofilm by Lactobacillus isolated from fine cocoa. BMC Microbiol. 2016, 16, 250.
- Gomaa, S.; Serry, F.; Abdellatif, H.; Abbas, H. Elimination of multidrug-resistant Proteus mirabilis biofilms using bacteriophages. Arch. Virol. 2019, 164, 2265–2275.
- Shaaban, M.; Abd El-Rahman, O.A.; Al-Qaidi, B.; Ashour, H.M. Antimicrobial and Antibiofilm Activities of Probiotic Lactobacilli on Antibiotic-Resistant Proteus Mirabilis. Microorganisms 2020, 8, 960.
- Campbell, K. Oral microbiome findings challenge dentistry dogma. Nature 2021.
- Wasfi, R.; El-Rahman, O.A.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillu ssp. inhibit growth, biofilm formation and gene expression of caries-inducingStreptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983.
- Tan, Y.; Leonhard, M.; Moser, D.; Schneider-Stickler, B. Inhibition activity of Lactobacilli supernatant against fungal-bacterial multispecies biofilms on silicone. Microb. Pathog. 2017, 113, 197–201.
- Vuotto, C.; Barbanti, F.; Mastrantonio, P.; Donelli, G. Lactobacillus brevisCD2 inhibitsPrevotella melaninogenicabiofilm. Oral Dis. 2013, 20, 668–674.
- Kaur, S.; Sharma, P.; Kalia, N.; Singh, J.; Kaur, S. Anti-biofilm Properties of the Fecal Probiotic Lactobacilli Against Vibrio spp. Front. Cell. Infect. Microbiol. 2018, 8, 120.
- Phukan, N.; Brooks, A.; Simoes-Barbosa, A. A Cell Surface Aggregation-Promoting Factor from Lactobacillus gasseri Contributes to Inhibition of Trichomonas vaginalis Adhesion to Human Vaginal Ectocervical Cells. Infect. Immun. 2018, 86.
- Song, Y.-G.; Lee, S.-H. Inhibitory effects of Lactobacillus rhamnosus and Lactobacillus casei on Candida biofilm of denture surface. Arch. Oral Biol. 2017, 76, 1–6.
- Manzoni, P. Use of Lactobacillus casei Subspecies Rhamnosus GG and Gastrointestinal Colonization by Candida Species in Preterm Neonates. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S190–S194.
- Matsubara, V.H.; Wang, Y.; Bandara, H.M.H.N.; Mayer, M.P.A.; Samaranayake, L.P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016, 100, 6415–6426.
- Bambirra, F.; Lima, K.; Franco, B.; Cara, D.; Nardi, R.; Barbosa, F.; Nicoli, J. Protective effect of Lactobacillus sakei 2a against experimental challenge with Listeria monocytogenes in gnotobiotic mice. Lett. Appl. Microbiol. 2007, 45, 663–667.
- Fangous, M.-S.; Gosset, P.; Galakhoff, N.; Gouriou, S.; Guilloux, C.-A.; Payan, C.; Vallet, S.; Héry-Arnaud, G.; Le Berre, R. Priming with intranasal lactobacilli prevents Pseudomonas aeruginosa acute pneumonia in mice. BMC Microbiol. 2021, 21, 195.
- Ku, S.; Park, M.S.; Ji, G.E.; You, H.J. Review on Bifidobacterium bifidum BGN4: Functionality and Nutraceutical Applications as a Probiotic Microorganism. Int. J. Mol. Sci. 2016, 17, 1544.
- Kim, Y.; Lee, J.W.; Kang, S.-G.; Oh, S.; Griffiths, M.W. Bifidobacterium spp. influences the production of autoinducer-2 and biofilm formation by Escherichia coli O157:H7. Anaerobe 2012, 18, 539–545.
- Valdez, R.M.A.; Ximenez-Fyvie, L.A.; Caiaffa, K.S.; dos Santos, V.R.; Cervantes, R.M.G.; Almaguer-Flores, A.; Duque, C. Antagonist effect of probiotic bifidobacteria on biofilms of pathogens associated with periodontal disease. Microb. Pathog. 2020, 150, 104657.
- Collado, M.C.; Jalonen, L.; Meriluoto, J.; Salminen, S. Protection mechanism of probiotic combination against human pathogens: In vitro adhesion to human intestinal mucus. Asia Pac. J. Clin. Nutr. 2006, 15, 570–575.
- Miyazaki, Y.; Yokota, H.; Takahashi, H.; Fukuda, M.; Kawakami, H.; Kamiya, S.; Hanawa, T. Effect of probiotic bacterial strains of Lactobacillus, Bifidobacterium, and Enterococcus on enteroaggregative Escherichia coli. J. Infect. Chemother. 2010, 16, 10–18.





