Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paulo Gentil | + 4144 word(s) | 4144 | 2021-12-24 05:03:42 | | | |
| 2 | Vivi Li | Meta information modification | 4144 | 2021-12-31 02:19:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gentil, P. Interval Training and the Immune System. Encyclopedia. Available online: https://encyclopedia.pub/entry/17671 (accessed on 07 February 2026).
Gentil P. Interval Training and the Immune System. Encyclopedia. Available at: https://encyclopedia.pub/entry/17671. Accessed February 07, 2026.
Gentil, Paulo. "Interval Training and the Immune System" Encyclopedia, https://encyclopedia.pub/entry/17671 (accessed February 07, 2026).
Gentil, P. (2021, December 30). Interval Training and the Immune System. In Encyclopedia. https://encyclopedia.pub/entry/17671
Gentil, Paulo. "Interval Training and the Immune System." Encyclopedia. Web. 30 December, 2021.
Copy Citation
Interval training (IT) is a popular training strategy recognized by its positive effects on metabolic and cardiovascular system. However, there seems no consensus regarding the effects of IT on immune system parameters. Therefore, researchers aimed to summarize the evidence regarding the effects of IT on the immune system. As our many findings, an IT acutely promote a transitory change on immune cell count followed by reduced function. The magnitude of these changes seems to vary in accordance with IT type. On the other hand, the regular practice of IT might contribute to improve immune function without apparent change on immune cell count.
immunity
immunologic monitoring
immunoglobulin A
aerobic capacity
physical activity
high-intensity interval exercise
leukocytes
infectious disease
1. Introduction
It is widely accepted that moderate-intensity continuous training (MICT) with short to moderate duration (<60 min) is associated with an enhanced immune defense [1]. However, acute bouts of high-intense or high-volume aerobic exercise might provide transitory negative changes on immune cell count and function (lasting between 3 h to 72 h depending on the immune outcome) [2][3]. This might lead to immunosuppression and increased risk for infectious diseases [1][4][5].
The underlying mechanisms to exercise-induced immunosuppression, referred to as the “open window”, are multifactorial and involve neuroendocrine and metabolic factors such as catecholamines, cortisol and growth hormones [3][6]. Immunosuppression usually occurs after intensive training protocols that result in increased levels of inflammation, metabolic and oxidative stress [4]. Therefore, it is important to study different aerobic training protocols since different physiological demands could have different impacts on immune function.
Interval training (IT) is an aerobic training strategy that usually consists in interspacing periods of high-intensity efforts with periods of rest or low-intensity exercise [7][8]. The rationality behind this strategy is to allow the accumulation of higher volume of vigorous exercise than those that could be achieved performing continuous exercise at high intensity [9]. Although current studies about the topic involve low-volume protocols [10][11][12][13], IT is usually performed near or at maximum individual’s capacity, which might result in higher metabolic and hormonal stress in comparison with MICT [14].
During the past century, IT gained popularity in sports preparation [15]. This training strategy was widely adopted by coaches and athletes to train at workloads closer to their specific performance competition [15]. However, in recent decades the recommendations of IT performance have been extended to non-athlete’s subjects as an effective strategy for health promotion [16][17]. Although compelling evidence from healthy and clinical populations have consistently shown that IT promotes metabolic and cardiovascular benefits in a similar or greater extent than MICT [10][18][19][20][21], there seems to be no consensus regarding the effects of IT on the immunological system.
2. Included Studies
Initially, 174 records were retrieved through searches strategy. After removing duplicates, 172 articles were screened for title and/or abstract analyses. Within these, 130 studies did not meet inclusion criteria and were removed. Subsequently, two researchers (DS and AFV) independently reviewed full text of the 42 remaining studies, in which three studies were removed because involved polarized training [22][23][24], three studies lack appropriate IT protocol description [25][26][27] and one study involved cold water immersion [28]. As result, 35 studies were included in final qualitative analysis. From these, 18 studies were included in quantitative analysis, where 12 studies were clinical trials, and six studies were randomized cross-over trials. When the study involved more than one IT intervention (e.g., different IT protocol or separated by sex), the data obtained from each intervention was calculated as an independent trial in meta-analysis. All these steps are described in Figure 1.
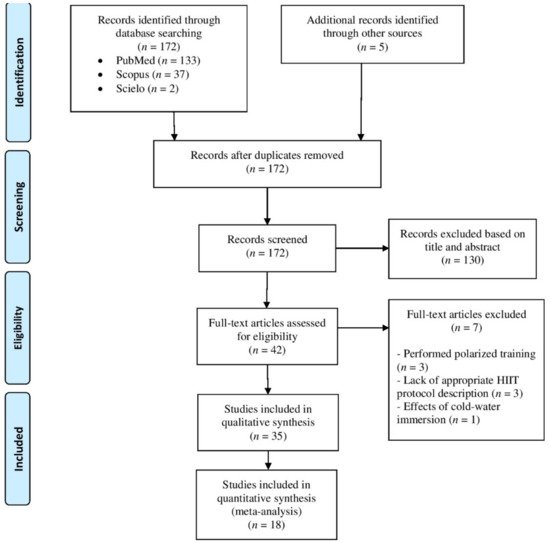
Figure 1. Flowchart of study selection.
3. Summary of Studies
Studies’ characteristics are summarized in Table 1 and Table 2. Twenty-three studies investigated exclusively the acute effects of IT, whereas 10 studies performed interventions lasting between 1 [29][30] and 26 weeks [31]. Two studies performed both acute and chronic investigations [32][33].
Table 1. Summarize of studies investigating the acute effects of interval training on immune outcomes.
| Study | Participants | Design | Modality/Interval Protocol | Results |
|---|---|---|---|---|
| Monje et al. 2020 [34] | 20 runners (10 men age: 21.9 ± 0.8 years; 10 women age: 25.8 ± 6.2 years) | Clinical trial | Running HIIT—10 bouts of 4 min at 90% of vV˙O2max interspersed by 2 min of passive recovery |
↑ salivary IgA concentration 20 min after exercise |
| Wahl et al. 2020 [35] 12 men triathletes and cyclists (age: 24.7 ± 3.4 years) Randomized cross-over trial |
Cycling HIIT—4 bouts of 4 min at 90–95% of peak power interspersed by 3 min of passive recovery |
↔ leucocyte count; ↓ lymphocyte count 30 min, and 60 min after exercise; ↑ neutrophil count 180 min after exercise; ↔ mixed cell count | ||
| Cycling HIIT—4 bouts of 4 min at 90–95% of peak power interspersed by 3 min at 45% of peak power |
↔ leucocyte count; ↑ lymphocyte count immediately after exercise followed by ↓ 30 min, 60 min and 180 min after exercise; ↑ neutrophil count 60 min and 180 min after exercise; ↔ mixed cell count | |||
| Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 7.5 min passive recovery |
↑ leucocyte count immediately, and 180 min after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 60 min and 180 min after exercise; ↑ neutrophil count 60 min and 180 min after exercise; ↑ mixed cell count immediately after exercise | |||
| Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 7.5 min at 45% of peak power |
↑ leucocyte count immediately, and 180 min after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 30 min, 60 min, and 180 min after exercise; ↑ neutrophil count 60 min, and 180 min after exercise; ↔ mixed cell count | |||
| De Oliveira Ottone et al. 2019 [36] | 12 inactive health men (age: 22.5 ± 3.9 years) | Clinical trial | Cycling HIIT—8 bouts of 60 s at 90% peak power interspersed by 75 s of active recovery (30 watts) |
↓ neutrophil oxidative burst in response to f-PMN 30 min after exercise; ↑ neutrophil phagocytic capacity, oxidative burst and redox status 24 h after exercise |
| Jamurtas et al. 2018 [37] | 12 health men (age: 22.4 ± 0.5 years) | Randomized cross-over trial | Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 4 min of active recovery |
↑ leucocyte count immediately after exercise |
| Souza et al. 2018 [38] | 10 obese men (age: 28.5 ± 2.7 years) | Randomized cross-over trial | Running HIIT—10 bouts of 1 min at 90% of Vmax interspersed by 1 min at 30% of Vmax |
↔ secretory IgA and IgA concentration |
| Rodrigues de Araujo et al. 2018 [39] | 32 men soccer players (age: 21.2 ± 4.2 years) | Clinical trial | Running SIT—7 bouts of 40 m “all-out” effort with direction changes interspersed by 25 s of active recovery (light jogging) |
↔ IgA concentration |
| Belviranli et al. 2017 [40] | 10 inactive health men (age: 20.0 ± 1.33 years) | Clinical trial | Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 4 min of active recovery (the load was determined according with the Monark Anaerobic Test Software) |
↑ leucocyte count immediately, 3h, and 6 h after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ lymphocyte count 3 h, and 6 h after exercise; ↑ neutrophil count 3 h, and 6 h after exercise; ↔ monocyte count; ↑ eosinophil count immediately after exercise followed by ↓ 3 h, and 6 h after exercise; ↑ basophil count immediately after exercise |
| Krüger et al. 2016 [41] | 23 untrained health men (age: 25.7 ± 3.2 years) | Randomized cross-over trial | Cycling HIIT—5 bouts of 3 min at 90% peak power output interspersed by 3 min of active recovery (without resistance) |
↑ lymphocyte CD3+, CD4+ and CD8+ count immediately, and 3 h after exercise; ↑ mobilization of low differentiated T cells, regulatory T cells and progenitor cells; ↑ apoptosis in high differentiated T cells |
| Tossige-Gomes et al. 2016 [42] | 10 inactive health men (age: 23.7 ± 1.1) | Clinical trial | Cycling HIIT—8 bouts of 1 min at 100% of peak power interspersed by 75 s of active recovery at 30 W |
↑ lymphocyte redox imbalance 30 min after exercise; ↓ lymphocyte proliferation in response to antigenic, but not to mitogenic stimulation immediately and 30 min after exercise |
| 6 inactive health men (age: 21.3 ± 1.8 years) | Cycling HIIT—8 bouts of 1 min at 100% of peak power interspersed by 75 s of active recovery at 30 W |
↔ lymphocyte viability | ||
| Turner et al. 2016 [43] | 9 health men (age: 22.1 ± 3.4 years) | Randomized cross-over trial | Cycling HIIT—10 bouts of 1 min at 90% of V˙O2max interspersed by 1 min at 40% of V˙O2max |
↑ leucocyte, lymphocyte count immediately after exercise; mobilization of cutaneous lymphocyte natural killer and lymphocyte CD8+ to blood |
| Dorneles et al. 2016 [44] | 12 overweight-obese men (age: 27.41 ± 9.20 years) | Randomized cross-over trial | Running HIIT—10 bouts of 1 min at 85–90% maximum power output interspersed by 75 s at 50% maximum power output |
↑ leucocyte, lymphocyte, and monocyte count immediately after exercise |
| 10 lean men (age: 26.5 ± 6.11 years) | Running HIIT—10 bouts of 1 min at 85–90% maximum power output interspersed by 75 s at 50% maximum power output |
↑ leucocyte immediately and 30 min after exercise; ↑lymphocyte and monocyte immediately after exercise | ||
| Arroyo-Morales et al. 2012 [45] | 50 active health subjects, 25 men (age: 22.4 ± 3.42 years) | Clinical trial | Arm-cycling SIT—3 bouts of 30 s “all-out” effort interspersed by 3 min (90 s of active recovery at 50% W work rate and 90 s of passive recovery) |
↔ secretory IgA |
| Friedman et al. 2012 [46] | 8 health subjects, 4 men (age: 24) | Clinical trial | SIT—2 sets of 3 bouts of 30 s “all-out” effort interspersed by 2 min of active recovery. Sets were separated by 6.75 min | ↑ lymphocyte CD8+, and CD8+/CD45RA+ count and ↑ lymphocyte CD8+, and CD8+/CD45RA+ migration immediately after exercise. ↑ lymphocyte CD8+, and CD8+/CD45RA+ count and ↔ lymphocyte CD4+, and CD4+/CD45RA+ migration immediately after exercise |
| Fisher et al. 2011 [33] | 8 active health men (age: 22 ± 2 years) | Clinical trial | Cycling HIIT—4 bouts with 30 s at 90% of maximum anaerobic power interspersed by 4 min of active recovery at 15% of maximum anaerobic power |
↑ leucocyte and neutrophil counts immediately and 3 h after exercise; ↑ lymphocyte count immediately after exercise; ↓ lymphocyte cell viability 3 h after exercise |
| Davison 2011 [47] | 9 active health men (age: 27 ± 5 years) | Randomized cross-over trial | Cycling SIT—4 bouts of 30 s “all-out” effort interspersed by 4 min of active recovery with light loads |
↔ secretory IgA and ↑ IgA concentration; ↑ neutrophil count immediately and 30 min after exercise; ↓ neutrophil oxidative burst in response to fMLP 30 min after exercise |
| Thomas et al. 2010 [48] | 10 health adolescent women (age 15.5 ± 0.6 years) | Clinical trial | Cycling SIT—8 bouts of 8 s “all-out” effort interspersed by 30 s of passive recovery |
↔ IgA concentration 5 min after exercise |
| Fahlman et al. 2001 [49] | 26 active health women (age: 24.2 ± 5.8 years) | Clinical trial | Cycling SIT—3 bouts of 30 s “all out” effort interspersed by 3 min (90 s of active recovery pedaling against light load and 90 s of passive recovery) |
↓ secretory IgA and ↔ IgA concentration 5 min after exercise |
| Walsh 1999 [50] | 8 trained men (age: 25 ± 1 years) | Clinical trial | Cycling HIIT –20 bouts of 1 min at 100% of V˙O2max interspersed by 2 min at 30% of V˙O2max |
↔ secretory IgA and IgA concentration after exercise |
| Walsh et al. 1998 [51] | 8 trained men (age: 25 ± 3 years) | Clinical trial | Cycling HIIT—20 bouts of 1 min at 100% of V˙O2max interspersed by 2 min at 30% of V˙O2max |
↑ leucocytes and neutrophil count 5 min, 1 h, 2.5 h, and 5 h after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 1 h after exercise |
| Hinton et al. 1997 [52] | 5 men runners (age: 23.0 ± 2.5 years) | Clinical trial | Running HIIT—15 bouts of 1 min at 90% of V˙O2max interspersed by 2 min of passive recovery |
↓ lymphocyte function immediately after exercise |
| Kargotich et al. 1997 [53] | 8 high performance men swimmers (age: 19.9 ± 2.2 years) | Clinical trial | Swimming HIIT—15 bouts of 100 m freestyle swimming interspersed by 2 min 25 m recovery swim |
↑ leucocyte and neutrophil count immediately after exercise; ↑ lymphocyte count immediately after exercise followed by ↓ 1 h, 2 h, and 2.5 h after exercise; ↑ monocyte count immediately and 30 min after exercise; ↔ eosinophil count |
| Gray et al. 1993 [54] | 8 men triathletes (age: 31.5 ± 4.5 years) | Clinical trial | Running HIIT—1 min at 100% of vV˙O2max interspersed by 1 min of active recovery until the exhaustion |
↑ leucocyte and lymphocyte count immediately after exercise; ↑ granulocyte and monocyte count 6 h after exercise |
| Mackinnon & Jerkin, 1993 [32] | 12 active health men (age: 17 to 25 years) | Clinical trial | Cycling SIT—5 bouts of 1 min “all out” effort interspersed by 5 min of passive recovery |
↓ secretory IgA and ↑ IgA concentration immediately after exercise |
| Fry et al. 1992 [55] | 14 men runners (age: 18–25 years) | Clinical trial | Running Treadmill HIIT—25 bouts of 1 min at one stage before that which the subject failed in the preliminary test) followed by 2 min active recovery |
↓ lymphocyte proliferative response immediately after exercise |
| 18 men kayakists (age: 18–25 years) | Paddling HIIT—25 bouts of 1 min at one stage before that which the subject failed in the preliminary test interspersed by 2 min of active recovery |
↓ lymphocyte proliferative response immediately after exercise | ||
| Fry et al. 1992 [56] | 7 men runners (age: 22.9 ± 5.6 years) | Cross-over clinical trial | Running HIIT—15 bouts of 1 min at 90% of Vmax interspersed by 2 min of active recovery |
↔ leucocytes, lymphocyte, neutrophil and monocyte count 5 min after exercise. ↔ the CD4+:CD8+ ratio and responsiveness of T cells to T cells mitogens |
| Running HIIT—15 bouts of 1 min at 120% of Vmax interspersed by 2 min of active recovery |
↑ leucocytes count, lymphocyte, neutrophil, monocyte count 5 min after exercise. ↓ the CD4+:CD8+ ratio and responsiveness of T cells to mitogens immediately after exercise | |||
HIIT, high intensity interval training; SIT, sprint interval training; IgA, immunoglobulin A; V˙O2max, maximal oxygen consumption; V˙O2max, velocity associated to maximal oxygen consumption; Vmax, maximal velocity achieved during the incremental test. fMLP, formyl-leucyl-methionyl-phenylalanine. ↑ significant increase; ↓ significant decrease; ↔ no significant change.
Table 2. Summarize of studies investigating the chronic effects of interval training on immune outcomes.
| Study | Participants | Duration/Design | Modality/Interval Protocol | Results |
|---|---|---|---|---|
| Bartlett et al. 2020 [57] | 10 subjects with prediabetes, 4 men (age: 71 ± 5 years) | Ten weeks clinical trial | Walking HIIT—60–90 s at 80–90% of V˙O2 reserve interspersed by 60–90 s of active recovery at 50–60% of VO2 reserve until complete 20 min. Frequency: 3 times per week. Supervised: Yes |
↑ neutrophil chemotaxis, mitogen stimulated ROS production and ↓ basal ROS production. ↔ neutrophil count |
| Toohey et al. 2020 [58] | 6 breast cancer survivors (age: 60 ± 8.12 years) | Twelve weeks randomized clinical trial | Cycling SIT—4 to 7 bouts of 30 s “all-out” effort interspersed by 2 min of active recovery. Frequency: 3 times per week. Supervised: Yes |
↔ IgA concentration |
| Dorneles et al. 2019 [30] | 7 sedentary obese men (age: 20 to 40 years) | One-week clinical trial | Running HIIT—10 bouts of 1 min at 85–90% maximum heart rate interspersed by 75 s at 50% maximum heart rate. Frequency: 3 times per week. Supervised: No reported |
↑ circulating of memory regulatory T cells and regulatory T cells |
| Werner et al. 2019 [31] | 29 inactive health subjects, 10 men (age: 48.4 ± 6.5 years) | Twenty-six weeks randomized controlled trial | Running HIIT—4 bouts of 4 min at 80–90% of heart rate reserve interspersed by 3 min at 65–70% of heart rate reserve. Frequency: 3 times per week. Supervised: No reported |
↔ total leucocyte counts (lymphocyte, neutrophil and monocyte); ↑ leucocyte telomerase length (lymphocyte, granulocyte) |
| Khammassi et al. 2020 [59] | 8 active health young adults (age: 18.9 ± 1.0 years) | Nine weeks randomized clinical trial | Running HIIT—3 sets of 6 to 8 30-s bouts at 100 to 110% of Vmax and 30 s of active recovery at 50% of Vmax. Frequency: 3 times per week. Supervised: No reported |
↔ total leucocyte counts (lymphocyte, neutrophil and monocyte) |
| Bartlett et al. 2018 [60] | 12 inactive elderly subjects with rheumatoid arthritis (age: 64 ± 7 years) | Ten weeks clinical trial | Walking HIIT—60–90 s at 80–90% of V˙O2 reserve interspersed by active recovery with similar duration at 50–60% of VO2 reserve until complete 20 min of session. Frequency: 3 times per week. Supervised: Yes |
↑ neutrophil function |
| Sheykhlouvand et al. 2018 [61] | 7 men canoe polo athletes (age: 24 ± 3 years) | Three weeks randomized clinical trial | Paddling HIIT—6 bouts of 1 min at 100 to 130% vV˙O2peak with 1:3 work to recovery ratio. Frequency: 3 times per week. Supervised: No reported |
↔ leucocyte counts |
| 7 men canoe polo athletes (age: 24 ± 3 years) | Paddling HIIT—6 to 9 bouts of 1 min at 100% vV˙O2peak with 1:3 work to recovery ratio. Frequency: 3 times per week. Supervised: No reported |
↔ leucocyte counts | ||
| Bartlett et al. 2017 [62] | 14 inactive health adults (age: 43 ± 11 years) | Ten weeks randomized clinical trial | Cycling HIIT—15 to 60 s above 90% of maximum heart rate interspersed by 45–120 s of active recovery until complete 18–25 min. Frequency: 3 times per week. Supervised: Yes |
↑ neutrophil and monocyte function |
| Tsai et al. 2016 [63] | 20 inactive health men (age: 23.0 ± 1.7 years) | Six weeks randomized clinical trial | Cycling HIIT—5 bouts of 3 min at 80% of V˙O2max interspersed by 3 min of active recovery at 40% of V˙O2max. Frequency: 5 times per week. Supervised: No reported |
↑ lymphocyte function |
| Navalta et al. 2014 [29] | 12 subjects, 8 men (age: 26 ± 4 years) | Three consecutive days clinical trial | Running HIIT—30 s at 100% of Vmax interspersed by active recovery with similar duration at 50% of Vmax until exhaustion. Frequency: 3 times per week. Supervised: No reported |
↑ lymphocyte apoptosis |
| Fisher et al. 2011 [33] | 8 active health men (age: 22 ± 2 years) | One-week clinical trial | Cycling HIIT—4 bouts with 30 s at 90% of maximum anaerobic power interspersed by 4 min of active recovery at 15% of maximum anaerobic power. Frequency: 3 times per week. Supervised: No reported |
↑ lymphocyte function |
| Mackinnon & Jerkin, 1993 [32] | 12 active health men (age: 17 to 25 years) | Eight weeks clinical trial |
Cycling SIT—5 bouts of 1 min “all out” effort interspersed by 5 min of passive recovery. Frequency: 3 times per week. Supervised: Yes |
↔ secretory IgA and IgA concentration |
HIIT, high intensity interval training; SIT, sprint interval training; IgA, immunoglobulin A; V˙O2max, maximal oxygen consumption; vV˙O2max, velocity associated to maximal oxygen consumption; Vmax, maximal velocity achieved during the incremental test. ROS, reactive oxygen species; ↑ significant increase; ↓ significant decrease; ↔ no significant change.
Among the 35 included studies, the numbers of participants by IT interventions ranged from 7 [30] to 50 [45], for a total of 509 participants from both sexes. Twenty-four studies involved exclusively men, three studies involved exclusively women [49][48][58] and eight studies investigated mixed-sex samples [29][31][34][45][46][57][60][62]. Participants’ age varied from 15.5 ± 0.6 [48] to 64.0 ± 7.0 years [60]. In most studies participants were apparently healthy, with the exception of studies that involved overweight-obese men [30][38][44], elderly with rheumatoid arthritis [60] and elderly with prediabetes [57]. The training status of the participants varied from sedentary with clinical conditions to high-performance athletes.
4. Intervention Characteristics
Regarding training intervention, the included studies used a diversity of modalities and IT protocols. Most used cycling (n = 18) or running (n = 11), while some studies used walking [57][60], arm-cycling [45], paddling [55][61] and swimming [53]. Twenty-five studies involved exclusively submaximal IT protocol (i.e., HIIT), 10 studies involved exclusively maximal IT protocol (i.e., SIT), usually Wingate-based protocols (i.e., repeated 30 s “all-out” effort) and one study involved both submaximal and maximal protocols [35], and compared different rest interval mode (e.g., passive or active) for HIIT and SIT.
The intensity of HIIT protocols was prescribed and controlled based on percentage of maximal velocity achieved during incremental test (Vmax) [29][59][38][55][56], velocity associated with V˙O2max [34][54] or V˙O2peak [61], percentage of V˙O2max [43][50][51][52][63], or reserve oxygen uptake (V˙O2reserve) [57][60], percentage of HRmax [30][62] or reserve heart rate HRreserve [31], and percentage of peak power [36][42][35][44][53] or maximum anaerobic power [33]. Eleven studies prescribed SIT protocol using “all out” efforts [49][32][47][35][37][39][40][45][46][48][58]. Characteristics of IT protocols are detailed in Table 1 and Table 2.
5. Qualitative Analysis of Acute Effects of IT on Immune Outcomes
5.1. Salivary Immunoglobulin A
A qualitative description of the acute effects of IT on immune measures are presented in Table 1. Six studies verified no change on absolute salivary IgA concentration after IT [49][38][39][45][48][50], while three studies verified transitory increase lasting up to 30 min after exercise [32][47][34]. Regarding secretory rate of IgA, four studies verified no change [47][38][45][50], and two studies verified decrease after exercise [49][32]. Considering IT type, the acute decrease on IgA secretion rate was only observed after SIT [49][32], while no HIIT intervention reduced this parameter [38][45][50] (Figure 2B).
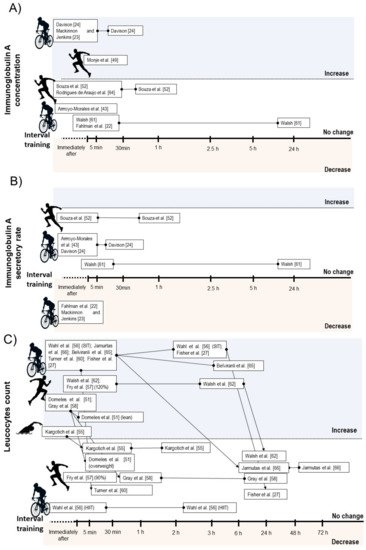
Figure 2. Illustration of time course of salivary immunoglobulin A concentration (A), salivary immunoglobulin secretory rate (B), and total leucocyte count (C) after acute interval training session.
5.2. Leucocyte Count
Ten studies verified transitory increases in total leucocyte count lasting up to 6 h after SIT [35][37][40] or HIIT [33][43][44][51][53][54][56]. One study verified no change on leucocyte count after a HIIT protocol with passive or active recovery [35]. Additionally, Fry et al. [56] reported a significant increase on leucocyte count immediately after HIIT when the high-intensity bouts were performed at 120% of Vmax, but not at 90% (Figure 2C).
Considering leucocyte subsets, nine studies showed increases on total lymphocyte counts immediately after a SIT [40][46] or HIIT session [33][43][44][51][53][54][56], while two HIIT intervention did not change lymphocyte count immediately after exercise [35][56]. Five intervention verified decrease on lymphocyte count between 30 min and 6 h after exercise [35][40][51][53]. From these, two involved SIT [35][40] and three involved HIIT [35][51][53]. Within the studies that did not observed lymphopenia during IT recovery, all involved HIIT [33][43][44][54] (Figure 3A).
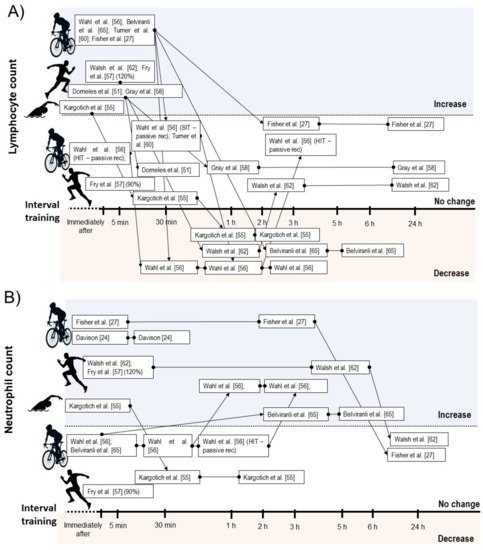
Figure 3. Illustration of time course of lymphocyte (A), and neutrophil count (B) after acute interval training session.
Seven studies reported increases on neutrophil count after SIT [47][35][40] or HIIT performance [33][51][53][56]. In some studies, the increased neutrophil count occurred immediately after exercise and remained elevated between 30 min and 5 h [47][33][51], while two studies verified delayed increase in this parameter starting between 1 h and 3 h after exercise [35][40] (Figure 3B). Five studies found increases on monocyte count immediately after SIT [35] or HIIT [44][53][54][56], while two studies involving SIT [40] and HIIT [35] verified no change on this measure. Regarding mixed cell count, two studies reported acute increases on eosinophil and basophil [35][40], and one study verified increases on granulocyte count after HIIT exercise [54]. One study involving HIIT verified no change on eosinophils count [53], while the study by Wahl et al. [35] showed no change and decrease on basophils and eosinophils count after HIIT and SIT protocol, respectively.
5.3. Leucocyte Function
Five studies involving HIIT [42][33][52][55][56] reported a transitory reduction in lymphocyte function or reduced cell viability after IT performance (lasting up to 3 h) in response to in-vitro stimulation. One study found mobilization of low differentiated T cells and regulatory T cells (Treg) immediately after HIIT, in parallel with apoptosis of high differentiated T cells three hours after exercise [41]. Two studies verified transitory reduced neutrophil function after SIT [47] and HIIT [36] performance (lasting up to 30 min) in response to in-vitro stimulation (Table 1).
6. Qualitative Analysis of Chronic Effects of IT on Immune Outcomes
A qualitative description of the chronic adaptations on immune measures in response to IT is presented in Table 2. Two studies involving SIT reported no change on salivary IgA (absolute concentration or secretory rate) after training [32][58]. Three studies involving HIIT found no significant change in leucocyte count [59][31][61]. Regarding leucocyte function, one study verified increases on peripheral lymphocyte T helper subsets (i.e., memory regulatory T cell and Treg) [30] after HIIT. Three studies involving HIIT provided significant improvements on neutrophil function [57][60][62] and two studies involving HIIT [33][63] verified improvements on lymphocyte function. In contrast, a study involving three consecutive days of HIIT performed until exhaustion reported a significant increase on lymphocyte migration and apoptosis after the third day of consecutive training session [29].
7. Quality Assessment
Considering the specificity of the TESTEX scale, only 14 studies were included in this analysis and the results are shown in the Table 3. The studies achieved an average score of 4.6 from a total of 15 points. Point estimate of outcomes and exercise volume were the most reported features in the included studies. Most studies failed to report if there were, or not, adverse events associated with exercise intervention or intention to treat analysis.
Table 3. Study quality and reporting of randomized clinical trial included studies.
| Reference | Study Quality | Score (0–5) |
Study Reporting | Score (0–10) |
Total Score (0–15) |
Study Quality Classification |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | 6b | 6c | 7 | 8a | 8b | 9 | 10 | 11 | 12 | |||||
| Khammassi et al. [59] | + | − | + | + | − | 3 | − | − | − | − | − | − | + | NA | − | + | 2 | 5 | Low |
| Toohey et al. [58] | + | + | + | − | + | 4 | + | − | + | − | + | + | + | − | + | + | 7 | 11 | High |
| Wahl et al. [35] | − | − | − | − | − | 0 | − | − | − | − | + | + | + | NA | − | + | 4 | 4 | Low |
| Dorneles et al. [30] | + | − | − | − | − | 1 | − | − | − | − | − | − | + | NA | + | + | 3 | 4 | Low |
| Werner et al. [31] | + | − | + | + | − | 3 | − | − | − | − | + | + | + | − | − | + | 4 | 7 | Low |
| de Souza et al. [38] | + | + | − | − | − | 2 | − | − | − | − | + | + | + | − | − | + | 4 | 6 | Low |
| Jamurtas et al. [37] | − | − | − | − | − | 0 | − | − | − | − | − | − | + | NA | − | + | 2 | 2 | Low |
| Sheykhlouvand et al. [61] | + | − | + | − | − | 2 | − | − | − | − | − | − | + | NA | − | + | 2 | 4 | Low |
| Bartlett et al. [62] | − | − | + | + | − | 2 | − | − | − | − | − | − | + | NA | − | + | 2 | 4 | Low |
| Krüger et al. [41] | + | − | − | − | − | 1 | − | − | − | − | − | − | − | NA | − | + | 1 | 2 | Low |
| Tsai et al. [63] | + | − | − | − | − | 1 | + | − | + | − | + | + | + | + | + | + | 8 | 9 | Fair |
| Turner et al. [43] | − | − | − | − | − | 0 | − | − | + | − | − | − | + | NA | − | + | 3 | 3 | Low |
| Davison. [47] | − | − | − | − | − | 0 | − | − | − | − | + | + | + | − | − | − | 3 | 3 | Low |
+, meet the criteria; −, do not meet the criteria; NA, not applicable.
8. Meta-Analysis
The effects of IT on immune parameters are present in Figure 4 and Figure 5. The within-group analysis found that IT significantly reduced IgA secretory rate immediately after exercise (n = 115; MD = −15.46 µg·min−1; 95%CI, −28.3 to 2.66; ∆% = −24%; p = 0.02) (Figure 4B). However, there was no significant change on absolute IgA concentration (n = 127; MD = 47.5 µg·mL−1; 95%CI, −10.6 to 105.6; ∆% = 23%; p = 0.11) (Figure 4A). There was significant increase on total leucocyte count immediately after exercise (n = 137; MD = 2.58 × 103 µL−1; 95%CI, 1.79 to 3.38; ∆% = 44%; p < 0.001) (Figure 5A). Additionally, IT promoted significant increase on lymphocyte count immediately after exercise (n = 125; MD = 1.3 × 103 µL−1;95%CI, 0.86 to 1.75; ∆% = 60%; p < 0.001) (Figure 5B), followed by significant reduction at the first recovery time point after post-exercise (30 to 180 min post-exercise) (n = 125; MD = −0.36 × 103 µL−1;−0.57 to −0.15; ∆% = −17%; p < 0.001) (Figure 5C). Substantial heterogeneity was detected in the analysis for IgA concentration (I2 = 88%; p < 0.001), IgA secretory rate (I2 = 62%; p = 0.01), leucocyte count (I2 = 80%; p < 0.001), lymphocyte count immediately after exercise (I2 = 80%; p < 0.001), and during recovery (I2 = 61%; p = 0.003).
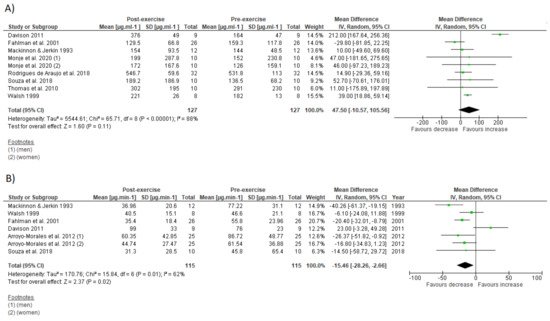
Figure 4. Forest plot of the acute effects of interval training on absolute immunoglobulin A concentration (A) and immunoglobulin secretory rate (B). SD standard deviation, CI confidence interval, IV random effects. The green squares represent the mean difference for each dataset. The black diamonds represent the estimated overall effect.
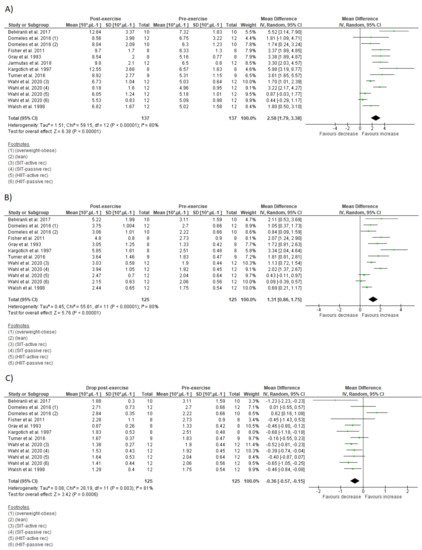
Figure 5. Forest plot of the acute effects of interval training on total leucocytes count (A), lymphocyte count immediately after exercise (B), and lymphocyte count at first drop during recovery (C). SD standard deviation, CI confidence interval, IV random effects. The green squares represent the mean difference for each dataset. The black diamonds represent the estimated overall effect.
Subgroup analysis detected a significant effect of IT type (HIIT vs. SIT) on IgA secretory rate decrease and lymphopenia for SIT, and on absolute IgA concentration increase for HIIT (Table 4). There was a significant effect of participant sex (men vs. women) on IgA secretory rate only for women and training modality (cycling vs. running) on lymphopenia only for cycling.
Table 4. Subgroup analysis of overall effects of interval training on immune outcomes.
| Outcome (Subgroup) | N° of Studies | MD (95% CI) | p-Value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) |
p-Value | ||||
| IgA concentration (µg·mL−1) | |||||
| IT type: SIT | 5 | 46.98 (56.73 to 150.68) | 0.37 | 94 | <0.001 |
| IT type: HIIT | 4 | 39.54 (19.92 to 59.16) | <0.001 | 0 | 1 |
| Sex: men | 6 | 65.62 (−6.43 to 137.66) | 0.07 | 91 | <0.001 |
| Sex: women | 3 | −18.91 (−66.24 to 28.42) | 0.43 | 0 | 0.59 |
| Modality: cycling | 5 | 53.22 (−33.53 to 139.96) | 0.23 | 94 | <0.001 |
| Modality: running | 4 | 22.07 (−17.34 to 61.47) | 0.27 | 0 | 0.92 |
| IgA secretory rate (µg·min−1) | |||||
| IT type: SIT | 6 | −17.33 (−33.68 to −0.98) | 0.03 | 68 | 0.007 |
| IT type: HIIT | 2 | −7.29 (−23.95 to 9.36) | 0.39 | 0 | 0.73 |
| Sex: men | 5 | −13.17 (−35.03 to 8.70) | 0.24 | 74 | 0.004 |
| Sex: women | 2 | −19.34 (−29.11 to −9.58) | <0.001 | 0 | 0.74 |
| Modality: cycling | - | - | - | - | - |
| Modality: running | - | - | - | - | - |
| Leucocyte count (103 µL−1) | |||||
| IT type: SIT | 5 | 3.14 (1.83 to 4.44) | <0.01 | 80 | <0.001 |
| IT type: HIIT | 9 | 2.31 (1.30 to 3.32) | <0.001 | 78 | <0.001 |
| Sex: men | - | - | - | - | - |
| Sex: women | - | - | - | - | - |
| Modality: cycling | 9 | 2.40 (1.47 to 3.33) | <0.001 | 84 | <0.001 |
| Modality: running | 3 | 2.46 (1.30 to 3.62) | <0.001 | 21 | 0.28 |
| Lymphocyte count (103 µL−1) | |||||
| IT type: SIT | 3 | 1.62 (0.89 to 2.35) | <0.001 | 66 | 0.05 |
| IT type: HIIT | 9 | 1.21 (0.67 to 1.74) | <0.001 | 81 | <0.001 |
| Sex: men | - | - | - | - | - |
| Sex: women | - | - | - | - | |
| Modality: cycling | 8 | 1.17 (0.65 to 1.70) | <0.001 | 82 | <0.001 |
| Modality: running | 3 | 1.14 (0.67 to 1.61) | <0.001 | 10 | 0.33 |
| Lymphocyte count (103 µL−1) recovery | |||||
| IT type: SIT | 3 | −0.51 (−0.77 to −0.26) | <0.001 | 18 | 0.30 |
| IT type: HIIT | 9 | −0.29 (−0.56 to 0.03) | 0.03 | 66 | 0.003 |
| Sex: men | - | - | - | - | - |
| Sex: women | - | - | - | - | - |
| Modality: cycling | 8 | −0.47 (−0.62 to −0.32) | <0.001 | 0 | 0.55 |
| Modality: running | 3 | 0.04 (−0.63, 0.72) | 0.9 | 85 | 0.001 |
SIT, sprint interval training; HIIT, high-intensity interval training. Significant p-values are indicated in bold.
9. Sensitivity Analysis
After sensitive analysis performance that checked outlies studies by successively removing the results of each study, changes were observed in effects of IT on absolute IgA concentration (p-value ranged from 0.001 to 0.14) and IgA secretory rate (p-value ranged <0.001 to 0.1) but not on total leucocyte count (p < 0.001), lymphocyte count immediately after exercise (p < 0.001), and during recovery (p-value ranged from <0.001 to 0.004).
References
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2018, 8, 201–217.
- Gonçalves, C.A.M.; Dantas, P.M.S.; Dos Santos, I.K.; Dantas, M.P.; Da Silva, D.C.P.; Cabral, B.G.D.A.T.; Guerra, R.O.; Júnior, G.B.C. Effect of acute and chronic aerobic exercise on immunological markers: A systematic review. Front. Physiol. 2020, 10.
- Pedersen, B.K.; Rohde, T.; Ostrowski, K. Recovery of the immune system after exercise. Acta Physiol. Scand. 1998, 162, 325–332.
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Jeffrey, M.G.; Woods, A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, K.; Hoffman-goetz, L.; et al. Part one: Immune function and exercise. EIR 2011, 17, 6–63.
- Nieman, D.C. Exercise immunology: Future directions for research related to athletes, nutrition, and the elderly. Int. J. Sports Med. 2000, 21, 61–68.
- Walsh, N.P. Recommendations to maintain immune health in athletes. Eur. J. Sport Sci. 2018, 18, 820–831.
- Viana, R.; de Lira, C.; Naves, J.P.A.; Coswig, V.S.; Del Vecchio, F.B.; Ramirez-Campillo, R.; Vieira, C.A.; Gentil, P. Can we draw general conclusions from interval training studies? Sports Med. 2018, 48, 2001–2009.
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013, 43, 313–338.
- Billat, V.L.; Slawinski, J.; Bocquet, V.; Demarle, A.; Lafitte, L.; Chassaing, P.; Koralsztein, J.-P. Intermittent runs at the velocity associated with maximal oxygen uptake enables subjects to remain at maximal oxygen uptake for a longer time than intense but submaximal runs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 81, 188–196.
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.; Rakobowchuck, M.; MacDonald, M.; McGee, S.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160.
- Little, J.P.; Jung, M.E.; Wright, A.E.; Wright, W.; Manders, R. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl. Physiol. Nutr. Metab. 2014, 39, 835–841.
- Gillen, J.B.; Percival, M.E.; Skelly, L.E.; Martin, B.J.; Tan, R.B.; Tarnopolsky, M.A.; Gibala, M.J. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS ONE 2014, 9, e111489.
- Little, J.P.; Safdar, A.; Wilkin, G.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022.
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Metab. 2014, 307, E539–E552.
- Billat, L.V. Interval training for performance: A scientific and empirical practice. Sports Med. 2001, 31, 13–31.
- Gibala, M.J. High-intensity interval training: A time-efficient strategy for health promotion? Curr. Sports Med. Rep. 2007, 6, 211–213.
- Souza, D.; Coswig, V.; De Lira, C.A.B.; Gentil, P. HIT-ing the barriers for exercising during social isolation. Biology 2020, 9, 245.
- Gibala, M.J.; Little, J.P.; Van Essen, M.; Wilkin, G.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint intervalversustraditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911.
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, O.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation 2007, 115, 3086–3094.
- Ramos, J.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692.
- Batacan, R.B.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2016, 51, 494–503.
- Born, D.-P.; Zinner, C.; Sperlich, B. The mucosal immune function is not compromised during a period of high-intensity interval training. Is it time to reconsider an old assumption? Front. Physiol. 2017, 8, 485.
- Born, D.-P.; Faiss, R.; Willis, S.J.; Strahler, J.; Millet, G.; Holmberg, H.-C.; Sperlich, B. Circadian variation of salivary immunoglobin A, alpha-amylase activity and mood in response to repeated double-poling sprints in hypoxia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 116, 1–10.
- McGawley, K.; Juudas, E.; Kazior, Z.; Ström, K.; Blomstrand, E.; Hansson, O.; Holmberg, H.-C. No additional benefits of block over evenly-distributed high-intensity interval training within a polarized microcycle. Front. Physiol. 2017, 8.
- Ide, B.N.; Souza-Junior, T.P.; McAnulty, S.R.; de Faria, M.A.C.; Costa, K.A.; Nunes, L.A.S. Immunological responses to a Brazilian Jiu-Jitsu high-intensity interval training session. J. Hum. Kinet. 2019, 70, 115–124.
- Owen, A.L.; Wong, D.P.; Dunlop, G.; Groussard, C.; Kebsi, W.; Dellal, A.; Morgans, R.; Zouhal, H. High-intensity training and salivary immunoglobulin a responses in professional top-level soccer players: Effect of training intensity. J. Strength Cond. Res. 2016, 30, 2460–2469.
- Chinda, D.; Umeda, T.; Shimoyama, T.; Kojima, A.; Tanabe, M.; Nakaji, S.; Sugawara, K. The acute response of neutrophil function to a bout of judo training. Luminescence 2003, 18, 278–282.
- Broatch, J.; Petersen, A.; Bishop, D. Postexercise cold water immersion benefits are not greater than the placebo effect. Med. Sci. Sports Exerc. 2014, 46, 2139–2147.
- Navalta, J.W.; Tibana, R.A.; Fedor, E.A.; Vieira, A.; Prestes, J. Three consecutive days of interval runs to exhaustion affects lymphocyte subset apoptosis and migration. BioMed Res. Int. 2014, 2014, 1–5.
- Dorneles, G.P.; Da Silva, I.; Boeira, M.C.; Valentini, D.; Fonseca, S.G.; Lago, P.D.; Peres, A.; Romão, P.R.T. Cardiorespiratory fitness modulates the proportions of monocytes and T helper subsets in lean and obese men. Scand. J. Med. Sci. Sports 2019, 29, 1755–1765.
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2018, 40, 34–46.
- Mackinnon, L.T.; Jenkins, D.G. Decreased salivary immunoglobulins after intense interval exercise before and after training. Med. Sci. Sports Exerc. 1993, 25, 678–683.
- Fisher, G.; Schwartz, D.D.; Quindry, J.; Barberio, M.D.; Foster, E.B.; Jones, K.W.; Pascoe, D.D. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J. Appl. Physiol. 2011, 110, 730–737.
- Monje, C.; Rada, I.; Castro-Sepulveda, M.; Peñailillo, L.; Deldicque, L.; Zbinden-Foncea, H. Effects of a high intensity interval session on mucosal immune function and salivary hormones in male and female endurance athletes. J. Sports Sci. Med. 2020, 19, 436–443.
- Wahl, P.; Mathes, S.; Bloch, W.; Zimmer, P. Acute impact of recovery on the restoration of cellular immunological homeostasis. Laryngo-Rhino-Otol. 2019, 41, 12–20.
- Ottone, V.D.O.; Costa, K.B.; Tossige-Gomes, R.; De Matos, M.A.; Brito-Melo, G.; Magalhaes, F.D.C.; Esteves, E.A.; Amorim, F.; Rocha-Vieira, E. Late neutrophil priming following a single session of high-intensity interval exercise. Laryngo-Rhino-Otol. 2019, 40, 171–179.
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The effects of acute low-volume HIIT and aerobic exercise on leukocyte count and redox status. J. Sports Sci. Med. 2018, 17, 501–508.
- De Souza, D.C.; Matos, V.; Dos Santos, V.O.A.; Medeiros, I.F.; Marinho, C.S.R.; Nascimento, P.R.P.; Dorneles, G.P.; Peres, A.; Müller, C.H.; Krause, M.; et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front. Physiol. 2018, 9, 567.
- De Araujo, V.R.; Lisboa, P.; Boaventura, G.; Caramez, F.; Pires, L.; Oliveira, E.; Moura, E.; Casimiro-Lopes, G. Acute high-intensity exercise test in soccer athletes affects salivary biochemical markers. Free Radic. Res. 2018, 52, 850–855.
- Belviranli, M.; Okudan, N.; Kabak, B. The effects of acute high-intensity interval training on hematological parameters in sedentary subjects. Med. Sci. 2017, 5, 15.
- Krüger, K.; Alack, K.; Ringseis, R.; Mink, L.; Pfeifer, E.; Schinle, M.; Gindler, K.; Kimmelmann, L.; Walscheid, R.; Muders, K.; et al. Apoptosis of T-cell subsets after acute high-intensity interval exercise. Med. Sci. Sports Exerc. 2016, 48, 2021–2029.
- Tossige-Gomes, R.; Costa, K.B.; Ottone, V.D.O.; Magalhães, F.D.C.; Amorim, F.T.; Rocha-Vieira, E. Lymphocyte redox imbalance and reduced proliferation after a single session of high intensity interval exercise. PLoS ONE 2016, 11, e0153647.
- Turner, J.E.; Wadley, A.J.; Aldred, S.; Fisher, J.P.; Bosch, J.A.; Campbell, J.P. Intensive exercise does not preferentially mobilize skin-homing T cells and NK cells. Med. Sci. Sports Exerc. 2016, 48, 1285–1293.
- Dorneles, G.; Haddad, D.O.; Fagundes, V.O.; Vargas, B.K.; Kloecker, A.; Romão, P.R.; Peres, A. High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight–obese individuals. Cytokine 2016, 77, 1–9.
- Arroyo-Morales, M.; Rodriguez, L.D.; Rubio-Ruiz, B.; Olea, N. Influence of gender in the psychoneuroimmunological response to therapeutic interval exercise. Biol. Res. Nurs. 2012, 14, 357–363.
- Friedman, R.A.; Navalta, J.W.; Fedor, E.A.; Kell, H.B.; Lyons, T.S.; Arnett, S.W.; Schafer, M.A. Repeated high-intensity Wingate cycle bouts influence markers of lymphocyte migration but not apoptosis. Appl. Physiol. Nutr. Metab. 2012, 37, 241–246.
- Davison, G. Innate immune responses to a single session of sprint interval training. Appl. Physiol. Nutr. Metab. 2011, 36, 395–404.
- Thomas, N.E.; Leyshon, A.; Hughes, M.G.; Jasper, M.A.; Davies, B.; Graham, M.R.; Bulloch, J.M.; Baker, J.S. Concentrations of salivary testosterone, cortisol, and immunoglobulin A after supra-maximal exercise in female adolescents. J. Sports Sci. 2010, 28, 1361–1368.
- Fahlman, M.M.; Engels, H.J.; Morgan, A.L.; Kolokouri, I. Mucosal IgA response to repeated Wingate tests in females. Int. J. Sports Med. 2001, 22, 127–131.
- Walsh, N. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 1999, 17, 129–134.
- Walsh, N.P.; Blannin, A.K.; Clark, A.M.; Cook, L.; Robson, P.; Gleeson, M. The effects of high-intensity intermittent exercise on the plasma concentrations of glutamine and organic acids. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 77, 434–438.
- Hinton, J.R.; Rowbottom, D.G.; Keast, D.; Morton, A.R. Acute intensive interval training and in vitroT-lymphocyte function. Int. J. Sports Med. 1997, 18, 130–135.
- Kargotich, S.; Keast, D.; Goodman, C.; Crawford, G.P.; Morton, A.R. The influence of blood volume changes on leucocyte and lymphocyte subpopulations in elite swimmers following interval training of varying intensities. Int. J. Sports Med. 1997, 18, 373–380.
- Gray, A.B.; Telford, R.D.; Collins, M.; Weidemann, M.J. The response of leukocyte subsets and plasma hormones to interval exercise—PubMed. Med. Sci. Sport Exerc. 1993, 25, 1252–1258.
- Fry, R.W.; Morton, A.R.; Keast, D. Acute intensive interval training and T-lymphocyte function. Med. Sci. Sports Exerc. 1992, 24, 339–345.
- Fry, R.W.; Morton, A.R.; Crawford, G.P.M.; Keast, D. Cell numbers and in vitro responses of leucocytes and lymphocyte subpopulations following maximal exercise and interval training sessions of different intensities. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 64, 218–227.
- Bartlett, D.B.; Slentz, C.A.; Willis, L.H.; Hoselton, A.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; Muoio, D.M.; et al. Rejuvenation of neutrophil functions in association with reduced diabetes risk following ten weeks of low-volume high intensity interval walking in older adults with prediabetes—A pilot study. Front. Immunol. 2020, 11.
- Toohey, K.; Pumpa, K.; McKune, A.; Cooke, J.; Welvaert, M.; Northey, J.; Quinlan, C.; Semple, S. The impact of high-intensity interval training exercise on breast cancer survivors: A pilot study to explore fitness, cardiac regulation and biomarkers of the stress systems. BMC Cancer 2020, 20, 1–11.
- Khammassi, M.; Ouerghi, N.; Said, M.; Feki, M.; Khammassi, Y.; Pereira, B.; Thivel, D.; Bouassida, A. Continuous moderate-intensity but not high-intensity interval training improves immune function biomarkers in healthy young men. J. Strength Cond. Res. 2020, 34, 249–256.
- Bartlett, D.B.; Willis, L.H.; Slentz, C.A.; Hoselton, A.; Kelly, L.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res. 2018, 20, 1–15.
- Sheykhlouvand, M.; Gharaat, M.; Khalili, E.; Agha-Alinejad, H.; Rahmaninia, F.; Arazi, H. Low-volume high-intensity interval versus continuous endurance training: Effects on hematological and cardiorespiratory system adaptations in professional canoe polo athletes. J. Strength Cond. Res. 2018, 32, 1852–1860.
- Bartlett, D.B.; Shepherd, S.O.; Wilson, O.J.; Adlan, A.; Wagenmakers, A.; Shaw, C.S.; Lord, J. Neutrophil and monocyte bactericidal responses to 10 weeks of low-volume high-intensity interval or moderate-intensity continuous training in sedentary adults. Oxidative Med. Cell. Longev. 2017, 2017, 1–12.
- Tsai, H.-H.; Chang, S.-C.; Chou, C.-H.; Weng, T.-P.; Hsu, C.-C.; Wang, J.-S. Exercise training alleviates hypoxia-induced mitochondrial dysfunction in the lymphocytes of sedentary males. Sci. Rep. 2016, 6, 35170.
More
Information
Subjects:
Sport Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
773
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




