Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pablo Diaz Fernadez | + 1998 word(s) | 1998 | 2021-11-30 03:15:09 | | | |
| 2 | Camila Xu | Meta information modification | 1998 | 2021-12-31 02:09:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Diaz Fernadez, P. Cephenemyiosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/17668 (accessed on 07 February 2026).
Diaz Fernadez P. Cephenemyiosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/17668. Accessed February 07, 2026.
Diaz Fernadez, Pablo. "Cephenemyiosis" Encyclopedia, https://encyclopedia.pub/entry/17668 (accessed February 07, 2026).
Diaz Fernadez, P. (2021, December 30). Cephenemyiosis. In Encyclopedia. https://encyclopedia.pub/entry/17668
Diaz Fernadez, Pablo. "Cephenemyiosis." Encyclopedia. Web. 30 December, 2021.
Copy Citation
Cephenemyia stimulator is a Palearctic species developing in the nasal cavity and pharynx of roe deer (Capreolus capreolus). It is widely spread in the range of distribution of this ungulate in Europe.
roe deer
Cephenemyia stimulator
myiasis
Europe
Spain
1. Introduction
Nasal bots are obligatory myiasis-causing larvae belonging to the subfamily Oestrinae (Family Oestridae), which includes several important genera infecting Cervidae (Cephenemyia and Pharyngomyia), other Artiodactyla species (Oestrus, Kirkioestrus and Gedoelstia), horses (Rhinoestrus), camels (Cephalopina), African elephants (Pharyngobolus) and Australian kangaroos (Tracheomyia) [1].
Cephenemyia spp. develops in the nasal and pharyngeal cavities of different species of Cervinae and Odocoileinae [2]. In this respect, the genus Cephenemyia is considered quite host-specific and rarely parasitizes unsuitable hosts [1][3].
Eight species in the genus Cephenemyia can infect cervids (Table 1); these species are confined to either the Neartic (new world) or Paleartic (old world) area excepting Cephenemyia trompe, which is distributed throughout the northern Holarctic region. Among them, Cephenemyia stimulator is a species specific to roe deer that is widely spread in the range of distribution of this ungulate in Europe [4].
Table 1. Cephenemyia species, distribution and main hosts.
| Species | Distribution | Hosts |
|---|---|---|
| C. trompe (Modeer, 1786) | Neartic/Paleartic | deer, moose, reindeer/caribou |
| C. ulrichii (Brauer, 1863) | Paleartic | moose |
| C. auribarbis (Meigen, 1824) | Paleartic | red deer |
| C. stimulator (Hunter, 1916) | Paleartic | roe deer |
| C. phobifer (Clark, 1815) | Neartic | white-tailed deer |
| C. apicata (Bennett & Sabrosky, 1962) | Neartic | mule deer |
| C. jellisoni (Townsend, 1941) | Neartic | mule deer, white-tailed deer, moose, elk |
| C. pratti (Hunter, 1916) | Neartic | mule deer, white-tailed deer |
2. Morphology of Flies and Larvae
The imagos of the different species of Cephenemyia are very similar and have a particular disposition of yellow and black hair. However, since the coloration varies among species, other features should be considered for achieving a reliable morphological identification [5].
The body of C. stimulator flies is covered with yellow and orange hairs, sometime acquiring a slightly reddish hue similar to that of bumblebees (Bombus spp). This coloration pattern is an adaptation for protecting themselves from possible predators by adopting a shape and color mimicking that of poisonous species [6]; moreover, they are fast flyers (calyptrates). The head is large in comparison to the rest of the body, with large eyes and short antennae [5][6][7]. Males have the phalosome covered with thin spines.
C. stimulator adults, such as other oestrid flies, do not feed during the adult stage, so they depend on an efficient assimilation and storage of nutrients during their parasitic larval stage [6]. Adults do not have functional mouthparts and their large eyes enable the localization of potential hosts, as well as suitable mates. Adults have a short lifespan and females emerge from the puparium with fully developed eggs ready for fertilization.
The three larval stages of C. stimulator are elongated and have twelve segments with the ventral region flatter than the dorsal one [8]. The first segment, the cephalic, is mobile and can be retracted within the first thoracic segment (segment II). They are covered with spines that, together with the large mouth hooks, serve to attach to the mucosa of the host, thus avoiding being expelled by the host’s defense mechanisms, such as cough and sneezing [9].
Larvae can be differentiated in three development stages according to their total length and color of the cuticle, as well as the shape and color of the posterior peritremes [10]. The first stage larvae (L1) were first described by Ullrich in 1936 [11] and more recently by Quintela [10]; they are small (2.27 ± 0.80 mm), white and flattened dorsoventrally (Figure 1a). The segments are surrounded by spines and both the dorsal and ventral areas of segments II and XII have numerous rows of denticles allowing researchers to differentiate this species from C. trompe, which only has rudimentary denticles in the dorsal part. In addition, the spines are more evident on the ventral and lateral surface of the thoracic and abdominal segments [12]. The posterior peritremes are cloverleaf-shaped, dark brown-colored, with small unpigmented peritremal buttons and scarce and large respiratory pores [10].

Figure 1. (a) First, (b) second and (c) third larval stages of Cephenemyia stimulator.
Second stage larvae (L2) are yellowish and their length ranges between 3 and 13 mm (average 10.03 ± 3.10) (Figure 1b). They are flattened dorsoventrally, similar to the rest of the larval stages; the dorsal surface is rounded and, in a sagittal cut, it has an ovoid appearance. The distribution of the spicules is similar to that of L1, but with a lower density in the dorsal region [11]; in addition, the X and XI segments lack spines, or have up to four rows of spines. Different authors pointed out that the number of spines on the dorsal face is higher than that on the ventral one and that, from the III to the XI segment, they present between five and eight rows of spines disposed in an irregular form [5][6][13].
Third stage larvae (L3) can reach 30 mm in length with an average of 24.73 ± 5.94 mm (Figure 1c). The posterior peritremes are reniform. Its shape and color are similar to those of L2 but the amount of melanin increases with maturation, leading to a darkening of the cuticle and the spines; dark spots appear on the body of mature larvae, especially in the last segment. Zumpt [5] indicated that the dorsal part of segment XI has several medial spines. The main features to be considered for the identification of L3 of C. stimulator are the shape and arrangement of the antennal segments (Figure 2a), the size of the mouth hooks (Figure 2b), the distribution pattern of the cuticular spines (Figure 2c) and the shape of the posterior peritremes (Figure 2d) [5][10].
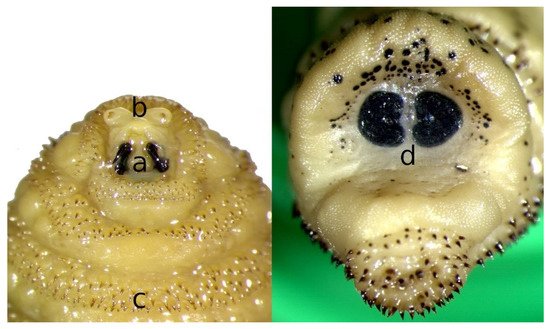
Figure 2. Morphological details of Cephenemyia stimulator third instar larvae: (a) mouth hooks, (b) antennal lobes, (c) cuticular spines and (d) posterior peritremes.
The general morphology and arrangement of the digestive and excretory systems of C. stimulator L3 are similar to those of O. ovis [14]. Micro-computed tomography revealed that the digestive system shows a uniformly narrow midgut that lacks gastric caeca and forms six loops before the beginning of the hindgut. The hindgut first runs anteriorly up to the fourth/fifth abdominal segment, where it turns and runs dorsally towards the posterior end of the body, opening into the anus. The two salivary glands are elongated, connected at their anterior ends by the common salivary duct, forming a ’glandular band’ by coming together at their posterior ends. The excretory system consists of two pairs of tubules that open into the transition between midgut and hindgut. The L3 of C. stimulator has a high abundance of fat body cells arranged in a kind of sheet that covers both sides and much of the dorsal aspect of the block formed by the digestive and excretory organs [14].
Finally, pupae of C. stimulator are from 16 to 20 mm long and present a black chitinous coating protecting the imago.
3. Life Cycle and Chronobiology
A new life cycle begins after copulation (Scheme 1), when fertilized females of C. stimulator detect a roe deer attracted by its odor or by the CO2 exhaled. Females can contain up to 500 larvae and, during their lifespan (16 days), they deposit or eject packets of 30–50 L1s in the nostrils of different specimens of roe deer. This strategy enables a higher survival rate, since a number of roe deer, with different age and immune status, can be infested; in addition, competition among larvae is lower [6][7][15]. L1s are surrounded by a thick, gelatinous fluid that promotes their adhesion and protects them from desiccation.
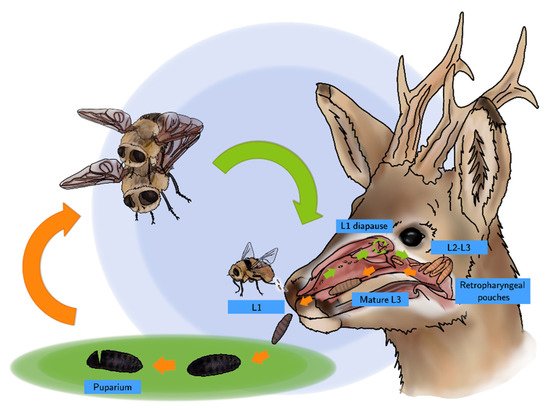
Scheme 1. Life cycle of C. stimulator.
The endogenous life cycle begins when the L1 moves into the nose or mouth towards the nasal cavities aided by their hooks and spines which constitute a defense mechanism against the host’s attempts to get rid of them through coughs, sneezes and sudden movements of the head. L1s can enter in a phase of hypobiosis or diapause as an adaptive response to adverse weather conditions; thus, their full development does not take place until environmental conditions become favorable for their survival. Diapause can occur at any stage of development, but usually occurs in the L1 or pupal phase [1][6][15]. Once L2 have been developed, they go towards the choanas, pharynx and larynx, the preferred location of L2s and L3s. In a number of roe deer, these larvae invade the retropharyngeal recesses that distend into “pouches” or diverticula harboring up to 30 larvae (Figure 3). In roe deer heavily infested by C. stimulator, larvae are grouped in the pharyngeal recesses as a cluster; the anterior part of the larva is fixed inside the foseta and the posterior end is oriented towards the opening of the recess. Paired retropharyngeal recesses were the preferred sites for the growing of both L2s and L3s of Cephenemyia, Cephalopina and Rhinoestrus [1].
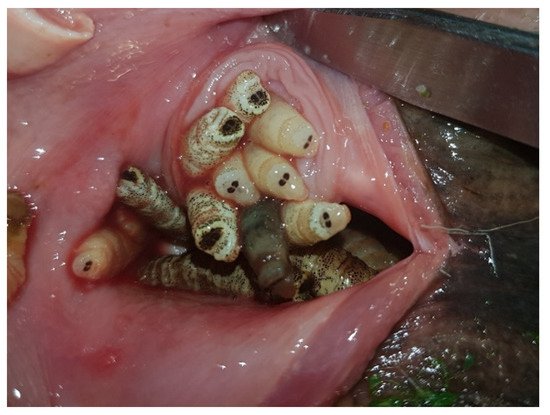
Figure 3. C. stimulator larvae in the retropharyngeal pouches of a roe deer (ventral view).
Once their growth is complete, mature L3s progress to the upper airways following an inverse process to that of the L1s and L2s. After reaching the nostrils, L3s exit the host aided by roe deer coughing or sneezing, or they simply crawl out themselves [6][15][16]. In the environment, L3s bury into the ground among the leaf litter since they are lucifuge and, finally, they pupate. Inside the puparium, L3s result in females and males of C. stimulator that emerge after about 2–3 weeks, as long as the weather conditions are favorable [6][7][15], starting a new cycle.
Regarding the chronobiology, mating and host-seeking activities of all species of oestrids occur on warm and sunny days, with temperatures between 20 and 30 °C [17]. It has been reported that the seasonal dynamics of C. stimulator depend directly on the climatic conditions that determine the geographical distribution and the chronobiology of this myiasis in each region [6][18].
Adults of C. stimulator are active from late May to mid-September, when the temperature exceeds 13 °C [18] and their activity reduces under lower temperatures or with precipitation [19].
Vegetation cover also seems to influence the intensity of infestation, as this is higher in areas with lower density of trees [20]. However, between 200 and 1000 m of altitude, this does not seem to influence the viability of C. stimulator [18].
The chronobiology of this myiasis has been described in several European countries. In roe deer from Poland, larvae of C. stimulator were observed throughout the year, with L2s predominating between April and July and L3s from April to August [21]. Similar observations were reported in Hungary, where roe deer harbored L1s from late July to April–May of the following year, many of them in diapause, L2s between April and May and L3s between April and August [4].
According to the climatic conditions (temperature, precipitation, insolation, etc.) of northwestern Spain, as well as the observation of the different larval stages in roe deer by different authors [7][13][15][22], the chronobiology of C. stimulator in this area is that represented in Scheme 2.
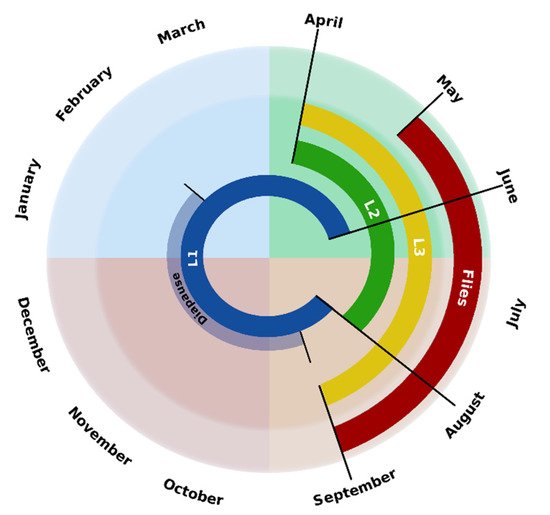
Scheme 2. Chronobiology of C. stimulator in northwestern Spain.
Between May and September, the days have a remarkable number of hours of light, there is absence of strong wind gusts and average temperatures range between 15 and 20 °C, being the most favorable conditions for the flight of the flies. From September to February the average temperatures range between 9 and 16 °C, registering minimum temperatures of 2–3 °C; therefore, in these months, there is larval diapause and only L1s are found. However, the temperatures recorded between April and July (averages from 12 to 20 °C) favor the development of the larvae, noting that, in this period, the active phase of the endogenous cycle occurs, since, in the roe deer, the three larval stages have been found. Finally, considering that, in April, L3s can already be observed and that the pupation period in the oestrids lasts 20–30 days, the first flies may be observed in May and, as in other Oestridae, their activity lasts throughout the summer.
Finding different instars of C. stimulator simultaneously in the same host could be explained by the production of several larval generations per year [20], successive reinfestations, the ability of first-instar larvae to become hypobiotic and overwinter into the host and/or also by an asynchronous and gradual development of the larvae parasitizing the same host [2].
References
- Angulo-Valadez, C.E.; Scholl, P.J.; Cepeda-Palacios, R.; Jacquiet, P.; Dorchies, P. Nasal bots… a fascinating world! Vet. Parasitol. 2010, 174, 19–25.
- Colwell, D.D.; Hall, M.J.R.; Scholl, P.J. A Synopsis of the Biology, Hosts, Distribution, Disease Significance and Management of the Genera. In The Oestrid Flies. Biology, Host-Parasite Relationships, Impact and Management; Colwell, D.D., Hall, M.J.R., Scholl, P.J., Eds.; CAB Int.: Oxfordshire, UK, 2006; p. 359.
- Leitner, N.; Schwarzmann, L.; Zittra, C.; Palmieri, N.; Eigner, B.; Otranto, D.; Glawischnig, W.; Fuehrer, H.-P. Morphological and molecular identification of nasopharyngeal bot fly larvae infesting red deer (Cervus elaphus) in Austria. Parasitol. Res. 2016, 115, 4417–4422.
- Papp, L.; Szappanos, A. Bagócslegyek Gasterophilidae, Oestridae, Hypodermatidae; Magyar Természettudományi Múzeum: Budapest, Hungary, 1992.
- Zumpt, F. Myiasis in Man and Animals in the Old World; Butterworths: London, UK, 1965; pp. 146–153, 217–229.
- Pajares, G. Estudio Sobre la Infestación por Larvas de Cephenemyia Stimulator (Diptera: Oestridae) en Corzos (Capreolus capreolus) del Norte de España. Ph.D. Thesis, Facultad de Veterinaria de Lugo, Universidade de Santiago de Compostela, Lugo, Spain, 2016.
- Calero-Bernal, R.; Habela, M. First report of Cephenemyia stimulator (Diptera, Oestridae) parasitizing Roe deer (Capreolus capreolus) in Extremadura (Spain). Galemys Span. J. Mammal. 2013, 25, 29–34.
- Minář, J. Family Oestridae. In Contributions to a Manual Palaearctic Diptera; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 2000; pp. 467–478.
- McMahon, D.C.; Bunch, T.D. Bot Fly Larvae (Cephenemyia spp. Oestridae) in Mule Deer (Odocoileus hemionus) from Utah. J. Wildl. Dis. 1989, 25, 636–638.
- Quintela, L. Estudio Morfológico de las Larvas Nasofaríngeas (Diptera: Oestridae) Encontradas en Corzos de la Provincia de Lugo. Trabajo Fin de Grado; Facultad de Veterinaria de Lugo. Universidad de Santiago de Compostela: Lugo, Spain, 2021.
- Ullrich, H. Untersuchungen uber die Biologie der Rachenbremse (genus Cephenemyia Latreille), uber die Pathogenen Einflusse der Rachenbremsenllarven auf ihre Wirtstiere und uber Bekampfungsmoglishkeiten der Rachenbremsenplage. Ph.D. Dissertation, Friedrich Wilhelm University, Berlin, Germany, 1936.
- Colwell, D.D.; Scholl, P.J. Cuticular sensilla on newly hatched larvae of Gasterophilus intestinalis and Oestrus ovis. Med. Veter-Èntomol. 1995, 9, 85–93.
- Martínez Calabuig, N. Prevalencia y Desarrollo Larvario de Cephenemyia sp. en Corzos del Norte de España. Trabajo Fin de Grado; Facultad de Veterinaria de Lugo. Universidade de Santiago de Compostela: Lugo, Spain, 2020.
- Martín-Vega, D.; Clark, B.; Ferrer, L.M.; López, S.; Panadero, R.; Cepeda-Palacios, R.; Colwell, D.D.; Hall, M.J.R. Major di-versity in the larval anatomy of the digestive and excretory systems of three Oestridae species revealed by micro-CT. Med. Vet. Entomol. 2021, 35, 106–210.
- Arias, M.S.; Sánchez-Andrade, R.; Paz-Silva, A.; Suárez, J.L.; Cazapal-Monteiro, C.; Prieto, J.M.; Casais, R.; Díez-Baños, P.; Morrondo, P. Assessment of Cephenemyia stimulator infection in roe deer (Capreolus capreolus) from Asturias (North Spain) by ELISA. Mappe Parassitol. 2012, 18, 129.
- Pajares, G.; Arias, M.S.; Pérez-Creo, A.; Prieto, A.; Callejo, A.; Díez-Baños, P.; Morrondo, P. Epidemiología de la cefenemiosis en corzos del noroeste de España. Boletín de la Asociación del Corzo Español. IV Simp. Sobre El Corzo En La Península Ibérica 2017, 15, 129–134.
- Anderson, J.R. Adult Biology. In The Oestrid Flies: Biology, Host-Parasite Relationships, Impact and Management; Colwell, D.D., Hall, M.J.R., Scholl, P.J., Eds.; CAB Int.: Wallingford, UK, 2006; pp. 140–164.
- Vaca, D. Biology of Nasopharyngeal Bot Fly Cephenemyia Stimulator (Diptera, Oestridae) and Its Distribution in the Czech Republic; COST Action 833: Brussels, Belgium, 2000; pp. 189–194.
- Cepelak and Macicka. Ksezönnemu vyskytu an ekológii streckov raticovej zveri na lesnej správe v Kamenici nad Hronom. Folia Venatoria 1979, 9, 293–299.
- Király, I.; Egri, B. Epidemiological characteristics of Cephenemyia stimulator (Clark, 1815) larvae infestation in European deer (Capreolus capreolus) in Hungary. Acta Zool. Acad. Sci. Hung. 2007, 53, 271–279.
- Dudziňski, W. Studies on Cephenemyia stimulator (Clark, 1815) (Diptera, Oestridae), the parasite of European roe deer, Capreolus capreolus (L.). I. Biology. Acta Parasitol. Pol. 1970, 18, 555–572.
- Arias, M.S.; Pajares, G.; Paz-Silva, A.; Díez-Baños, N.; Suárez, J.L.; Díez-Baños, P.; Sánchez-Andrade, R.; Morrondo, P. Antigen characterization from second instar larvae of Oestridae flies for the detection of anti-Cephenemyia stimulator antibodies by ELISA in roe deer (Capreolus capreolus). Med. Vet. Entomol. 2014, 28, 83–89.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
31 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




