Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Héctor J. Rodríguez-Casanovas | + 1165 word(s) | 1165 | 2021-12-30 04:52:46 | | | |
| 2 | Jessie Wu | Meta information modification | 1165 | 2021-12-31 02:14:08 | | | | |
| 3 | Jessie Wu | Meta information modification | 1165 | 2021-12-31 02:14:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rodríguez-Casanovas, H. Virucidal Activity of Different Mouthwashes. Encyclopedia. Available online: https://encyclopedia.pub/entry/17661 (accessed on 07 February 2026).
Rodríguez-Casanovas H. Virucidal Activity of Different Mouthwashes. Encyclopedia. Available at: https://encyclopedia.pub/entry/17661. Accessed February 07, 2026.
Rodríguez-Casanovas, Héctor. "Virucidal Activity of Different Mouthwashes" Encyclopedia, https://encyclopedia.pub/entry/17661 (accessed February 07, 2026).
Rodríguez-Casanovas, H. (2021, December 30). Virucidal Activity of Different Mouthwashes. In Encyclopedia. https://encyclopedia.pub/entry/17661
Rodríguez-Casanovas, Héctor. "Virucidal Activity of Different Mouthwashes." Encyclopedia. Web. 30 December, 2021.
Copy Citation
This entry describes the effect of mouthwash on the stability of the viral envelope and its ability to reduce the viral load.
mouthwash
SARS-CoV-2
1. Background
The salivary glands may be a major source of SARS-CoV-2 in saliva [1]. COVID-19 can be found in patients’ saliva at a rate of 91.7 percent, and the live virus can be grown in salivary samples [2]. This suggests that SARS-CoV-2 transmitted by asymptomatically infected individuals may originate from infected saliva [3].
Since the beginning of the pandemic, the use of antiseptic mouth rinses has been suggested to help diminish the risk of transmission of SARS-CoV-2 [4][5][6]. This might be extremely beneficial in dentistry in granting a more secure field to work in and also in daily routine for individuals using mouthwash regularly.
Currently, there is enough in vitro evidence to reinforce the use of mouthwashes to potentially reduce the viral load of SARS-CoV-2 and other coronaviruses [7].
2. Current Research on Different Compounds Mouthwash
To evaluate the virucidal potential of different compounds and commercial mouthwash solutions, a method was designed to test the stability of the SARS-CoV-2 envelope to protect its RNA from RNase treatment directly from nasopharyngeal samples obtained from COVID-19 epidemiological surveillance. A model for the proposed mechanism of action by the mouthwash solutions on the viral envelope is described in Figure 1.
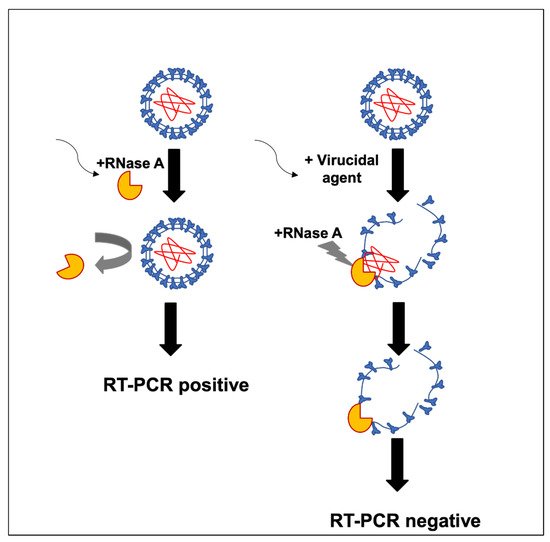
Figure 1. Model of the mechanism of action by mouthwash products on the viral envelope.
Previously diagnosed positive samples of SARS-CoV-2 RNA by RT-PCR were incubated in the presence of RNase to test whether enveloped viral particles, presumably infective, would protect the RNA from the enzymatic degradation. Figure 2 shows the stability of the viral RNA after the RNase treatment, suggesting the viral envelope protects the viral genome from enzymatic digestion.
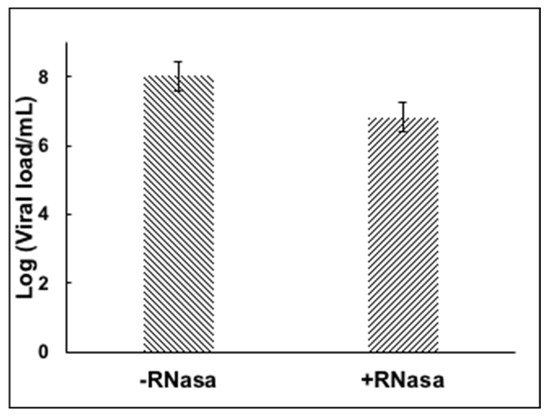
Figure 2. RNase treatment of nasopharyngeal sample containing SARS-CoV-2.
There is a decrease in the amount of viral load in the RNase treated sample, presumably due to broken viral particles and naked RNA present in the swab sample that was degraded during the RNase treatment. However, most of the RNA was preserved and quantified. The assay was tested with an iodopovidone solution (IPV), previously reported to have strong virucidal activity against SARS-CoV-2 in in vitro experiments [8]. A 1-min incubation of IPV with the nasopharyngeal swab positive sample reduced the viral load completely, more than 6 logs reduction efficacy (Figure 3), suggesting that IPV degrades or compromises the integrity of the viral envelope, exposing the RNA to the RNase enzyme to degrade it, and presumably inactivating the virus. The virucidal activity of several commercial mouthwash products was subsequently tested by the RNA inactivating assay. We observed a reduction of about 6 logs with a D-limonene (0.2%) and CPC (0.05%) containing formula §§.
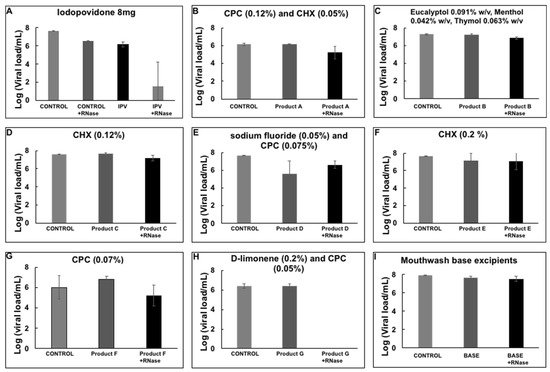
Figure 3. Viral load reduction of SARS-CoV-2 from nasopharyngeal samples by mouthwash products. (A) Iodopovidone *; (B) Product A **; (C) Product B ††; (D) Product C †††; (E) Product D ***; (F) Product E ‡‡‡; (G) Product F ‡‡; (H) Product G §§; (I) Mouthwash base excipients. Plots show the Mean and SD of 4–5 experiments.
However, none of the other solutions showed a reduction in viral load with the assay presented here (Figure 3).
Viral samples were incubated with D-limonene, CPC, or CHX, common compounds found in mouthwash solutions, to determine whether they had any impact on the reduction of viral load. After incubation with the different compounds already mentioned, D-limonene and CHX resulted in a reduction of viral load of about 1.5 logs (~95% reduction) (Figure 4). However, RNA was still present and resisted degradation through the RNase treatment.
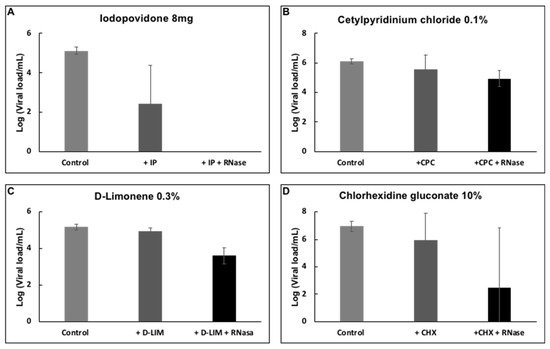
Figure 4. Effect of (A) IPV * 4%, (B) CPC † 0.1%, (C) D-limonene ‡ 10%, and (D) CHX § 10% on the detection of SARS-CoV-2 RNA by RT-PCR. Plots show the Mean and SD of 4–5 experiments.
3. D-limonene and CPC of Mouthwater
One of the main current concerns in dentistry is the risk of infection with SARS- CoV-2 while working with patients. A high risk of infection by SARS-CoV-2 while sharing settings that are enclosed, confined, or crowded, has also been described [9]. This has been increased due to new variants, as the Delta variant infections are on average ~1000 times greater compared to A/B lineage infections during the initial epidemic wave in China in early 2020, implying that Delta could replicate more quickly and be more contagious during the early stages of infection [10].
New alternatives of reducing the risk of infection by decreasing the viral loads of contagious individuals are being evaluated in different centers worldwide. Different protocols and recommendations have been published addressing key points to reduce this risk.
For dentists, using mouthwash before a dental appointment is one of the most promising ways to lower the risk of SARS-CoV-2 infection. Considering the way SARS-CoV-2 is transmitted, reducing its risk of infection has become a safety priority for dentists, as well as for the general population.
In May 2020, the Cochrane Library published a review with recommendations for the re-opening of dental services [11], reporting 82% of sources recommended the use of pre-operative mouthwashes at the dental office. This recommendation was issued without robust evidence, but other published articles do suggest that it is a recommendation that should be followed [12].
Mouthwashes directly inactivating SARS-CoV-2 would be crucial to creating a protocol to reduce the spread of COVID-19 [13].
Few articles have examined the inactivating effect of chlorhexidine gluconate (CHX) on SARS-CoV-2 [14][15], and some have suggested the effects of cetylpyridinium chloride (CPC) on other viruses [16], but there are currently no data on direct inactivation effects of CPC on SARS- CoV-2 [6]. On the other hand, previous studies on virucidal activities have been tested against some coronaviruses [17][18]. Benzalkonium chloride and hydrogen peroxide have also been tested against coronaviruses [16]. The relevance of using CDCM on day 1 (4 h after the initial dose) to reduce the SARS-CoV-2 viral load in saliva was supported by a multicenter, randomized, double-blind controlled trial of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in people with asymptomatic to moderate COVID-19 symptoms; the mouthwash was commercially available with cyclodextrin and citrox (bioflavonoids) (CDCM) [19]. A randomized clinical trial found that mouthwash containing CPC + Zinc and CHX resulted in substantial reduction of the SARS-CoV-2 viral load in saliva for up to 60 min after rinsing, whereas mouthwash with hydrogen peroxide (HP) resulted in a significant reduction for up to 30 min [20]. According to a study using an anionic phthalocyanine derivative (APD) in a mouthwash protocol, the mechanical action of the mouthwash containing a chemical with antiviral activity against SARS-CoV-2 may minimize patient symptoms and virus spread [21].
An important reduction of about 6 logs (or more than 99.99%) in the virucidal activity via a solution containing D-limonene and CPC. This finding is a strong advantage when compared to other mouthwashes included in the study.
The antiviral activity against SARS-CoV-2 of the following oral care products: iodopovidone (8 mg); * D-limonene, a terpene extracted from citrus peels (0.3%); † cetylpyridinium chloride (0.1%) (CPC); ‡ chlorhexidine gluconate (10%) (CHX); § a CPC (0.12%) and CHX (0.05%) containing formula; ** a formula containing essential oils; †† a CPC containing formula (0.07%); ‡‡ a D-limonene (0.2%) and CPC (0.05%) containing formula; §§ a solution containing sodium fluoride (0.05%) and CPC (0.075%); *** a solution containing CHX (0.12%); ††† a CHX (0.2%) containing formula ‡‡‡.
However, only iodopovidone and a D-limonene (0.2%) and CPC (0.05%) containing formula have shown a significant reduction of SARS-CoV-2 viral load using the biochemical assay.
References
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Chen, Z. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011, 85, 4025–4030.
- To, K.K.; Tsang, O.T.; Yip, C.C.; Chan, K.H.; Wu, T.C.; Chan, J.M.; Leung, W.S.; Chik, T.S.; Choi, C.Y.; Kandamby, D.H.; et al. Consistent detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020, 71, 841–843.
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020, 99, 989.
- Herrera, D.; Serrano, J.; Roldán, S.; Sanz, M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin. Oral Investig. 2020, 24, 2925–2930.
- O’Donnell, V.B.; Thomas, D.; Stanton, R.; Maillard, J.Y.; Murphy, R.C.; Jones, S.A.; Humphreys, I.; Wakelam, M.J.O.; Fegan, C.; Wise, M.P.; et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function 2020, 1, zqaa002.
- Seneviratne, C.J.; Balan, P.; Ko, K.; Udawatte, N.S.; Lai, D.; Ng, D.; Venkatachalam, I.; Lim, K.S.; Ling, M.L.; Oon, L.; et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 2021, 49, 305–311.
- Mateos Moreno, M.V.; Obrador, A.M.; Márquez, V.A.; Ferrer García, M.D. Oral antiseptics against Coronavirus: In vitro and clinical evidence. J. Hosp. Infect. 2021, 113, 30–43.
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020, 9, 669–675.
- Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. Scientific Brief. 9 July 2020. Available online: https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 9 August 2021).
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Xiong, Q.; Liu, Z.; Guo, Q.; Zou, L.; Zhang, H.; et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Medrxiv 2021, 7, 21260122.
- Jan, C.; Craig, R.; Magaly, A.-M.; Miriam, B.; Thibault, C.; Manas, D.; Anne-Marie, G.; Beatriz, G.; Thomas, L.; Derek, R.; et al. 16 May 2020 Recommendations for the Re-Opening of Dental Services: A Rapid Review of International Sources (Substantial Update 16 May 2020) Cochrane Database of Systematic Reviews. Available online: https://oralhealth.cochrane.org/news/recommendations-re-opening-dental-services-rapid-review-international-sources (accessed on 9 August 2021).
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927.
- Muñoz-Basagoiti, J.; Perez-Zsolt, D.; León, R.; Blanc, V.; Raïch-Regué, D.; Cano-Sarabia, M.; Izquierdo-Useros, N. Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro. J. Dent. Res. 2021, 100, 1265–1272.
- Komine, A.; Yamaguchi, E.; Okamoto, N.; Yamamoto, K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 475–477.
- Meister, T.L.; Brüggemann, Y.; Todt, D.; Conzelmann, C.; Müller, J.A.; Groß, R.; Steinmann, E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome Coronavirus 2. J. Infect. Dis. 2020, 222, 1289–1292.
- Baker, N.; Williams, A.J.; Tropsha, A.; Ekins, S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm. Res. 2020, 37, 104.
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251.
- Khokhar, M.; Roy, D.; Purohit, P.; Goyal, M.; Setia, P. Viricidal treatments for prevention of coronavirus infection. Pathog. Glob. Health 2020, 114, 349–359.
- Carrouel, F.; Valette, M.; Gadea, E.; Esparcieux, A.; Illes, G.; Langlois, M.E.; Perrier, H.; Dussart, C.; Tramini, P.; Ribaud, M.; et al. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: A multicentre, randomized, double-blind controlled trial. Clin. Microbiol. Infect. 2021, 27, 1494–1501.
- Eduardo, F.P.; Corrêa, L.; Heller, D.; Daep, C.A.; Benitez, C.; Malheiros, Z.; Stewart, B.; Ryan, M.; Machado, C.M.; Hamerschlak, N.; et al. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 2021, 7, e07346.
- da Silva Santos, P.S.; da Fonseca Orcina, B.; Machado, R.; Vilhena, F.V.; da Costa Alves, L.M.; Zangrando, M.; de Oliveira, R.C.; Soares, M.; Simão, A.; Pietro, E.; et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: Randomised trial. Sci. Rep. 2021, 11, 19937.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
COVID-19
Revisions:
3 times
(View History)
Update Date:
31 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




