
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberta Pennati | + 1737 word(s) | 1737 | 2021-12-24 09:38:53 | | | |
| 2 | Yvaine Wei | + 1 word(s) | 1738 | 2021-12-30 03:11:06 | | | | |
| 3 | Yvaine Wei | Meta information modification | 1738 | 2021-12-30 09:26:41 | | |
Video Upload Options
The swimming larva represents the dispersal phase of ascidians, marine invertebrates belonging to tunicates. Due to its adhesive papillae, the larva searches the substrate, adheres to it, and undergoes metamorphosis, thereby becoming a sessile filter feeding animal. The papillae of H. roretzi, previously described as simple and conform, exhibit dynamic changes during settlement. This opens up new considerations on papillae morphology and evolution and deserves to be further investigated.
1. Introduction
2. The 3D Reconstruction of Halocynthia roretzi Larva
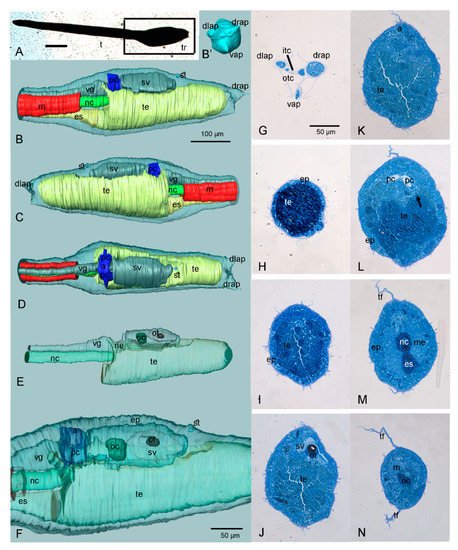
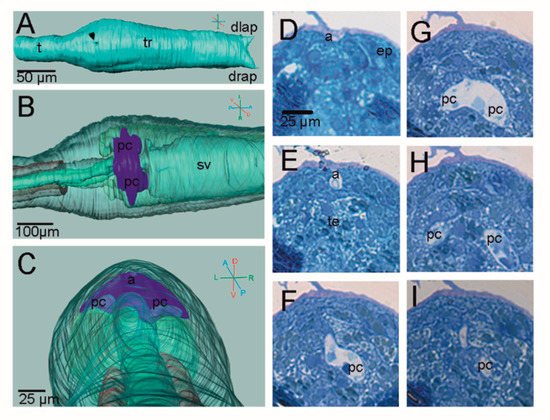
3. Adhesive Papillae
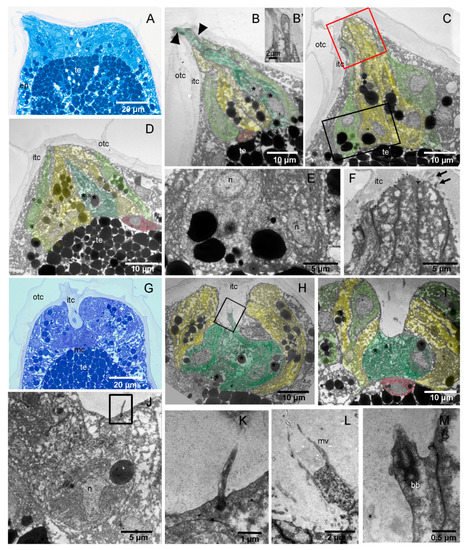
4. H. roretzi Exhibits Minimal Adultation
H. roretzi has been long considered very similar to the well-described larvae of the genera Ciona and Phallusia, which are phlebobranch model species [25]. These larvae are simple in their anatomy with no sign of adultation; the pharynx primordium occupies most of the trunk where the mesenchyme is organized in two lateral-ventral pockets [26]. The tail contains 40 notochord cells organized in a central row, flanked by 36 muscle cells on both sides. Dorsally to the notochord, the posterior neural tube is formed by a hollow of ependymal cells [22].
Indeed, it was already known that the larva of H. roretzi presents minimal caudalisation, since the tail has 42 rather than the conventional 36 or 38 tail muscle cells [27]. In this species, muscle cells are added to the posterior tip of the tail through the induction of more secondary muscle cells [28].
5. The Adhesive Papillae of H. roretzi Undergo Dynamic Changes during Adhesion
References
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965–968.
- Sepúlveda, R.; Rozbaczylo, N.; Ibáñez, C.; Flores, M.; Cancino, J. Ascidian-associated polychaetes: Ecological implications of aggregation size and tube-building chaetopterids on assemblage structure in the Southeastern Pacific Ocean. Mar. Biodivers 2014, 45, 733–741.
- Voultsiadou, E.; Kyrodimou, M.; Antoniadou, C.; Vafidis, D. Sponge epibionts on ecosystem-engineering ascidians: The case of Microcosmus sabatieri. Estuar. Coast. Shelf Sci. 2010, 86, 598–606.
- Mercurio, S.; Messinetti, S.; Manenti, R.; Ficetola, G.F.; Pennati, R. Embryotoxicity characterization of the flame retardant tris(1-chloro-2-propyl)phosphate (TCPP) in the invertebrate chordate Ciona intestinalis. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 339–347.
- Messinetti, S.; Mercurio, S.; Pennati, R. Effects of bisphenol A on the development of pigmented organs in the ascidian Phallusia mammillata. Invertebr. Biol. 2018, 137, 329–338.
- Messinetti, S.; Mercurio, S.; Pennati, R. Bisphenol A affects neural development of the ascidian Ciona robusta. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 5–16.
- Lambert, G.; Karney, R.C.; Rhee, W.Y.; Carman, M. Wild and cultured edible tunicates: A review. Manag. Biol. Invasions 2016, 7, 59–66.
- Coleman, F.C.; Williams, S.L. Overexploiting marine ecosystem engineers: Potential consequences for biodiversity. Trends Ecol. Evol. 2002, 17, 40–44.
- Wang, K.; Dantec, C.; Lemaire, P.; Onuma, T.A.; Nishida, H. Genome-wide survey of miRNAs and their evolutionary history in the ascidian, Halocynthia roretzi. BMC Genom. 2017, 18, 314.
- Brozovic, M.; Martin, C.; Dantec, C.; Dauga, D.; Mendez, M.; Simion, P.; Percher, M.; Laporte, B.; Scornavacca, C.; Di Gregorio, A.; et al. ANISEED 2015: A digital framework for the comparative developmental biology of ascidians. Nucleic Acids Res. 2016, 44, D808–D818.
- Lambert, G. Invasive sea squirts: A growing global problem. J. Exp. Mar. Biol. Ecol. 2007, 342, 3–4.
- Brown, F.D.; Swalla, B.J. Evolution and development of budding by stem cells: Ascidian coloniality as a case study. Dev. Biol. 2012, 369, 151–162.
- Alié, A.; Hiebert, L.S.; Scelzo, M.; Tiozzo, S. The eventful history of nonembryonic development in tunicates. J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 250–266.
- Berrill, N.J.; Watson, D.M.S.; II-Studies in Tunicate development. Part V-The evolution and classification of Ascidians. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1936, 226, 43–70.
- Rubinstein, N.D.; Feldstein, T.; Shenkar, N.; Botero-Castro, F.; Griggio, F.; Mastrototaro, F.; Delsuc, F.; Douzery, E.J.P.; Gissi, C.; Huchon, D. Deep Sequencing of Mixed Total DNA without Barcodes Allows Efficient Assembly of Highly Plastic Ascidian Mitochondrial Genomes. Genome Biol. Evol. 2013, 5, 1185–1199.
- Tsagkogeorga, G.; Turon, X.; Hopcroft, R.R.; Tilak, M.-K.; Feldstein, T.; Shenkar, N.; Loya, Y.; Huchon, D.; Douzery, E.J.P.; Delsuc, F. An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models. BMC Evol. Biol. 2009, 9, 187.
- DeBiasse, M.B.; Colgan, W.N.; Harris, L.; Davidson, B.; Ryan, J.F. Inferring Tunicate Relationships and the Evolution of the Tunicate Hox Cluster with the Genome of Corella inflata. Genome Biol. Evol. 2020, 12, 948–964.
- Jeffery, W.R.; Swalla, B.J. Evolution of alternate modes of development in ascidians. BioEssays 1992, 14, 219–226.
- Zaniolo, G.; Manni, L.; Burighel, P. Ovulation and embryo-parent relationships in Botrylloides leachi (Ascidiacea, Tunicata). Invertebr. Reprod. Dev. 1994, 25, 215–225.
- Zaniolo, G.; Manni, L.; Brunetti, R.; Burighel, P. Brood pouch differentiation in Botrylloides violaceus, a viviparous ascidian (Tunicata). Invertebr. Reprod. Dev. 1998, 33, 11–23.
- Manni, L.; Lane, N.J.; Joly, J.-S.; Gasparini, F.; Tiozzo, S.; Caicci, F.; Zaniolo, G.; Burighel, P. Neurogenic and non-neurogenic placodes in ascidians. J. Exp. Zool. B. Mol. Dev. Evol. 2004, 302, 483–504.
- Burighel, P.; Cloney, R.A. Urochordata: Ascidiacea. In Microscopic Anatomy of Invertebrates, Vol 15. Hemichordata, Chaetognatha, and the Invertebrate Chordates; Harrison, F.W., Rupert, E.E., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 1997; Volume 15.
- Dolcemascolo, G.; Pennati, R.; De Bernardi, F.; Damiani, F.; Gianguzza, M. Ultrastructural comparative analysis on the adhesive papillae of the swimming larvae of three ascidian species. Invertebr. Surviv. J. 2009, 6, S77–S86.
- Caicci, F.; Zaniolo, G.; Burighel, P.; Degasperi, V.; Gasparini, F.; Manni, L. Differentiation of papillae and rostral sensory neurons in the larva of the ascidian Botryllus schlosseri (Tunicata). J. Comp. Neurol. 2010, 518, 547–566.
- Hudson, C.; Yasuo, H. Similarity and diversity in mechanisms of muscle fate induction between ascidian species. Biol. Cell. 2008, 100, 265–277.
- Katz, M.J. Comparative anatomy of the tunicate tadpole, Ciona intestinalis. Biol. Bull. 1983, 164, 1–27.
- Passamaneck, Y.J.; Di Gregorio, A. Ciona intestinalis: Chordate development made simple. Dev. Dyn. 2005, 233, 1–19.
- Nishida, H. Determinative mechanisms in secondary muscle lineages of ascidian embryos: Development of muscle-specific features in isolated muscle progenitor cells. Development 1990, 108, 559–568.
- Pennati, R.; Zega, G.; Groppelli, S.; De Bernardi, F. Imunohistochemical analysis of the adhesive papillae of Botrylloides leachi (Chordata, Tunicata, Ascidiacea): Implications for their sensory function. Ital. J. Zool. 2007, 74, 325–329.
- Pennati, R.; Groppelli, S.; De Bernardi, F.; Mastrototaro, F.; Zega, G. Immunohistochemical analysis of adhesive papillae of Clavelina lepadiformis (Müller, 1776) and Clavelina phlegraea (Salfi, 1929) (Tunicata, Ascidiacea). Eur. J. Histochem. 2009, 53, e4.




