
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luka Roškar | + 1847 word(s) | 1847 | 2021-12-29 07:58:33 | | | |
| 2 | Luka Roškar | -23 word(s) | 1824 | 2021-12-30 00:56:54 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1824 | 2021-12-30 10:29:04 | | |
Video Upload Options
Endometrial cancer (EC) is the most frequent gynecological malignancy in developed countries and requires a relatively invasive diagnostic evaluation and operative therapy as the primary therapeutic approach. Angiogenesis is one of the main processes needed for cancer growth and spread. The production of angiogenic factors (AFs) appears early in the process of carcinogenesis. The detection of AFs in plasma and tissue and a better understanding of the angiogenic properties of EC may contribute not only to earlier but also more specific diagnosis and consequently tailored and individual therapeutic approaches. AFs and their receptors also have high potential as binding sites for targeted cancer therapy.
1. Introduction
2. Angiogenesis in Cancer
During the initial stage of tumor growth and development (i.e., before the tumor’s size exceeds 1–2 mm3), the tumor is independent of the vascular network, as nutrients and oxygen can be obtained via diffusion. During the later stages of carcinogenesis, such nutrient supply becomes insufficient. Due to rapid tumor growth, high interstitial pressure, and larger distances between cancer cells and capillaries, hypoxia occurs in solid tumors. Hypoxia is an important controller of the angiogenic switch that is mainly regulated by hypoxia-inducible factor-1α (HIF-1α), a transcription factor that activates the transcription of a set of key genes involved in cell survival under hypoxic conditions, e.g., those involved in initiating angiogenesis [22]. In this way, new vasculature is formed in and around the tumor that provides essential nutrients and oxygen for the tumor cells and allows continuous growth and unlimited proliferation. Simultaneously, this vascular network is used for metastatic spread to other organs [4,23]. The density of the capillary network has been shown to be a prognostic factor for EC. Blood microvessel density was associated with deeper myometrial invasion, positive lymphovascular invasion, positive lymph node metastasis, and poor overall survival in EC patients [24].
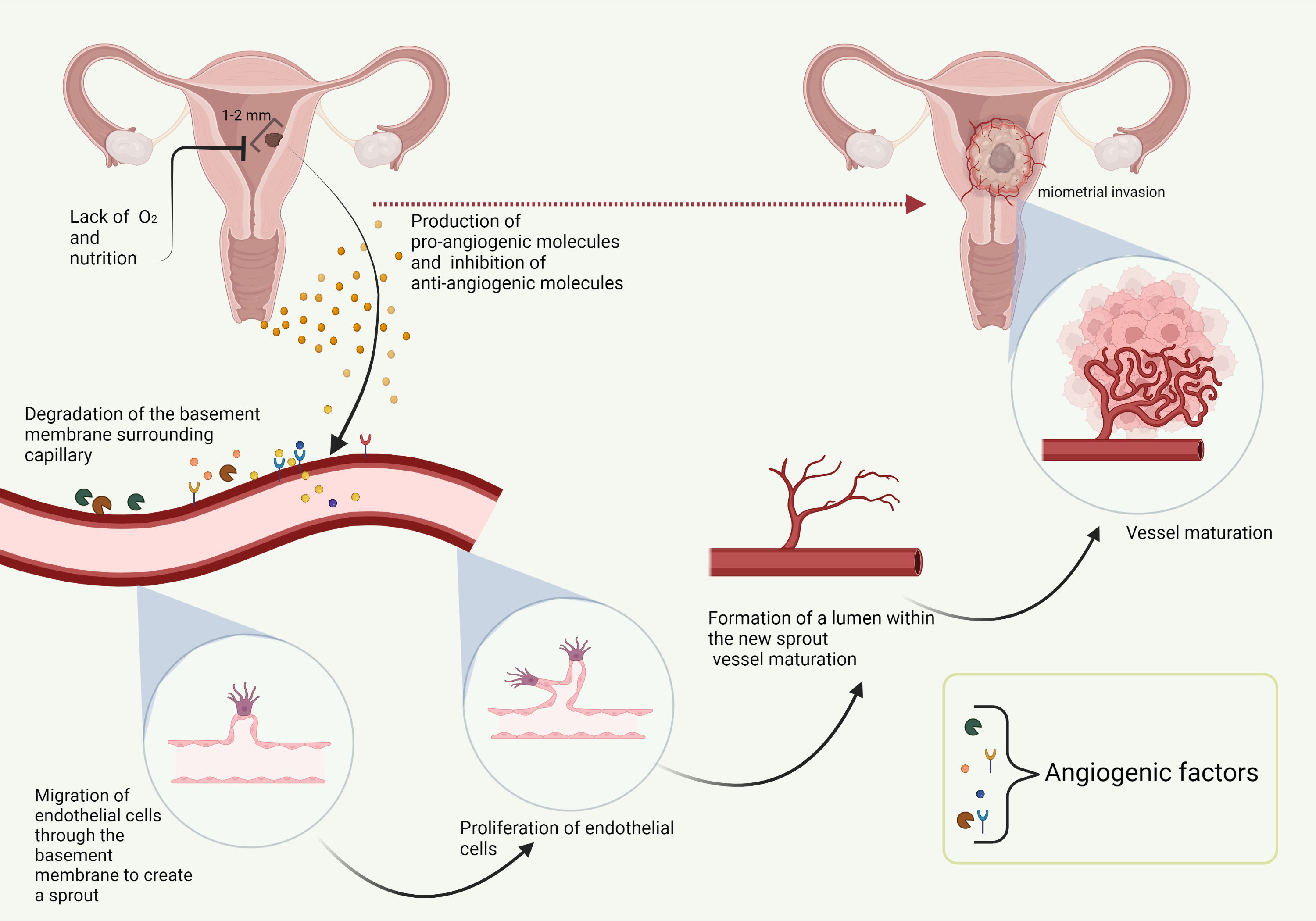 Figure 1. Angiogenesis in endometrial cancer. Created with BioRender.com.
Figure 1. Angiogenesis in endometrial cancer. Created with BioRender.com.
3. Diagnostic Value of AFs
Normal angiogenesis is regulated by both molecular activators and inhibitors. In cancer, the balance between them is disturbed in favor of angiogenic activators. More than a few dozen different proteins have been identified as pro-AFs, including growth factors, cytokines, proteases, protease inhibitors, trace elements, oncogenes, and endogenous modulators [25]. The production of AFs by cancer cells alters AF levels in the surrounding tissue and blood plasma. Altered AF levels may thus represent potential markers that could detect cancer from blood plasma samples in the early and prognostically favorable stages of cancer [26]. AF plasma concentrations could also represent an important additional diagnostic tool for a more precise diagnosis of EC, which could also guide decision-making regarding the extent of surgical treatment.
4. Anti-Angiogenic Treatment of EC
5. Conclusions
References
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2017, 110, 354–361.
- Arem, H.; Park, Y.; Pelser, C.; Ballard-Barbash, R.; Irwin, M.L.; Hollenbeck, A.; Gierach, G.L.; Brinton, L.A.; Pfeiffer, R.M.; Matthews, C.E. Prediagnosis Body Mass Index, Physical Activity, and Mortality in Endometrial Cancer Patients. J. Natl. Cancer Inst. 2013, 105, 342–349.
- Freuer, D.; Linseisen, J.; O’Mara, T.A.; Leitzmann, M.; Baurecht, H.; Baumeister, S.-E.; Meisinger, C. Body Fat Distribution and Risk of Breast, Endometrial, and Ovarian Cancer: A Two-Sample Mendelian Randomization Study. Cancers 2021, 13, 5053.
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257.
- Roškar, L.; Klančič, T.; Knific, T.; Rižner, T.; Smrkolj, Š. Tie-2, G-CSF, and Leptin as Promising Diagnostic Biomarkers for Endometrial Cancer: A Pilot Study. J. Clin. Med. 2021, 10, 765.
- Stelloo, E.; Bosse, T.; Nout, R.A.; Mackay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015, 28, 836–844.
- Lee, Y.C.; Lheureux, S.; Oza, A.M. Treatment strategies for endometrial cancer: Current practice and perspective. Curr. Opin. Obstet. Gynecol. 2017, 29, 47–58.
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974.
- McMeekin, D.S.; Sill, M.W.; Benbrook, D.; Darcy, K.M.; Stearns-Kurosawa, D.J.; Eaton, L.; Yamada, S.D. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: A Gynecologic Oncology Group study. Gynecol. Oncol. 2007, 105, 508–516.
- Waldner, M.J.; Neurath, M.F. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 5–13.
- Eng, L.; Azad, A.K.; Habbous, S.; Pang, V.; Xu, W.; Der Zee, A.H.M.-V.; Savas, S.; Mackay, H.J.; Amir, E.; Liu, G. Vascular Endothelial Growth Factor Pathway Polymorphisms as Prognostic and Pharmacogenetic Factors in Cancer: A Systematic Review and Meta-analysis. Clin. Cancer Res. 2012, 18, 4526–4537.
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204.
- Oza, A.M.; Selle, F.; Davidenko, I.; Korach, J.; Mendiola, C.; Pautier, P.; Chmielowska, E.; Bamias, A.; DeCensi, A.; Zvirbule, Z.; et al. Efficacy and Safety of Bevacizumab-Containing Therapy in Newly Diagnosed Ovarian Cancer: ROSiA Single-Arm Phase 3B Study. Int. J. Gynecol. Cancer 2016, 27, 50–58.
- van Beijnum, J.R.; Nowak-Sliwinska, P.; Huijbers, E.J.M.; Thijssen, V.L.; Griffioen, A.W. The Great Escape; the Hallmarks of Resistance to Antiangiogenic Therapy. Pharmacol. Rev. 2015, 67, 441–461.
- Aghajanian, C.; Filiaci, V.; Dizon, D.S.; Carlson, J.W.; Powell, M.A.; Secord, A.A.; Tewari, K.S.; Bender, D.P.; O’Malley, D.M.; Stuckey, A.; et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol. 2018, 150, 274–281.
- Leslie, K.K.; Filiaci, V.L.; Mallen, A.R.; Thiel, K.W.; Devor, E.J.; Moxley, K.; Richardson, D.; Mutch, D.; Secord, A.A.; Tewari, K.S.; et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: An NRG Oncology study. Gynecol. Oncol. 2021, 161, 113–121.
- Dizon, D.S.; Sill, M.W.; Schilder, J.M.; McGonigle, K.F.; Rahman, Z.; Miller, D.S.; Mutch, D.G.; Leslie, K.K. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2014, 135, 441–445.
- Bender, D.; Sill, M.W.; Lankes, H.A.; Reyes, H.D.; Darus, C.J.; Delmore, J.E.; Rotmensch, J.; Gray, H.J.; Mannel, R.S.; Schilder, J.M.; et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015, 138, 507–512.
- Powell, M.A.; Sill, M.W.; Goodfellow, P.J.; Benbrook, D.M.; Lankes, H.A.; Leslie, K.K.; Jeske, Y.; Mannel, R.S.; Spillman, M.A.; Lee, P.S.; et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2014, 135, 38–43.
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992.
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163.
- Coleman, R.L.; Sill, M.W.; Lankes, H.A.; Fader, A.N.; Finkler, N.J.; Hoffman, J.S.; Rose, P.G.; Sutton, G.P.; Drescher, C.W.; McMeekin, D.S.; et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2012, 127, 538–543.
- Moore, K.N.; Sill, M.W.; Tenney, M.E.; Darus, C.J.; Griffin, D.; Werner, T.L.; Rose, P.G.; Behrens, R. A phase II trial of trebananib (AMG 386; IND#111071), a selective angiopoietin 1/2 neutralizing peptibody, in patients with persistent/recurrent carcinoma of the endometrium: An NRG/Gynecologic Oncology Group trial. Gynecol. Oncol. 2015, 138, 513–518.




