Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Piotr Kurcok | + 2711 word(s) | 2711 | 2021-12-22 07:32:57 | | | |
| 2 | Jason Zhu | Meta information modification | 2711 | 2021-12-30 02:27:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kurcok, P. β-butyrolactone. Encyclopedia. Available online: https://encyclopedia.pub/entry/17633 (accessed on 07 February 2026).
Kurcok P. β-butyrolactone. Encyclopedia. Available at: https://encyclopedia.pub/entry/17633. Accessed February 07, 2026.
Kurcok, Piotr. "β-butyrolactone" Encyclopedia, https://encyclopedia.pub/entry/17633 (accessed February 07, 2026).
Kurcok, P. (2021, December 29). β-butyrolactone. In Encyclopedia. https://encyclopedia.pub/entry/17633
Kurcok, Piotr. "β-butyrolactone." Encyclopedia. Web. 29 December, 2021.
Copy Citation
Over 35 years ago, researchers originally reported on the anionic ring-opening polymerization (ROP) of β-butyrolactone (BBL) into poly(β-butyrolactone) (poly(BBL)), which is an amorphous, atactic analogue of isotactic poly([R]-β-hydroxybutyrate) (PHB), a promising and widely used natural biodegradable polyester.
β-butyrolactone
anionic ring-opening polymerization
poly(hydroxyalkanolate)s
1. Homo- and Copolymerization of β-butyrolactone (BBL) with Anionic Initiators of Different Nucleophilicity
The ring opening of β-butyrolactone (BBL) can proceed by two different pathways: O-acyl cleavage (a) and O-alkyl cleavage (b), depicted in Scheme 1. From both pathways, it can be deduced that acyl cleavage leads to the formation of an alkoxide chain-end group, while a carboxylate results from alkyl cleavage.
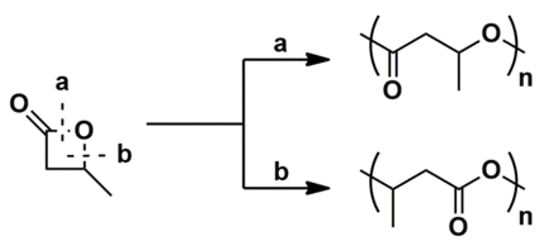
Scheme 1. Two possible mechanisms for the ROP of BBL.
Over 35 years ago, β-butyrolactone was polymerized by a homogeneous solution of potassium in THF. This solution results from the reaction of a crown ether (18-crown-6) in THF with a preformed potassium mirror [1][2]. The presence of a crown ether was found to be essential for the anionic ROP of this dormant monomer. Thus, alkali metal naphthalenides in THF, such as potassium naphthalenide complexes with crown ethers or cryptands, are another class of effective initiators for ROP of BBL. Accordingly, researchers prepared poly(BBL) within high yields and with a narrow molecular mass distribution. The mechanism of this polymerization consists of an α-proton abstraction from monomer, formation of the corresponding enolate, and subsequent scission of the alkyl-oxygen bond with formation of a potassium crotonate (Scheme 2). This species initiates propagation, which proceeds through an active carboxylate group [3][4].
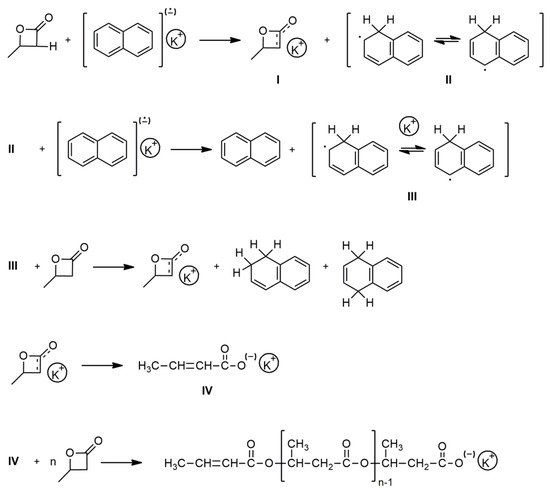
Scheme 2. Anionic ROP of BBL in the presence of potassium naphthalenide/18-crown-6 complex.
A similar initiation mechanism was observed in anionic polymerization of BBL in the presence of a potassium hydride/18-crown-6 complex. The α-proton abstraction of the monomer was found to proceed at the initiation step of this polymerization. The salt of the crotonic acid formed initiates further propagation, leading to polyester functionalized with unsaturated dead end groups [5].
Over this time, it has also been shown that sodium and potassium alkoxides (strong nucleophiles) in aprotic solvents react with the BBL according to the mechanism of nucleophilic substitution at the carbonyl carbon with the acyl-oxygen bond of the lactone ring scission and formation of an unstable β-hydroxyester alkoxide, which is finally transformed into to the respective alkyl crotonate (Scheme 3). The alkali metal hydroxide formed in this reaction is the real polymerization initiator, and its reaction with the monomer molecule leads to the formation of a hydroxyacid salt. Under the reaction conditions, the salt is partially eliminated to the unsaturated acid salt. Thus, the chain growth in the studied polymerization occurs only at carboxylate centers [6][7][8][9].
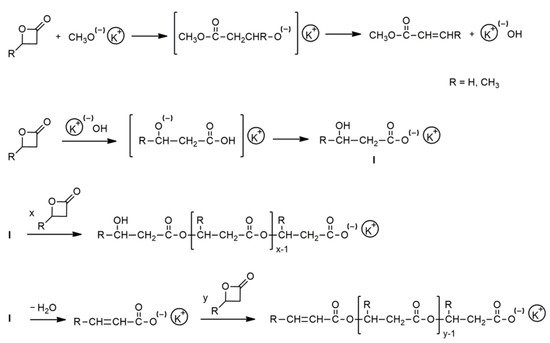
Scheme 3. Anionic ROP of BBL in the presence of CH3-OK/18-crown-6 complex.
The complexity of the initiation mechanism of anionic BBL polymerization, in relation to the ambient reactivity of the initiator used, BBL purity, and the effect of experimental parameters such as solvent and temperature, has been further studied in detail. The influence of monomer purity on the polymerization reaction reveals that an additional reaction of BBL with an oxidizing agent, such as potassium permanganate, produces a monomer of higher purity, as demonstrated by the higher rate of BBL polymerization and improved control over the polymerization process that is initiated with either tetrabutylammonium acetate or carboxylic acid/phosphazene base (P1-t-Bu, P2-t-Bu and P4-t-Bu) systems [10].
The anionic polymerization of BBL initiated with acetic acid salts in selected solvents showed a significant dependence of the activity of the initiator and polymer chain-growth centers on the size of the counterion used for the process carried out in a solvent with relatively low polarity (tetrahydrofuran). In addition, acceleration or retardation of the polymerization was found, depending on the initiator (counterion)/solvent system used. For a carboxylate with a small counterion in a solvent with high polarity and for a carboxylate with a large counterion in a solvent with low polarity, acceleration of the reaction was observed. However, for salts with a large counterion in a highly polar solvent, the opposite effect was observed [11]. Based on the information obtained, the method for the synthesis of high molar mass (Mn > 100,000) poly(BBL) with low dispersity by anionic ROP carried out in bulk and initiated with tetrabutylammonium acetate was described [12].
The mechanism of BBL polymerization initiated with both strong nucleophiles, e.g., alkali metal methoxide, and weak nucleophiles, such as alkali metal carboxylates, inspired us to clarify the mechanism of initiation of the polymerization of this monomer in the presence of solvent-activated (DMSO) initiators , such as alkali metal phenolates, i.e., weak nucleophiles/bases with an alkoxide structure of active centers [13]. The obtained results of the research on this system clearly show that the structure of the active centers of the anionic initiator does not affect the initiation mechanism, and the main factor defining the opening mechanism of the BBL ring is the basicity and nucleophilicity of the initiator (Scheme 4).
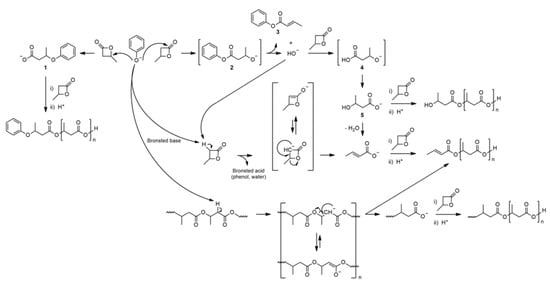
Scheme 4. Anionic ROP of BBL initiated by sodium phenoxide (for simplicity, the counterion is omitted).
The lowest basic phenoxide (sodium p-nitrophenoxide) reacts mainly as a weak nucleophile, i.e., the nucleophilic attack occurs at the C4 carbon of the lactone, similarly to weak-nucleophile-initiated polymerization. In the case of using sodium p-methoxyphenoxide, the most basic of the tested phenoxides (but also the most nucleophilic), the initiation proceeds predominantly according to the addition/elimination mechanism typical of strong nucleophiles, e.g., alkali metal alkoxides. It is important that depending on the basicity and nucleophilicity of the initiator used, the initiation of anionic polymerization of BBL varies, but ultimately, the centers of chain growth are carboxylate centers, which is important, for example, when planning the synthesis of block copolymers [13].
Current studies on anionic ROP of BBL have opened a new perspective for the preparation of bioactive polymer conjugates containing biodegradable polymer moieties. PHA and their synthetic analogues, due to their in vivo and in vitro biodegradation, as well as cell and tissue compatibility, can be used in medical applications, especially as drug delivery systems, implants, including heart-valve tissue engineering, vascular tissue engineering, bone tissue engineering, cartilage tissue engineering, as well as nerve conduit tissue biomaterials [14]. Researchers have shown that poly(BBL) oligomers are non-toxic and can potentially serve to modify pharmacological properties and/or serve as carriers able to vectorize drugs in the form of chemical conjugates for drug delivery. The methods for oligomer preparation are simple, and drug sensitivity tests have proved that these conjugates are nontoxic. Moreover, poly(BBL) oligomers do not induce the cellular cytoprotective response [15][16].
Over 90 years after Fleming’s discovery, penicillins are still widely used as antibiotics, and a variety of their new derivatives have been synthesized, tested, and commercialized. This β-lactam antibiotic covalently bonded to atactic poly(BBL) was originally synthetized via ring-opening polymerization of racemic BBL initiated by a supramolecular complex of penicillin G potassium salt, thus showing that the conjugation of penicillins is a promising method of pharmacokinetic modification of such antibiotics [17][18].
The anticancer activity of acetylsalicylic acid with oligo p(BBL) conjugates, as well as their characteristics and in vitro biological studies, has also been reported [19]. Research has demonstrated that acetylsalicylic acid (aspirin) attached via hydrolysable ester bonds to non-toxic, well-defined BBL oligomers was more effective than aspirin in growth inhibition of human colon adenocarcinoma cells, HT-29, and human colon carcinoma cells, HCT 116, in vitro.
Ibuprofen is a well-known anti-inflammatory, analgesic, and antipyretic drug that has recently been found to slow down proliferation of colon cancer cells effectively. A convenient method of synthesis of ibuprofen conjugates via anionic oligomerization of BBL initiated with ibuprofen sodium salt in DMSO, as well as their antiproliferative activity against HT-29 and HCT 116 colon cancer cells, has been studied. Research has demonstrated that such modification increased the anticancer potential of the drug. A significant difference between the antiproliferative properties of ibuprofen conjugate, as compared to free ibuprofen, indicates changes in the mechanism of action or bioavailability of the drug caused by the attachment of 3-hydroxybutyrate oligomers, as well as enhancement of cellular uptake of ibuprofen conjugate [20].
The above-mentioned examples demonstrate that conjugation of poly(BBL) oligomers with selected drugs could be one strategy aimed at improving or modifying biopharmaceutical properties of the drug.
2. Studies on the Biodegradable Polymer Systems for Controlled Release of Bioactive Substances for Cosmetology
Using the concept of activated initiators in anionic polymerization of BBL and extension on other β-substituted β-lactones containing a bounded biologically active substance, innovative, biodegradable polymer systems were developed for controlled release of bioactive substances for application in cosmetology. Two approaches were elaborated to enable chemical bonding of the biologically active substances of antioxidative properties with the chains of oligomeric 3-hydroxybutyrate and its copolymers. In the first method, as initiators of the ring-opening polymerization of β-butyrolactone, the sodium or potassium salts of the selected phenolic acids with antioxidative properties were used.
Conjugates were obtained in which the biologically active compounds of antioxidative properties, including lipoic acid, as well as selected phenolic acids, were chemically bonded as end groups of the oligomeric poly(BBL) [21][22]. In further research, within the cooperation of the team managed by Professor Janusz Jurczak from the Institute of Organic Chemistry of the Polish Academy of Sciences, a method for synthesis of β-substituted β-lactones containing biologically active substances was developed, which has not yet been described in literature [23]. Using the β-substituted β-lactones containing a chemically bonded bioactive substance as monomers or comonomers in anionic polymerization obtained bioactive (co)oligoesters containing more molecules of the bioactive substance linked as side groups along the polymer chain [24]. It was demonstrated that the studied conjugates are non-toxic and well-tolerated by epidermal cells. The conducted tests of permeability confirmed that the obtained bioconjugates penetrated deep into skin layers, but no in vitro transdermal penetration was observed [22].
3. Studies on the Biodegradable Polymer Systems for Controlled Release of Bioactive Substances for Agriculture and Environmental Protection
Simultaneous to the research on the systems for controlled release of bioactive substances for cosmetology, studies on systems for controlled release of bioactive substances for potential application in agriculture were conducted. Research covered bioactive substances belonging to pesticide groups, which aimed to develop systems to allow for extended duration of release of a pesticide for the purpose of delivering its optimum amount to a target area. As a result, this would limit significant losses of active ingredients caused by weather conditions, as well as the adverse impact of such compounds on the environment, which can be seen in conventional forms of pesticide use. Preliminary research resulted in the development of methods that allow us to chemically bind the bioactive substances, i.e., pesticides with biodegradable oligomers of poly(BBL)s. The developed methods are based on anionic oligomerization of BBL or other β-substituted β-lactones containing linked bioactive substance selected from the pesticide group and possessing a carboxyl group. Just like in the case of the above-mentioned conjugates for cosmetology, the application of these methods allowed us to obtain and characterize two types of pesticide-oligomer conjugates:
- (i)pesticide-oligo BBL conjugates in which each oligo(BBL) chain contains one molecule of pesticide (a herbicide or an antibacterial substance) as an end group, which is connected by ester bond [25][26],
- (ii)homo- and (co)oligoesters containing an increased amount of bioactive substance connected to the oligomer chains as the end group and side groups along the polymer chain. The amount of pesticide molecules bonded to the chain of (co)oligoesters may be controlled by changing the composition of the β-lactone monomers used in the process of (co)oligomerization [27].
Initial tests on the obtained conjugates were performed to determine the usefulness of the developed systems for application in agriculture. An important element was research into hydrolytic degradation of pesticide-oligomer conjugates, which confirmed that the ester bond between the bioactive substance and the oligomer chain is subject to hydrolysis. This allowed for the gradual release of the bioactive compounds from the oligomer chain in their original form, while maintaining their biological activity. Moreover, the tests conducted in both greenhouse and field conditions, in cooperation with the Plant Protection Institute in Sośnicowice and the Jan Długosz University in Częstochowa, Poland, demonstrated that the herbicidal effectiveness of the formulations containing the selected polymer-herbicide conjugates on selected dicotyledonous weeds was comparable to that of the commercially available formulation containing the same active substance [28].
The benefit of the developed system is an extended period of action, which permits a reduction in the use of that type of ingredients during one season.
The positive results of these preliminary tests of the oligomer-pesticide conjugates obtained through anionic oligomerization provided the impulse for continuing research in this area and to search for synthesis methods of such conjugates that might be more economically advantageous. This resulted in the development of a method for synthesis of this type of conjugates through transesterification of commercially available aliphatic biopolyesters with selected pesticides. The developed method is relatively simple, provides a high percentage value of attachment of pesticide to PHA, and may be applied to the synthesis of conjugates both using pesticides containing a carboxyl group and bioactive substances containing hydroxyl groups [29][30].
Such methods are promising both from an economical point of view and from the point of view of scale-up of synthesis, owing to the application of commercially available polyhydroxyalkanoates and pesticides used in preparations currently available on the market, the relatively short period of reaction of biopolyesters with pesticides (transesterification in 2 min), and the elimination of solvents.
4. Varying Copolymer Composition Affords Copolyesters with Adjustable Properties
The block polymerization of BBL with β-propiolactone proceeds fast, with a high yield in the presence of potassium solutions in THF containing 18-crown-6. Respective block copolymers with the expected molar mass and composition are formed in this way. Their glass transition and melting temperatures, as well as their melting enthalpies, determined by DSC, show a strict correlation with block copolymer composition [31].
The “living” poly(BBL) with carboxylate active centers was applied as an initiator for synthesis of poly(pivalolactone) (PPVL) block copolymers. The obtained diblock copolymers with tailored molecular weight and composition contain an amorphous phase with Tg = 5 °C, associated with the poly(BBL) block, and a high-melting crystalline phase, the amount of which increases with PPVL content [32].
Copolyesters with designed architecture were obtained via anionic ring-opening copolymerization of BBL with β-ethoxymethyl-β-propiolactone initiated with tetrabutylammonium acetate. Depending on the reaction conditions, diblock or random copolymers were obtained, and their structure was evaluated at the molecular level [33].
Diblock copolymers consisting of natural PHAs and poly(BBL) were also prepared. Macroinitiators obtained by the controlled degradation of natural PHAs (PHB, PHBV, or PHO) in the presence of KOH/18-crown-6 complex were then used in anionic ROP of BBL [34]. Combining the anionic polymerization of the of BBL with the coordination ring-opening polymerization of the ε-caprolactone enabled synthesis of the respective diblock copolymer. According to NMR and DSC analysis, the poly(BBL) block is atactic and totally amorphous, in contrast to the polycaprolactone block, which is semicrystalline. However, a partial miscibility of the two blocks in the amorphous phase was detected [35]. The polyethylene oxide (PEG)-containing poly(BBL)-b-PEG-b-poly(BBL) triblock copolymers were obtained via anionic polymerization of racemic BBL, initiated with respective PEG macroinitiators with carboxylate moieties. The structure of resulting triblock copolymers was proved by SEC and NMR spectroscopy [36]. Star-like copolymers composed of hydrophilic PEG and hydrophobic poly(BBL) segments linked by phosphoester moiety were also obtained [37]. A brush copolymer composed of a biodegradable hydrophobic poly(BBL) chain and hydrophilic PEG brushes was synthesized by a three-step procedure consisting of ring-opening anionic polymerization of BBL, yielding polyester with two hydroxyl functionalities at one chain terminus, followed by synthesis of PHB-derived microinitiator species, and ATRP of polyethylene glycol methyl ether methacrylate. The self-aggregation behavior of the brush copolymer in an aqueous medium was evidenced by optical-absorption-probe technique and dynamic/static light-scattering measurements [38]. ATRP was also used for synthesis of the poly(BBL) copolymers modified by introduction of hydrophobic or hydrophilic segments via grafting technique [39]. The anionic grafting from the reaction of BBL on poly(methyl methacrylate) (PMMA) was used for the synthesis of graft copolymers of PMMA with poly(BBL) side chains [40]. The partially saponified PMMA bearing carboxylate anions complexed by 18-crown-6 potassium counterion was used as a macroinitiator of BBL polymerization (Scheme 5). The compatibilizing effect of such graft copolymers on bacterial PHB/PMMA blends was observed [41].
References
- Jedliński, Z.; Kurcok, P.; Kowalczuk, M.; Kasperczyk, J. Anionic Polymerization of 4-Methyl-2-Oxetanone. Makromol. Chem. 1986, 187, 1651–1656.
- Jedliński, Z.; Kowalczuk, M.; Kurcok, P. Anionic Ring Opening Polymerization by Alkali Metal Solutions. Makromol. Chem. Macromol. Symp. 1986, 3, 277–293.
- Jedliński, Z.; Kowalczuk, M.; Glowkowski, W.; Grobelny, J.; Szwarc, M. Novel polymerization of β-butyrolactone initiated by potassium naphthalenide in the presence of a crown ether or a cryptand. Macromolecules 1991, 24, 349–352.
- Jedliński, Z.; Kowalczuk, M.; Kurcok, P. Polymerization of β-Lactones Initiated with Potassium Naphthalenide. A Convenient Route to Telechelic Polymers. J. Macromol. Sci. Pure Appl. Chem. 1992, A29, 1223–1230.
- Kurcok, P.; Matuszowicz, A.; Jedliński, Z. Anionic Polymerization of β-Lactones Initiated with Potassium Hydride. A Convenient Route to Polyester Macromonomers. Macromol. Rapid Commun. 1995, 16, 201–206.
- Jedliński, Z.; Kowalczuk, M.; Kurcok, P. What is the Real Mechanism of Anionic Polymerization of β-Lactones by Potassium Alkoxides? A Critical Approach. Macromolecules 1991, 24, 1218–1219.
- Kurcok, P.; Kowalczuk, M.; Hennek, K.; Jedliński, Z. Anionic Polymerization of β-Lactones Initiated with Alkali-Metal Alkoxides: Reinvestigation of the Polymerization Mechanism. Macromolecules 1992, 25, 2017–2020.
- Kurcok, P.; Jedliński, Z.; Kowalczuk, M. Reactions of β-Lactones with Potassium Alkoxides and Their Complexes with 18-Crown-6 in Aprotic Solvents. J. Org. Chem. 1993, 58, 4219–4220.
- Kurcok, P.; Kowalczuk, M.; Jedliński, Z. Response to ‘On the Ambident Raectivity of β-Lactones in Their Reactions with Alcoholates Initiating Polymerization. Macromolecules 1994, 27, 4833–4835.
- Kawalec, M.; Coulembier, O.; Gerbaux, P.; Sobota, M.; De Winter, J.; Dubois, P.; Kowalczuk, M.; Kurcok, P. Traces Do Matter—Purity of 4-Methyl-2-Oxetanone and Its Effect on Anionic Ring-Opening Polymerization as Evidenced by Phosphazene Superbase Catalysis. React. Funct. Polym. 2012, 72, 509–520.
- Kawalec, M.; Śmiga-Matuszowicz, M.; Kurcok, P. Counterion and Solvent Effects on the Anionic Polymerization of β-Butyrolactone Initiated with Acetic Acid Salts. Eur. Polym. J. 2008, 44, 3556–3563.
- Kurcok, P.; Śmiga, M.; Jedliński, Z. β-Butyrolactone Polymerization Initiated with Tetrabutylammonium Carboxylates. A Novel Approach to Biomimetic Polyesters Synthesis. J. Polym. Sci. Polym. Chem. 2002, 40, 2184–2189.
- Domiński, A.; Konieczny, T.; Zięba, M.; Klim, M.; Kurcok, P. Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism Polymers 2019, 11, 1221.
- Peptu, C.; Kowalczuk, M. 8—Biomass-Derived Polyhydroxyalkanoates: Biomedical Applications in Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amterdam, The Netherlands, 2018; pp. 271–313.
- Piddubnyak, V.; Kurcok, P.; Matuszowicz, A.; Głowala, M.; Fiszer-Kierzkowska, A.; Jedliński, Z.; Juzwa, M.; Krawczyk, Z. Oligo-3-hydroxybutyrates as potential carriers for drug delivery. Biomaterials 2004, 25, 5271–5279.
- Elustondo, P.A.; Angelova, P.R.; Kawalec, M.; Michalak, M.; Kurcok, P.; Abramov, A.Y.; Pavlov, E.V. Polyhydroxybutyrate Targets Mammalian Mitochondria and Increases Permeability of Plasmalemmal and Mitochondrial Membranes. PLoS ONE 2013, 8, e75812.
- Adamus, G.; Kowalczuk, M. Electrospray multistep ion trap mass spectrometry for the structural characterisation of poly containing a β-lactam end group. Rapid Commun. Mass Spectr. 2000, 14, 195–202.
- Schmidt, M.; Bast, L.K.; Lanfer, F.; Richter, L.; Hennes, E.; Seymen, R.; Krumm, C.; Tiller, J.C. Poly(2-oxazoline)–Antibiotic Conjugates with Penicillins. Bioconjugate Chem. 2017, 28, 2440–2451.
- Juzwa, M.; Rusin, A.; Zawidlak-Wegrzyńska, B.; Krawczyk, Z.; Obara, I.; Jedliński, Z. Oligo(3-hydroxybutanoate) conjugates with acetylsalicylic acid and their antitumour activity. Eur. J. Med. Chem. 2008, 43, 1785–1790.
- Zawidlak-Węgrzyńska, B.; Kawalec, M.; Bosek, I.; Łuczyk-Juzwa, M.; Adamus, G.; Rusin, A.; Filipczak, P.; Głowala-Kosińska, M.; Wolańska, K.; Krawczyk, Z.; et al. Synthesis and Anti-proliferative Properties of Ibuprofen-Oligo Conjugates. Eur. J. Med. Chem. 2010, 45, 1833–1842.
- Maksymiak, M.; Dębowska, R.; Jelonek, K.; Kowalczuk, M.; Adamus, G. Structural characterization of biocompatible lipoic acid—oligo(3-hydroxybutyrate) conjugates by ESI-mass spectrometry. Rapid Commun. Mass Spectr. 2013, 27, 773–783.
- Maksymiak, M.; Dębowska, R.; Bazela, K.; Dźwigałowska, A.; Orchel, A.; Jelonek, K.; Dołęgowska, B.; Kowalczuk, M.; Adamus, G. Designing of biodegradable and biocompatible release and delivery systems of selected antioxidants used in cosmetology. Biomacromolecules 2015, 6, 3603–3612.
- Bałakier, T.; Chaładaj, W.; Jurczak, J.; Adamus, G.; Kowalczuk, M. An effective protocol for the synthesis enantiomerically pure 4-substituted oxetane-2-ones. Tertahedreon 2013, 69, 4990–4993.
- Maksymiak, M.; Bałakier, T.; Jurczak, J.; Kowalczuk, M.; Adamus, G. Bioactive (co)oligoesters with antioxidant properties—Synthesis and structural characterization at the molecular level. RSC Adv. 2016, 6, 7751–7761.
- Kwiecień, I.; Adamus, G.; Kowalczuk, M. Electrospray ionisation mass spectrometry molecular-level structural characterisation of novel phenoxycarboxylic acid–oligo(3-hydroxybutyrate) conjugates with potential agricultural applications. Rapid Commun. Mass Spectr. 2012, 26, 2673–2682.
- Kwiecień, I.; Adamus, G.; Bartkowiak, A.; Kowalczuk, M. Synthesis and structural characterization at the molecular level of oligo(3-hydroxybutyrate) conjugates with antimicrobial agents designed for food packaging materials. Des. Monomers Polym. 2014, 17, 311–321.
- Kwiecień, I.; Bałakier, T.; Jurczak, J.; Kowalczuk, M.; Adamus, G. Molecular architecture of novel potentially bioactive (co)oligoesters containing pesticide moieties established by electrospray ionization multistage mass spectrometry. Rapid Commun. Mass Spectr. 2015, 29, 533–544.
- Kowalski, W.J.; Glazek, M.; Silowiecki, A.; Kowalczuk, M.; Romanowska, I.; Wloka, D. Controlled Release of 2,4-D and Dicamba 3-hydroxybutyric Acid Oligomers. In Pesticide Formulation and Delivery Systems: 32nd Volume, Innovating Legacy Products for New Uses; Bernards, M., Devisetty, B., Eds.; ASTM International: West Conshohocken, PA, USA, 2013; pp. 15–30.
- Kwiecień, I.; Radecka, I.; Kowalczuk, M.; Adamus, G. Transesterification of PHA to oligomers covalently bonded with (bio)active compounds containing either carboxyl or hydroxyl functionalities. PLoS ONE 2015, 10, e0120149.
- Kwiecień, I.; Radecka, I.; Kwiecień, M.; Adamus, G. Synthesis and structural characterization of bioactive PHA and γ-PGA oligomers for potential applications as a delivery system. Materials 2016, 9, 307.
- Jedliński, Z.; Kowalczuk, M.; Kurcok, P.; Brzoskowska, L.; Franek, J. Anionic Block Polymerization of β-Lactones Initiated by Potassium Solutions 1. Synthesis of Poly(4-methyl-2-oxetanone-block-2-oxetanone). Makromol. Chem. 1987, 188, 1575–1582.
- Scandola, M.; Focarete, L.; Gazzano, M.; Matuszowicz, A.; Sikorska, W.; Adamus, G.; Kurcok, P.; Kowalczuk, M.; Jedliński, Z. Crystallinity-Induced Biodegradation of Novel -b-Pivalolactone Copolymers. Macromolecules 1997, 30, 7743–7748.
- Adamus, G. Molecular Level Structure of (R,S)-3-Hydroxybutyrate/(R,S)-3-Hydroxy-4-ethoxybutyrate Copolyesters with Dissimilar Architecture. Macromolecules 2009, 42, 4547–4557.
- Adamus, G.; Sikorska, W.; Janeczek, H.; Kwiecień, M.; Sobota, M.; Kowalczuk, M. Novel block copolymers of atactic PHB with natural PHA for cardiovascular engineering: Synthesis and characterization. Eur. Polym. J. 2012, 48, 621–631.
- Kurcok, P.; Dubois, P.; Sikorska, W.; Jedliński, Z.; Jérôme, R. Macromolecular Engineering. 24. Controlled Synthesis of β-Butyrolactone-b-ε-Caprolactone Block Copolymers by Anionic and Coordination-Polymerization. Macromolecules 1997, 30, 5591–5595.
- Kawalec, M.; Kurcok, P.; Adamus, G.; Kowalczuk, M. Synthesis of Poly via anionic ROP. Macromol. Symp. 2007, 253, 59–64.
- Koseva, N.S.; Kurcok, P.; Adamus, G.; Troev, K.D.; Kowalczuk, M. Polyester-based Copolymers for Biomaterials Fabrication. Macromol. Symp. 2007, 253, 24–32.
- Koseva, N.S.; Novakov, C.P.; Rydz, J.; Kurcok, P.; Kowalczuk, M. Synthesis and Characterization of PHB-PEG Brush Copolymer through ATRP in a Macroinitiator-Macromonomer Feed System. Design. Monomers Polym. 2010, 13, 579–595.
- Neugebauer, D.; Rydz, J.; Goebel, I.; Dacko, P.; Kowalczuk, M. Synthesis of Graft Copolymers Containing Biodegradable Poly(3-hydroxybutyrate) Chains. Macromolecules 2007, 40, 1767–1773.
- Kowalczuk, M.; Adamus, G.; Jedlinski, Z. Synthesis of new graft polymers via anionic grafting of beta.-butyrolactone on poly(methyl methacrylate). Macromolecules 1994, 27, 572–575.
- Ceccurulli, G.; Scandola, M.; Adamus, G. Compatibilizing effect of a graft copolymer on bacterial poly(3-hydroxybutyrate)/poly(methyl methacrylate) blends. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1390–1399.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
30 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




