You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | palmiro poltronieri | + 3826 word(s) | 3826 | 2021-12-28 03:36:52 | | | |
| 2 | Amina Yu | Meta information modification | 3826 | 2021-12-30 02:29:49 | | | | |
| 3 | palmiro poltronieri | Meta information modification | 3826 | 2022-01-01 07:44:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Poltronieri, P. Lemongrass Essential Oil. Encyclopedia. Available online: https://encyclopedia.pub/entry/17628 (accessed on 03 January 2026).
Poltronieri P. Lemongrass Essential Oil. Encyclopedia. Available at: https://encyclopedia.pub/entry/17628. Accessed January 03, 2026.
Poltronieri, Palmiro. "Lemongrass Essential Oil" Encyclopedia, https://encyclopedia.pub/entry/17628 (accessed January 03, 2026).
Poltronieri, P. (2021, December 29). Lemongrass Essential Oil. In Encyclopedia. https://encyclopedia.pub/entry/17628
Poltronieri, Palmiro. "Lemongrass Essential Oil." Encyclopedia. Web. 29 December, 2021.
Copy Citation
Cymbopogon spp. are fast-growing C4 perennial sedges from the grass family Poaceae and are primarily cultivated for their essential oils. The genus lemongrass comprises about 180 species, such as Cymbopogon citratus, Cymbopogon flexuosus, Cymbopogon winterianus, Cymbopogon martinii, Cymbopogon nardus, and Cymbopogon refractus.
Lemongrass (Cymbopogonspp.) oil is a cocktail of various terpenes and terpenoids, out of which the major components belong to cyclic and acyclic monoterpenes. The monoterpenes are derived from geranyl diphosphate (GPP).
anticancer
antimicrobial
antioxidants
cancer signalling
citral
essential oil
1. Antimicrobial Potential
Singh et al. [1] studied 1114 strains of different microbes including moulds, yeasts, and bacteria from 29 genera and 105 species, and circled out about 425 LEO-sensitive microbial isolates. It is suggested that a low concentration of LEO inhibits microbial growth and development (bacteriostatic, fungistatic, and virustatic), while a higher concentration renders irreversible destruction leading to microbial death (bactericidal, fungicidal, and virucidal) [1][2]. Furthermore, one study addressed LEO antimicrobial potential against 42 microorganisms, including 20 bacteria, 15 fungi, and 7 yeasts [3].
In microbial studies, IC50 (half maximal inhibitory concentration) and MIC (minimum inhibitory concentration) values are two important markers that can be considered to determine the antimicrobial potential of any chemical. The IC50 of a drug is the concentration that can bring a 50% reduction in the microbial activity, therefore it may be cytostatic but bacteria can recover soon thereafter. In addition, concentrations are not always assessed precisely but are usually tested on a scalar dilution, from 1 to 10, and so on. However, it is more informative to know the MIC of a drug or plant extract, i.e., the concentration at which no visible growth of a microbe is detected. The MIC of LEO and citral against planktonic Staphylococcus aureus were noticed to be 0.0781% (v/v) and 0.0313% (v/v), respectively [2]. Similarly, LEO had higher efficacy (MIC—0.65 % v/v) against Acinetobacter baumannii strains over citral (MIC—0.14% v/v) [3].
1.1. Antibacterial Activity
The antibacterial characteristic of LEO is well established [4][5][6][7][8][9][10]. It has been suggested that LEO induces the destruction of bacterial biofilms and hinders further bacterial growth and development [11]. Furthermore, LEO components can destabilize the bonds between the lipid bilayer and neutralize the bacteria through membrane disintegration [12]. LEO can confer structural changes, as well, in different bacteria. It was reported to cause complete disfiguration and distortion in the Pseudomonas spp. [13]. The MIC values for LEO and citral against P. aeruginosa were calculated as >40% and 10%, respectively. Furthermore, LEO blocks biofilm formation in bacterial colonies [11], for example, 0.125% (v/v) of LEO can restrict biofilm formation in methicillin-resistant Staphylococcus aureus strains [14]. It can disrupt the cell membrane and inhibit cytoplasmic metabolism, making LEO effective against both Gram-negative and Gram-positive bacteria [15][6][12]. Cymbopogon khasianus essential oil inhibited the growth of Escherichia coli with the MIC and MBC (Minimum Bactericidal Concentration) values of 20 μg/mL each. It can also retard the growth of Bacillus subtilis, Salmonella enterica typhimurium, Staphylococcus aureus, Klebsiella pneumoniae with a MIC range of 25–50 μg/mL [16]. Multiple recent studies against MDR (multidrug-resistant) bacteria [17][18] show that, while a low concentration of LEO retards growth and biofilm formation, a higher concentration can confer complete elimination of Salmonella Heidelberg.
The bacteriostatic and bactericidal characteristics of LEO primarily depend on the bacteria and oil concentration [19][20]. However, several other factors, such as oil composition, extraction method, plant developmental stage, and environmental variables including temperature can influence the oil’s effectiveness. Therefore, lemongrass oils from different species might exhibit effects of different nature and intensity. Nevertheless, the host organism can also decide oil effectiveness to a certain extent depending on its morpho-physiological attributes [21]. Therefore, EOs react differently with Gram-positive and Gram-negative bacteria, owing to their dissimilar cell-wall structures [19][22]. Costa et al. [23] examined Cymbopogon flexuosus EO (μL mL−1) against Listeria monocytogenes, Staphylococcus aureus, and Salmonella typhimurium and determined their MICs and MBCs as of 3.9 μL mL−1 each. The MICs of citral against Cronobacter sakazakii strains ranged from 0.27 to 0.54 mg/mL. Scanning electron microscopy analysis further confirmed that C. sakazakii cell membranes were damaged by citral [24].
1.2. Antifungal Activity
The antifungal activity of LEO has been reported against multiple fungi [25][26][27][28]. Volatiles from lemongrass oil, such as phenols, flavonoids, and flavones, are effective against numerous fungal strains [29][30][31]. Helal et al. [32] reported that LEO caused plasma membrane disruption and disorganization of mitochondria and resulted in Ca2+, K+, and Mg2+ leakage. The loss of ions can further affect signal transduction and fungal germination. Moreover, Alviano et al. [33] observed that LEO components induce cell size reduction and inhibit the spore germination in Candida albicans. LEO can directly act upon the fungal lipid bilayer owing to its readily volatile and lipophilic nature. It can form a charge–transfer complex with the lipid bilayer, destabilizing the membrane and inhibiting further membrane synthesis, and retards fungal spore formation and cellular respiration [33][34]. Boukhatem et al. [3] found that the vapor form of LEO inhibits mycotic growth and development more effectively than the liquid phase, probably because of the direct accumulation of LEO vapors on fungal mycelium.
LEO components including citral, geraniol, myrcene, limonene, and linalool, have significant antifungal activity [35][25][5][16][26][32][33][34][36][37][38]. Geraniol increases the outward leakage rate of potassium ions, while citral damages the microtubules and exhibits cytotoxicity in fungi [39]. Linalool, a monoterpene alcohol, comprises numerous fungicidal properties [40]. It retards the overall development and propagation of different fungi through the respiratory restriction of their aerial mycelia [41]. Additionally, other aldehydes of LEO can confer antimycotic activity through cross-linkage reaction within the fungal membrane [41].
EOs can remain effective for a longer duration against fungal spore production, ensuring improved shelf life for food products [33][34][38]. It was also suggested that lemongrass oil induces reactive oxygen species (ROS) production in fungi and afflicts severe oxidative damage that leads to subsequent cellular death [42]. This can also enable EOs as a sustainable alternative in the food preservation and packaging industries [43][44][45]. Edible coating of EOs, including LEO on stored fruits, meat, and dairy products, discourages fungal attack and food spoilage through restricting fungal growth and reproduction [40][43][44][45][46]. The edible coatings of EOs have increased antimicrobial potential over the free EOs due to their altered surface charge and amplified action on multiple target sites in the mycotic membrane [47]. This extends edibility and maintains physicochemical qualities, including the tastes and odours of such products [36][48]. However, different EOs have different antimicrobial mechanisms, and thus acquisition of resistance by microbes against the wide array of compounds in EOs is rare [49].
1.3. Antiviral Activity
In addition to its fungal and bacterial efficacy, LEO is also effective against numerous viruses. The antiviral activity of lemongrass oil was tested against herpes simplex virus-I (HSV-I) [50] and murine norovirus (MNV) [51]. The studies demonstrated that 0.1% and 2% of LEO concentration were sufficiently potent to inhibit the replication of HSV-I and MNV, respectively. Furthermore, LEO can weaken the HIV transcription and virus reactivation by interfering with the Tat/TAR-RNA complex. As the Tat protein enhances the efficiency of viral transcription, LEO’s interference with the Tat/TAR-RNA complex results in the downregulation of HIV activity [52]. Lemongrass volatiles were also able to cause more than 50% inhibition of the tobacco mosaic virus at a 100 µg/mL concentration [1]. Human mastadenovirus (HAdV) causes numerous ailments, such as respiratory infection, gastroenteritis, hepatitis, meningoencephalitis, pneumonia, and multiple others. Lemongrass extracts also induced cytotoxicity in a human lung adenocarcinoma cell line and monkey kidney cell lines and showed antiviral activity against HAdV [53]. In the recent pandemic upsurge, the efficacy of LEO was suggested against influenza and coronaviruses SARS-CoV-2, as well, enhancing the relevance and importance of lemongrass oil even more [54][53].
2. Antioxidant-Related Effects
Plant and animal cells produce various oxidative compounds, such as H2O2, O2−, and OH−, which can damage lipids, proteins, and DNA and can induce several health complications including cancer, aging, and neurological disorders in humans [55]. However, another group of compounds, known as antioxidants, has the potential to counter these effects [56][57]. Lemongrass possesses antioxidants that render protective measures against reactive species [58][59]. Lemongrass extracts have been reported to reduce reactive species concentration, lipid peroxidation, and decolourisation of 2,2-diphenyl-1-picrylhydrazyl [60][61]. Lemongrass extract can also buttress the endogenous antioxidant defence system in alveolar macrophages cells through augmenting superoxide dismutase activity and glutathione formation [61][62]. A vast array of plant extracts has been studied for their beneficial antioxidant properties. In particular, plant antioxidant potential may protect cells and organs from radical oxygen species. The natural extracts are usually compared to controls, such as butylated hydroxy anisol (BHA) or buthylhydrotoluene (BHT), for their antioxidant profile. Radical scavenging activity is evaluated by their activity towards a stable free radical, 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS). To date, there are no published reports on the antioxidant power of LEO. These data could be useful in comparing different cultivars or different methods of cultivation. Plant extracts have been studied, on cultured cells, for their antioxidant properties, their enzymes, and their redox potentials. In particular, regulation of redox status in cells may affect the levels of methyl group donor S-adenosylmethionine (SAM). SAM is a cofactor for histone methyltransferases and DNA methyltransferases. The consumption of glutathione, as occurs during oxidative stress, with an increase of its oxidized form, glutathione disulfide, may inhibit S-adenosylmethionine (SAM) synthetase, with a reduction of SAM synthesis [63] influencing the epigenetic modifications of proteins and DNA. Citral was shown to increase intracellular oxygen radicals, while inhibition of glutathione synthesis increased citral’s anticancer effect [63]. Citral was shown to modulate oxidative stress preferentially in cancer cells and to induce the endoplasmic reticulum stress exerting thus an antiproliferative action [64]. Lemongrass has a competitive advantage over other synthetic antioxidants, such as butylated hydroxytoluene, since they can induce haemorrhage: in this view, lemongrass oil is regarded as ‘safe’ for human consumption [64]. This opens a new vista for lemongrass oil in the food preservation and safety industries, including the meat, and dairy industries [41]. In the food industry, the oxidation of lipids is an important determinant of meat and dairy products. However, their highly rich nutritional profiles are prone to lipid peroxidation and quality deterioration. In this regard, coating such products with LEO minimizes lipid peroxidation and increases their shelf life and quality [65]. Furthermore, the antioxidant nature of citral is exploited in animal skin cancer models [66][67]. Soares et al. [68] reported that LEO, which was characterised in its major components, showed high antioxidant activity compared to the methanolic and aqueous extracts of lemongrass. In detail, they showed higher antioxidant capacity of LEO compared to the aqueous extracts of leaves: Using the 2,2-diphenyl-1-picrylhydrazyl free radical as a control, they obtained similar antioxidant power, at levels of 41 μg/mL. The antioxidant activity of LEO can further be enhanced through mixing it with other potent antioxidative agents. On this note, a mixture of LEO with Ocimum gratissimum, and Thymus vulgaris oil had enhanced effects against Bipolaris oryzae and Alternaria alternata [68][69]. A review on the antioxidant, antimicrobial and antifungal properties of LEO and recent updates on the possible applicative uses has been recently published [70].
3. Anticancer Activity
According to the World Health Organization (WHO), cancer caused an approximated 10 million deaths, or one in six deaths, in 2020. This situation is not going to be relieved, as there is estimated to be an increase of 45% in cancer mortality rate between 2008–2030. Among cancers, the most common types are breast cancer, lung cancer, colorectal cancer, prostate cancer, skin, and stomach cancer. The ongoing conventional chemotherapies, radiotherapy treatment, and surgeries have shown a large number of involuntary side effects due to insufficient knowledge of treatment specificity, and are not recommended for long-term usage [71][72].
Medicinal plants emerge as potential candidates in the cancer world and raise hopes for the scientific community. Scientists are constantly looking for natural sources to uncover the potential plant-based therapeutic agents having immense anticancer properties [73]. In this sense, the essential oil of lemongrass counts among such plants for its cytotoxicity on human cancer cells. Its active ingredients, including geraniol, geranyl acetate, α-bisabolol, and iso-intermedeol have individually been found to impart cytotoxic effects on cancer cells [74]. Lemongrass EO has exhibited inhibition of human mouth epidermal carcinoma (KB) and murine leukemia cell lines (P388) [75].
Among LEO terpenes, citral, the major component of lemongrass oil, plays a potential role as antiproliferative against several types of cancer cells, such as the two human prostate cancer cell lines, LNCaP and PC-3 [74], HL60, U937 ovarian cancer cells [75][76][77], cervical cancer cell lines [78], and the breast cancer cell line, MCF-7 [79]. Interestingly, citral does not exert cytotoxicity to normal epithelial cells but exhibits toxic effects against human breast cancer cell lines, confirming its cancer-specific efficacy [80].
Although a large number of studies evinced the anticancer activity of lemongrass, scarce data are available on its mode of action. Studies based on different cancer cell types substantiated citral efficacy via activated procaspase-3, induction of apoptosis, and cell cycle arrest in the G2/M phase [81][82]. Citral consists of a double bond in conjugation with an aldehyde (α, β-unsaturated) group in its core structure, which serves as a potent caspase 3 activator, responsible for pro-apoptotic activity [81]. Moreover, citral-induced apoptotic activity was associated with DNA fragmentation and induced caspase 3 activity against hematopoietic cancer cell lines and ovarian cancer cell lines.
The Src-tyrosine kinase is expressed in small cell lung cancer and can phosphorylate transcription factor Stat3(Y705) [81], which sequentially enhances the expression of downstream genes engaged in the antiapoptotic activity, i.e., Bcl-xL and Mcl-1 [83]. An experimental study showed the inhibitory effects of LEO and citral on phosphorylation of Src(Y416) blocking its activation, resulting in reduced phosphorylation of Stat3 (Y705). Non-phosphorylated Stat3 disrupts cell growth and the signal pathways that upregulate the expression of Bcl-xL and Mcl-1 [84]. Citral-dependent apoptosis induction has also been observed against prostate cancer cell lines. Citral induced gene activation initiates AMPK (an enzyme necessary in the fatty acid metabolism) phosphorylation resulting in the activation of BAX and the downregulation of Bcl-2, which initiates an apoptosis cascade in prostate cancer cell lines [85]. Citral-mediated breast tumour growth inhibition via the inhibition of ALDH1A3 was reported [86]. The up-regulation of retinoic acid (RA) signalling by ALDH1A3 can cause breast cancer growth, and citral inhibited the expression of RA-inducible genes mediated by ALDH1A3 [86]. Microtubule affinity regulating kinase 4 (MARK4), an AMP-activated protein kinase [87], is reported to mediate apoptosis, inflammation, and distinct regulatory pathways [88]. Alterations in MARK4 expression hamper the cell cycle and eventually cause cancer. Citral potentially binds to MARK4 and inhibits its kinase activity, and is being considered an effective strategy to prevent the growth of cancer cells and other MARK4 associated diseases [89]. Citral has been reported inducing the phosphorylation of p53 protein and the expression of Bax, while reducing the expression of the antiapoptotic factors Bc-2 and Bcl-xL in human colorectal cancer lines, i.e., HT116 and HT29 [90]. Citral interferes with the ERK1/2 pathway and reduces the translocation of ERK1/2 protein to the nucleus. There is a certain possibility of the involvement of ERK1/2 in melanoma carcinogenesis and the progression in presence of mutated N-Ras and B-Raf. Therefore, citral could negatively affect cancer growth by inhibiting the final step of the MAPK cascade [91].
Geraniol, the second major constituent of LEO, has garnered heed for its potentiality in cancer treatment. It has been reported that geraniol induces the production of ROS and inhibits the phosphorylation of tyrosine kinases, which, in turn, induce apoptosis of cancer cells [92]. Several studies have been conducted to gain insights into its anticancer activity [93][94][95]. Ornithine decarboxylase (ODC) plays a prime role in the synthesis of polyamines, providing stabilization to DNA structure [96]. A decrease in ODC activity after geraniol treatment has been observed in the intestinal adenocarcinoma Caco-2 cell line, which, in turn, caused DNA synthesis inhibition and cell cycle arrest in the S phase [96]. Polyamine metabolism is a potential target in the development of cancer-preventive drugs, therefore, geraniol mediated decline in ODC activity might have a useful clinical role [97]. Geraniol-induced inhibition of the proliferation of A453 and A549 human lung cancer cell lines has been reported. Geraniol alters the tubulin polymerization and disrupts the active property of both the studied cell lines, resulting in cell apoptosis. Geraniol arrested the G0/G1 phase in A431 cells, with no effects on sub-diploid cells, and the G2/M phase of A549 with the increased population of sub-diploid cells, in a dose-dependent manner. The inhibitory effects of geraniol might be interrelated with the observed alteration in the ODC activity [98]. Geraniol caused inhibition of cell cycle progression, exerting altered expression of cyclins D1, A, B1, CDK2, and the cyclin kinase inhibitor proteins p21 and p27 [99]. Geraniol has been reported to induce the expression of pro-apoptotic proteins Bcl-2, Bax, Bak, and caspase3/8/9 in several human cancer cell lines [95][100]. Moreover, the considerable increase in these proteins indicates that geraniol induces apoptosis through the mitochondrial intrinsic pathway [101]. The antiangiogenic activity of geraniol has been confirmed by both in-vivo and in-vitro studies. Geraniol suppresses the endothelioma cell line and reduces Ki67-positive cells and CD3-microvessela by suppressing the expression of VEGFR-2 in Balb/c mice [102]. This activity might play a role in reducing tumour growth, as the tumour needs a new blood vessel to grow. Geraniol arrested the proliferation of two pancreatic cancer cell lines, MIA PaCa-2 and BxPC, in hamsters when injected with PC-1 pancreatic ductal adenocarcinoma cells. In both the cell lines, it arrested the G1 phase of the cell cycle along with increasing the expression of the cyclin kinase inhibitor proteins p21cip1 and p27kip1 while suppressing those of cyclin A, B1, and CDK2 [103][104]. Figure 1 indicates a series of distinctive signalling pathways activated in cancer cells via different components of LEO.
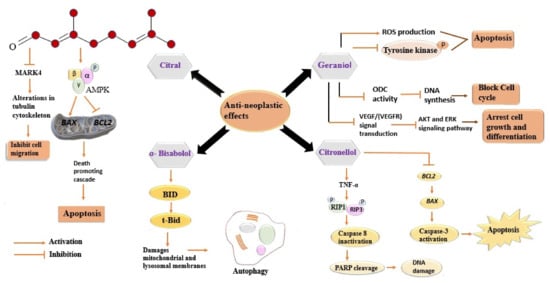
Figure 1. Distinctive signalling pathways activated in cancer cells via different components of LEO. Every component acts differently against cancer cells and involves diverse signalling pathways. All the involved pathways lead to inhibition of cell migration, cell cycle, and DNA synthesis. All these events eventually cause cell death (apoptosis). MARK4, Microtubule affinity-regulating kinase 4; AMPK, 5′ adenosine monophosphate-activated protein kinase; BAX, BCl2- associated X protein; BCL2, B-cell lymphoma 2; BID, BH3-only activator protein; tBid, truncated Bid, ROS, reactive oxygen species; ODC, ornithine decarboxylase; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; AKT, Ak strain transforming; ERK, extracellular regulated kinase; TNF-α, tumour necrosis factor; RIP1, receptor-interacting serine-threonine protein kinase 1; RIP3, receptor-interacting serine-threonine protein kinase 3; PARP, poly ADP ribose polymerase; DNA, deoxyribonucleic acid.
D-limonene, another constituent of LEO, was also reported to possess antineoplastic activity. D-limonene enhances the activity of carcinogen metabolizing enzymes, such as cytochrome P450, responsible for the conversion of carcinogens into less harmful forms and blocks their interaction with DNA [105]. Treatment of D-limonene on LS174T human colon cancer cells inhibited the P13K/Akt pathway and induced cell apoptosis. An increase in PARP cleavage and activation of caspase-3 indicates the involvement of the mitochondrial apoptotic pathway [106]. Limonene-mediated induction of apoptosis via increased expression of Bax and caspase-3 and decreased Bcl-2 expression has been reported in T24 bladder cancer cells. Moreover, limonene arrested the cell cycle in the G2/M phase and wound healing and transwell assay using Matrigel has confirmed the limonene mediated suppression of cancer cell migration and invasion [107]. Another component of the EO of lemongrass, citronellol, is also found to exert cytotoxic effects on several cancer cell lines [108][109][110]. Citronellol showed its anticancer activity via increased reactive oxygen species production, alterations in mitochondrial permeability, DNA fragmentation, changes in cytochrome c activities, and activation of caspase, against the MCF-7 human mammary tumour cell line [111]. The cytotoxicity of α-bisabolol has been reported against human and rat malignant glioma cancer cell lines. In α-bisabolol-treated cell lines, the rapid loss of inner transmembrane potential and an increase in cytochrome-c translocation indicate that α-bisabolol can trigger apoptosis through mitochondrial intrinsic pathway [112]. Another experimental study has confirmed the cytotoxic effect of α-bisabolol against several cancer cell lines. α-bisabolol arrested cell cycle and initiated cancer cell death via a BID (BH3-only activator protein)-dependent mechanism [113]. It induces the permeability of the outer mitochondrial membrane and plays a crucial role during apoptosis [114]. α-bisabolol-induced damage to lysosomal and mitochondrial membranes via BID resulted in autophagy and regulated cell death, enlightening its modes of action [113][114][115][116].
All of the mentioned components of LEO have presented their chemo-preventative effects via arrest of different phases of the cell cycle, suppression of cyclins and cyclin-dependent kinases, DNA fragmentation, and antiangiogenic activity, against different cancer cell lines. Several distinct signalling pathways and mechanisms of action have been reported in experimental studies exhibiting the anticancer activities of these mentioned component.
Epigenetic modifications play a key role in cancer proliferation. Several chromatin remodelling complex components are found mutated, silenced, or overexpressed in cancers. Epigenetic mechanisms may be taken into account to explain the regulation of various protein-coding and protein non-coding genes. One type of regulation of gene transcription is based on the level of DNA methylation on promoters, controlled by DNA methyltransferases. A second mechanism is dependent on chromatin accessibility mediated by remodelling complexes, recruitment of Polycomb repressing complexes, and histone-modifying enzymes [63]. An involvement of non-coding RNAs (nc-RNAs) is at the basis of these mechanisms; therefore, the expression of long and small non-coding RNAs determines whether an antioncogenic pathway is repressed or downregulated, or oncogenes are set free to induce cell transformation. Therefore, chromatin accessibility (opening or compaction), access to transcription machinery, and promoter methylation are the principal mechanisms that are targeted by plant extracts, essential oils, and individual bioactives. Various classes of bioactives were shown to exert anticancer effects through the downregulation of microRNAs, most often oncomiRs, and the release of mRNAs coding for antiproliferative proteins. The review by Sabo [117] and that by Cherng [118] showed the relationship between natural compounds and nc-RNAs in cancer cells, and the potential use of their bioactives in cancer therapies. Xu and colleagues described, for the first time, the involvement of pinene, one component of LEO, in the upregulation of p27/CDKN1B, a cell cycle-blocking protein, through the downregulation of miR-221 [118]. The most well-known group of nc-RNAs are microRNAs [119]. When they are abundant, they silence mRNA transcription by sequestering them and destining them to degradation. Long non-coding RNAs, such as competing endogenous RNAs (ceRNAs), may sponge a group of miRNAs and the relative abundance thereof determines whether the miRNA can exert its effects or is bound to the sponge [119]. This has been clearly reviewed for stilbenes [120] and other plant bioactives [120][121].
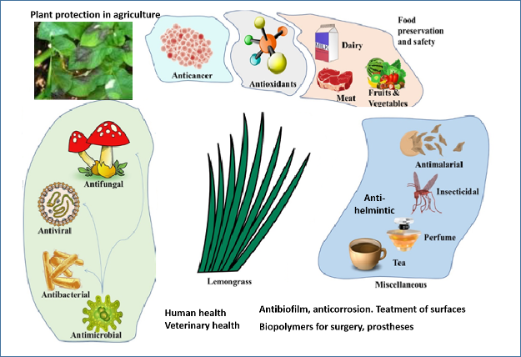
In conclusion, the bioactive LEO phytocomponents are useful for a myriad of medicinal properties including anti-microbial, anticancer, antioxidant, insecticidal, and antimalarial activities (Figure 2). Although LEO and its constituents have shown anticancer activity in vitro, and, in some cases, in animal studies, only a few researchers to date have tested the delivery of its bioactive components combined with nanoparticles or delivery systems. a variety of applications, from food safety and food preservation, in terms of antioxidant potential as well as for antifungal properties, to applications in agriculture and veterinary medicine, and as coatings on biopolymers for surgery (maxillofacial silicone specimens in dentistry, other medical implants) have been recently proposed. The ability of LEO terpenes to stop bacteria and fungi from growing in biofilms has also a wide array of applicative uses in medicine and surgical devices, and in industrial solutions to biocorrosion, biofouling, biodegradation, water microbiology, and the control of bacterial quorum sensing signals [103]. The ability to modify the materials used in medical devices allows the application of LEO components to make such surfaces resistant to biofilm formation.
References
- Singh, B.R.; Singh, R.K. Antimicrobial activity of lemongrass (Cymbopogon citratus) oil against microbes of environmental, clinical and food origin. Int. Res. J. Pharm. Pharmacol. 2011, 1, 228–236.
- Lu, M.; Han, Z.; Xu, Y.; Yao, L. In Vitro and In Vivo Anti-Tobacco Mosaic Virus Activities of Essential Oils and Individual Compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778.
- Negrelle, R.R.B.; Gomes, E.C. Cymbopogon citratus (DC.) Stapf: Chemical composition and biological activities. Rev. Bras. Pl. Med. 2007, 9, 80–92.
- Meena, S.; Kumar, S.R.; Rao, D.K.V.; Dwivedi, V.; Shilpashree, H.B.; Rastogi, S.; Shasany, A.K.; Nagegowda, D.A. De Novo sequencing and analysis of lemongrass transcriptome provide first insights into the essential oil biosynthesis of aromatic grasses. Front. Plant Sci. 2016, 7, 1129.
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829.
- Vazirian, M.; Kashani, S.T.; Ardekani, M.R.S.; Khanavi, M.; Jamalifar, H.; Fazeli, M.R.; Toosi, A.N. Antimicrobial activity of lemongrass (Cymbopogon citratus (DC) Stapf.) essential oil against food-borne pathogens added to cream-filled cakes and pastries. J. Essent. Oil Res. 2012, 24, 579–582.
- Cai, Z.; Remadevi, R.; Al Faruque, M.; Setty, M.; Fan, L.; Haque, A.N.M.A.; Naebe, M. Fabrication of a cost-effective lemongrass (Cymbopogon citratus) membrane with antibacterial activity for dye removal. RSC Adv. 2019, 9, 34076–34085.
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martin-Belloso, O. Antimicrobial Activity of Essential Oils on Salmonella Enteritidis, Escherichia coli, and Listeria innocua in Fruit Juices. J. Food Prot. 2006.
- Balakrishnan, B.; Paramasivam, S.; Arulkumar, A. Evaluation of the lemongrass plant (Cymbopogon citratus) extracted in different solvents for antioxidant and antibacterial activity against human pathogens. Asian Pac. J. Trop. Dis. 2014, 4.
- Mishra, D.; Khare, P.; Singh, D.K.; Luqman, S.; Kumar, P.V.A.; Yadav, A.; Das, T.; Saikia, B.K. Retention of antibacterial and antioxidant properties of lemongrass oil loaded on cellulose nanofibre-poly ethylene glycol composite. Ind. Crop. Prod. 2018, 114, 68–80.
- Moore-Neibel, K.; Gerber, C.; Patel, J.; Friedman, M.; Ravishankar, S. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. J. Appl. Microbiol. 2012, 112, 485–492.
- Kotzekidou, P.; Giannakidis, P.; Boulamatsis, A. Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and on the fate of inoculated pathogens in chocolate. LWT Food Sci. Technol. 2008, 41, 119–127.
- Aiemsaard, J.; Aiumlamai, S.; Aromdee, C.; Taweechaisupapong, S.; Khunkitti, W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011, 91, 31–37.
- Adukwu, E.C.; Allen, S.C.; Phillips, C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (C itrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012, 113, 1217–1227.
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538.
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755.
- Takaisi-Kikuni, N.; Krüger, D.; Gnann, W.; Wecke, J. Microcalorimetric and electron microscopic investigation on the effects of essential oil from Cymbopogon densiflorus on Staphylococcus aureus. Microbios. 1996, 88, 55–62.
- Peichel, C.; Nair, D.V.T.; Dewi, G.; Donoghue, A.M.; Reed, K.M.; Johny, A.K. Effect of lemongrass (Cymbopogon citratus) essential oil on the survival of multidrug-resistant Salmonella enterica serovar Heidelberg in contaminated poultry drinking water. J. Appl. Poult. Res. 2019, 28, 1121–1130.
- Dewi, G.; Nair, D.V.T.; Peichel, C.; Johnson, T.J.; Noll, S.; Johny, A.K. Effect of lemongrass essential oil against multidrug-resistant Salmonella Heidelberg and its attachment to chicken skin and meat. Poult. Sci. 2021, 101116.
- Hassoun, A.; Emir Çoban, Ö. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36.
- Swamy, M.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462.
- Devi, M.A.; Sahoo, D.; Singh, T.B.; Rajashekar, Y. Toxicity, repellency and chemical composition of essential oils from Cymbopogon species against red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J. fur Verbraucherschutz Leb. 2020, 15, 181–191.
- Costa, K.A.D.; Moura, R.; Millezi, A.F. Antimicrobial and antibiofilm activity of Cymbopogon flexuosus essential oil microemulsions. Rev. Ceres 2019, 66, 372–379.
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006.
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2020, 104620.
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480.
- Rkin, R.; Korukluoglu, M. Effectiveness of Cymbopogon citratus l. essential oil to inhibit the growth of some filamentous fungi and yeasts. J. Med. Food. 2009, 12, 193–197.
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258.
- Dong, L.M.; Thuy, D.T.K. Evaluation of the synergistic effect of ethanol and lemongrass oil against Aspergillus niger. J. Microbiol. Biotech. Food Sci. 2021, 1312–1316.
- Abd-El Fattah, S.M.; Yahia Hassan, A.; Bayoum, H.M.; Eissa, H.A. The use of lemongrass extracts as antimicrobial and food additive potential in yoghurt. J. Am. Sci. 2010, 6, 582–594.
- Júnior, R.C.; Capucho, E.; Garcia, T.M.; Del Valle, T.A.; Campana, M.; Zilio, E.M.C.; Azevedo, E.B.; Morais, J.P.G. Lemongrass essential oil in sugarcane silage: Fermentative profile, losses, chemical composition, and aerobic stability. Anim. Feed Sci. Tech. 2020, 260, 114371.
- Helal, G.A.; Sarhan, M.M.; Shahla, A.N.K.A.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J. Basic Microbiol. 2007, 47, 5–15.
- Alviano, W.S.; Mendonça-Filho, R.R.; Alviano, D.S.; Bizzo, H.R.; Souto-Padrón, T.; Rodrigues, M.L.; Bolognese, A.M.; Alviano, C.S.; Souza, M.M.G. Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol. Immunol. 2005, 20, 101–105.
- da Silva, C.d.B.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E.S. Antifungal activity of the lemongrass oil and citral against Candida spp. Brazilian J. Infect. Dis. 2008, 12, 63–66.
- Li, M.; Liu, B.; Bernigaud, C.; Fischer, K.; Guillot, J.; Fang, F. Lemongrass (Cymbopogon citratus) Oil: A promising miticidal and ovicidal agent against Sarcoptes scabiei. PLoS Negl. Trop. Dis. 2020, 14, e0008225.
- Tchinda, E.S.; Jazet, P.M.D.; Tatsadjieu, L.N.; Ndongson, B.D.; Amvam, P.H.Z.; Menut, C. Antifungal Activity of the Essential Oil of Cymbopogon citratus (Poaceae) Against Phaeoramularia angolensis. J. Essent. Oil Bear. Plants 2009, 12, 218–224.
- Mishra, A.K.; Dubey, N.K. Evaluation of some essential oils for their toxicity against fungi causing deterioration of stored food commodities. Appl. Environ. Microbiol. 1994, 60, 1101–1105.
- Masniyom, P.; Benjama, O.; Maneesri, J. Effect of turmeric and lemongrass essential oils and their mixture on quality changes of refrigerated green mussel (Perna viridis). Int. J. Food Sci. Technol. 2012, 47, 1079–1085.
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337.
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108.
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Tayebi, K. The food preservative potential of essential oils: Is lemongrass the answer? J. Fur Verbrauch. Leb. 2014, 9, 13–21.
- Lee, J.E.; Seo, S.M.; Huh, M.J.; Lee, S.C.; Park, I.K. Reactive oxygen species mediated-antifungal activity of cinnamon bark (Cinnamomum verum) and lemongrass (Cymbopogon citratus) essential oils and their constituents against two phytopathogenic fungi. Pestic. Biochem. Phys. 2020, 168, 104644.
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Song, K.B.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT 2017, 75, 742–750.
- Muhammad, I.; Riffat, T.; Asif, J.; Atif, J.; Raja, M.U.; Anjum, M. Lemongrass essential oil as an alternate approach to manage seed associated fungi of wheat and rice. Int. J. Agric. Biol. 2017, 19, 1301–1306.
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martin-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12.
- Frazão, G.G.S.; Blank, A.F.; de Aquino Santana, L.C.L. Optimisation of edible chitosan coatings formulations incorporating Myrcia ovata Cambessedes essential oil with antimicrobial potential against foodborne bacteria and natural microflora of mangaba fruits. LWT Food Sci.Technol. 2017, 79, 1–10.
- Silveira, S.M.D.; Júnior, A.C.; Scheuerman, G.N.; Secchi, F.L.; Vieira, C.R.W. Chemical composition and antimicrobial activity of essential oils from selected herbs cultivated in the South of Brazil against food spoilage and foodborne pathogens. Cienc. Rural. 2012, 42, 1300–1306.
- Belewu, M.; Ahmed El-Imam, A.M.; Adeyemi, K.; Belewu, M.A.; Ahmed El-Imam, A.M.; Adeyemi, K.D.; Oladunjoye, S.A. Eucalyptus Oil and Lemon Grass Oil: Effect on Chemical Composition and Shelf-Life of Soft Cheese. Environ. Nat. Resour. Res. 2012, 2, 114–118.
- Yousuf, B.; Srivastava, A.K. Flaxseed gum in combination with lemongrass essential oil as an effective edible coating for ready-to-eat pomegranate arils. Int. J. Biol. Macromol. 2017, 104, 1030–1038.
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206.
- Kim, Y.; Khan, A.L.; Waqas, M.; Lee, I.; Lee, I. Silicon Regulates Antioxidant Activities of Crop Plants under Abiotic-Induced Oxidative Stress: A Review. Front. Plant Sci. 2017, 8, 1–7.
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, e1700436.
- Chiamenti, L.; Silva, F.P.D.; Schallemberger, K.; Demoliner, M.; Rigotto, C.; Fleck, J.D. Cytotoxicity and antiviral activity evaluation of Cymbopogon spp hydroethanolic extracts. Braz. J. Pharm. Sci. 2019, 55, e18063.
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684.
- Olorunnisola, S.K.; Asiyanbi, H.T.; Hammed, A.M.; Simsek, S. Biological properties of lemongrass: An overview. Int. Food Res. J. 2014, 21, 455–462.
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161.
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300.
- Anggraeni, N.I.; Hidayat, I.W.; Saadah, D.; Rachman, E. Bioactivity of essential oil from lemongrass (Cymbopogon citratus Stapf) as antioxidant agent. AIP Conf. Proc. 2018, 1927, 30007.
- Karpagam, G.N.; Gayathri, R.; Vishnupriya, V. Bioactivity analysis of lemongrass oil. Asian J. Res. Chem. 2016, 9, 903.
- Selim, S.A. Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas Y Aceites 2011, 62, 55–61.
- Mirghani, M.E.S.; Liyana, Y.; Parveen, J. Bioactivity analysis of lemongrass (Cymbopogan citratus) essential oil. Int. Food Res. J. 2012, 19, 569–575.
- Tiwari, M.; Dwivedi, U.N.; Kakkar, P. Suppression of oxidative stress and pro-inflammatory mediators by Cymbopogon citratus D. Stapf extract in lipopolysaccharide stimulated murine alveolar macrophages. Food Chem. Toxicol. 2010, 48, 2913–2919.
- Farooqi, A.A.; Fayyaz, S.; Poltronieri, P.; Calin, G.; Mallardo, M. Epigenetic deregulation in cancer: Enzyme players and non-coding RNAs. Semin. Cancer Biol. 30 July epub ahead of print. 2020.
- Kapur, A.; Felder, M.; Fass, L.; Kaur, J.; Czarnecki, A.; Rathi, K.; Zeng, S.; Osowski, K.; Howell, C.; Xiong, M.; et al. Modulation of oxidative stress and subsequent induction of apoptosis and endoplasmic reticulum stress allows citral to decrease cancer cell proliferation. Sci. Rep. 2016, 6, 1–14.
- Ndhlala, A.R.; Moyo, M.; van Staden, J. Natural Antioxidants: Fascinating or Mythical Biomolecules? Molecules 2010, 15, 6905–6930.
- Hartatie, E.S.; Prihartini, I.; Widodo, W.; Wahyudi, A. Bioactive Compounds of Lemongrass (Cymbopogon citratus) essential oil from different parts of the plant and distillation methods as natural antioxidant in broiler meat. A. IOP Conf. Ser. Mater. Sci. Eng. 2019, 532, 012018.
- Hedges, L.J.; Lister, C.E. Nutritional Attributes of Herbs. Crop & Food Research Confidential Report No. 1891; New Zealand Institute for Crop & Food Research Limited: Christchruch, New Zealand, 2007; pp. 1–85.
- Soares, M.O.; Vinha, A.F.; Barreira, S.V.P.; Coutinho, F.; Aires-Gonçalves, S.; Oliveira, M.B.P.P.; Pires, P.C.; Castro, A. Evaluation of Antioxidant and Antimicrobial Properties of the Angolan Cymbopogon Citratus Essential Oil With a View to Its Utilization as Food Biopreservative. J. Agric. Sci. 2013, 5, 36–45.
- Nguefack, J.; Nguikwie, S.K.; Fotio, D.; Dongmo, B.; Zollo, P.H.A.; Leth, V.; Nkengfack, A.E.; Poll, L. Fungicidal potential of essential oils and fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris to control Alternaria padwickii and Bipolaris oryzae, two seed-borne fungi of rice (Oryza sativa L.). J. Essent. Oil Res. 2007, 19, 581–587.
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86.
- Nguyen, C.V. Anticancer Activity of Natural Health Products (Dandelion Root, Lemongrass, and Hibiscus Extracts); A Study of Efficacy, Interaction, and Mechanism of Action. Master Thesis, Windsor University, Ontario, CA, USA, 2019.
- Parveen, A.; Akash, M.S.H.; Rehman, K.; Kyun, W.W. Anticancer activities of medicinal plants: Modulation of p53 expression and induction of apoptosis. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 257–271.
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. Biol. Interact. 2009, 179, 160–168.
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120.
- Bailly, C. Targets and pathways involved in the antitumor activity of citral and its stereo-isomers. Eur. J. Pharmacol. 2020, 871, 172945.
- Liu, Y.; Whelan, R.J.; Pattnaik, B.R.; Ludwig, K.; Subudhi, E.; Rowland, H.; Claussen, N.; Zucker, N.; Uppal, S.; Kushner, D.M.; et al. Terpenoids from Zingiber officinale (Ginger) Induce Apoptosis in Endometrial Cancer Cells through the Activation of p53. PLoS ONE 2012, 7, e53178.
- Idrees, M.; Hakkim, F.L.; Naikoo, G.A.; Hassan, I.U. Recent advances in extraction, characterization, and potential use of citral. In Natural Bio-active Compounds; Aktar, M.S., Swami, M.K., Eds.; Springer Nature: Switzerland, 2019; Volume 3.
- Halabi, M.; Sheikh, B. Anti-proliferative effect and phytochemical analysis of Cymbopogon citratus extract. Biomed. Res. Int. 2014, 2014, 906239.
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical Composition and in Vitro Cytotoxic Screening of Sixteen Commercial Essential Oils on Five Cancer Cell Lines. Chem. Biodivers. 2020, 17, e1900478.
- Patel, P.B.; Thakkar, V.R.; Patel, J.S. Cellular effect of curcumin and citral combination on breast cancer cells: Induction of apoptosis and cell cycle arrest. J. Breast Cancer 2015, 18, 225–234.
- Dudai, N.; Weinstein, Y.; Krup, M.; Rabinski, T.; Ofir, R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005, 71, 484–488.
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Quang, T.N.; Dat, N.T. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. Biomed. Res. Int. 2020, 2020, 5924856.
- Harada, D.; Takigawa, N.; Kiura, K. The Role of STAT3 in Non-Small Cell Lung Cancer. Cancers 2014, 6, 708–722.
- Frank, D.A. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007, 251, 199–210.
- Maruoka, T.; Kitanaka, A.; Kubota, Y.; Yamaoka, G.; Kameda, T.; Imataki, O.; Dobashi, H.; Bandoh, S.; Kadowaki, N.; Tanaka, T. Lemongrass essential oil and citral inhibit Src/Stat3 activity and suppress the proliferation/survival of small-cell lung cancer cells, alone or in combination with chemotherapeutic agents. Int. J. Oncol. 2018, 52, 1738–1748.
- Balusamy, S.R.; Perumalsamy, H.; Veerappan, K.; Huq, M.; Rajeshkumar, S.; Lakshmi, T.; Kim, Y.J. Citral induced apoptosis through modulation of key genes involved in fatty acid biosynthesis in human prostate cancer cells: In silico and in vitro study. BioMed Res. Int. 2020, 2020, 6040727.
- Thomas, M.L.; De Antueno, R.; Coyle, K.M.; Sultan, M.; Cruickshank, B.M.; Giacomantonia, M.A.; Giacomantonia, C.A.; Duncan, R.; Marcato, P. Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol. Oncol. 2016, 10, 1485–1496.
- Liu, Z.; Gan, L.; Chen, Y.; Luo, D.; Zhang, Z.; Cao, W.; Zhou, Z.; Lin, X.; Sun, C. Mark4 promotes oxidative stress and inflammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes. Sci. Rep. 2016, 6, 21382.
- Feng, M.; Tian, L.; Gan, L.; Liu, Z.; Sun, C. Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways. Biol. Cell 2014, 106, 294–307.
- Naz, F.; Khan, F.I.; Mohammad, T.; Khan, P.; Manzoor, S.; Hasan, G.M.; Lobb, K.A.; Luqman, S.; Islam, A.; Ahmad, F.; et al. Investigation of molecular mechanism of recognition between citral and MARK4: A newer therapeutic approach to attenuate cancer cell progression. Int. J. Biol. Macromol. 2018, 107, 2580–2589.
- Sheikh, B.Y.; Sarker, M.M.R.; Kamarudin, M.N.A.; Mohan, G. Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed. Pharmacother. 2017, 96, 834–846.
- Sanches, L.J.; Marinello, P.C.; Panis, C.; Fagundes, T.R.; Morgada-Diaz, J.A.; de-Freitas-Junior, J.C.M.; Cecchini, R.; Cecchini, A.L.; Luiz, R.C. Cytotoxicity of citral against melanoma cells: The involvement of oxidative stress generation and cell growth protein reduction. Tumor Biol. 2017, 39, 1010428317695914.
- Kim, S.H.; Park, E.J.; Lee, C.R.; Chun, J.N.; Cho, N.H.; Lee, S.; Kim, T.W.; Park, H.H.; So, I.; Jeon, J.H. Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. Int. J. Oncol. 2012, 40, 1683–1690.
- Galle, M.; Crespo, R.; Kladniew, B.R.; Villegas, S.M.; Polo, M.; De Bravo, M.G. Suppression by Geraniol of the Growth of A549 Human Lung Adenocarcinoma Cells and Inhibition of the Mevalonate Pathway in Culture and In Vivo: Potential Use in Cancer Chemotherapy. Nutr. Cancer 2014, 66, 888–895.
- Wittig, C.; Scheuer, C.; Parakenings, J.; Menger, M.D.; Laschke, M.W. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling. PLoS ONE 2015, 10, e0131946.
- Crespo, R.; Rodenak-Kladniew, B.E.; Castro, M.A.; Soberon, M.V.; Lavarias, S.M. Induction of oxidative stress as a possible mechanism by which geraniol affects the proliferation of human A549 and HepG2 tumor cells. Chem. Biol. Interact. 2020, 320, 109029.
- Maczka, W.; Wínska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303.
- Murray-Stewart, T.R.; Woster, P.M.; Casero Jr, R.A. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016, 473, 2937–2953.
- Fatima, K.; Wani, Z.A.; Meena, A.; Luqman, S. Geraniol exerts its antiproliferative action by modulating molecular targets in lung and skin carcinoma cells. Phytother. Res. 2021, 35, 3861–3874.
- Madan, K.; Devaki, T. Geraniol, a component of plant essential oils-a review of its pharmacological activities. Int. J. Pharm. Pharm. Sci. 2015, 7, 67–70.
- Shen, X.; Cui, X.; Cui, H.; Jin, Y.; Jin, W.; Sun, H. Geraniol and lupeol inhibit growth and promote apoptosis in human hepatocarcinoma cells through the MAPK signaling pathway. J. Cell. Biochem. 2018, 120, 5033–5041.
- Kuzu, B.; Cüce, G.; Çınar Ayan, İ.; Gültekin, B.; Tuba Canbaz, H.; Gül Dursun, H.; Şahin, Z.; Keskin, İ.; Serpil Kalkan, S.S. Evaluation of Apoptosis Pathway of Geraniol on Ishikawa Cells. Nutr. Cancer 2020. 22 September epub ahead of print.
- Shanmugapriya, S.; Subramanian, P.; Kanimozhi, S. Geraniol Inhibits Endometrial Carcinoma via Downregulating Oncogenes and Upregulating Tumour Suppressor Genes. Indian, J. Clin. Biochem. 2017, 32, 214–219.
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review). Int. J. Oncol. 2016, 48, 1772–1782.
- Hajizadeh, M.; Maleki, H.; Barani, M.; Fahmindehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Res. Pharma. Sci. 2019, 14, 448.
- Jia, S.S.; Xi, G.P.; Zhang, M.; Chen, Y.B.; Lei, B.; Dong, X.S.; Yang, Y.M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354.
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON./Off. J. Balk. Union. Oncol. 2020, 25, 280–285.
- Jayaganesh, R.; Pugalendhi, P.; Murali, R. Effect of citronellol on NF-kB inflammatory signaling molecules in chemical carcinogen-induced mammary cancer in the rat model. J. Biochem. Mol. Toxicol. 2020, 34, e22441.
- Yu, W.; Lai, Y.J.; Ma, J.; Ho, C.T.; Hung, S.W.; Chen, Y.H.; Chen, C.T.; Kao, J.Y.; Wat, T.D. Citronellol induces necroptosis of human lung cancer cells via TNF-α pathway and reactive oxygen species accumulation. In Vivo 2019, 33, 1193–1201.
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476.
- Rajendran, J.; Pachaiappan, P.; Thangarasu, R. Citronellol, an Acyclic Monoterpene Induces Mitochondrial-Mediated Apoptosis through Activation of Proapoptotic Factors in MCF-7 and MDA-MB-231 Human Mammary Tumor Cells. Nutr. Cancer 2020, 73, 1448–1458.
- Cavalieri, E.; Mariotto, S.; Fabrizi, C.; de Prati, A.C.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 2004, 315, 589–594.
- Rigo, A.; Ferrarini, I.; Lorenzetto, E.; Darra, E.; Liparulo, I.; Bergamini, C.; Sissa, C.; Cavalieri, E.; Vinante, F. BID and the α-bisabolol-triggered cell death program: Converging on mitochondria and lysosomes. Cell. Death Dis. 2019, 10, 1–13.
- Rigo, A.; Vinante, F. The antineoplastic agent α-bisabolol promotes cell death by inducing pores in mitochondria and lysosomes. Apoptosis 2016, 21, 917–927.
- Uno, M.; Kokuryo, T.; Yokoyama, Y.; Senga, T.; Nagino, M. α-Bisabolol inhibits invasiveness and motility in pancreatic cancer through KISS1R activation. Anticancer Res. 2016, 36, 583–589.
- Fang, D.; Wang, H.; Li, M.; Wei, W. α-bisabolol enhances radiotherapy-induced apoptosis in endometrial cancer cells by reducing the effect of XIAP on inhibiting caspase-3. Biosci. Rep. 2019, 39, BSR20190696.
- Sabo, A.A.; Dudau, M.; Constantin, G.L.; Cop, T.C.; Geifus, C.M.; Naccarati, A.; Dragomir, M.P. Two Worlds Colliding: The Interplay Between Natural Compounds and Non-Coding Transcripts in Cancer Therapy. Front. Pharmacol. 2021, 12, 652074.
- Cherng, J.M.; Shieh, D.E.; Chiang, W.; Chang, M.Y.; Chiang, L.C. Chemopreventive effects of minor dietary constituents in common foods on human cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 1500–1504.
- Lee, H.Y.; Son, S.W.; Moeng, S.; Choi, S.Y.; Park, J.K. The role of noncoding RNAs in the regulation of anoikis and anchorage-independent growth in cancer. Int. J. Mol. Sci. 2021, 22, 627.
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 38, BSR20180980.
- Poltronieri, P.; Xu, B.; Giovinazzo, G. Resveratrol and other Stilbenes: Effects on Dysregulated Gene Expression in Cancers and Novel Delivery Systems. Anticancer Agents Med. Chem. 2021, 21, 567–574.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
3 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




