Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mario Inclán | + 2495 word(s) | 2495 | 2021-12-27 09:56:35 | | | |
| 2 | Vivi Li | Meta information modification | 2495 | 2021-12-29 02:40:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Inclán, M. Fluorescent Chemosensors Based on Polyamine Ligands. Encyclopedia. Available online: https://encyclopedia.pub/entry/17595 (accessed on 07 February 2026).
Inclán M. Fluorescent Chemosensors Based on Polyamine Ligands. Encyclopedia. Available at: https://encyclopedia.pub/entry/17595. Accessed February 07, 2026.
Inclán, Mario. "Fluorescent Chemosensors Based on Polyamine Ligands" Encyclopedia, https://encyclopedia.pub/entry/17595 (accessed February 07, 2026).
Inclán, M. (2021, December 28). Fluorescent Chemosensors Based on Polyamine Ligands. In Encyclopedia. https://encyclopedia.pub/entry/17595
Inclán, Mario. "Fluorescent Chemosensors Based on Polyamine Ligands." Encyclopedia. Web. 28 December, 2021.
Copy Citation
Polyamine ligands are water-soluble receptors that are able to coordinate, depending on their protonation degree, either metal ions, anionic, or neutral species. Furthermore, the presence of fluorescent signaling units allows an immediate visual response/signal. For these reasons, they can find applications in a wide variety of fields, mainly those where aqueous media is necessary, such as biological studies, wastewater analysis, soil contamination, etc.
sensing
polyamines
fluorescence
water solubility
molecular recognition
nanoparticles
1. Introduction
Since the first fluorescent chemosensor was reported in 1867, by Goppelsröder, a great number of these systems has been developed, many of which have been applied in fields such as analytical chemistry, biology, physiology, pharmacology, and environmental sciences. Particularly, the development of fluorescent chemosensors for analytes that are present in aqueous solution has aroused great interest due to their potential applications in important areas, such as biology, environmental, or medicinal chemistry [1][2][3].
Polyamine chains are characterized by their solubility in water and their ability to coordinate either metal ions or neutral/anionic species as a function of their protonation degree [4][5][6][7][8][9][10][11]. The functionalization of polyamines with fluorescent groups has allowed obtaining different chemosensors for metal ions and/or anions [12]. The deprotonation of polyammonium groups is accompanied by a characteristic quenching of the fluorescence emission, which is associated with a photoinduced electron transfer (PET) from the nitrogen lone pair to the excited fluorophore. Protonation engages the lone pair in the amine, causing thereby the recovery of the fluorescence emission (Figure 1).
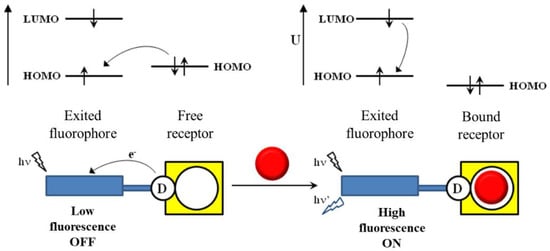
Figure 1. Scheme of the recognition of an analyte by blocking the PET process. Figure adapted from reference [1].
2. Open Chain Polyamine Derivatives
2.1. Linear Polyamines
Open-chain polyamines can be easily functionalized with fluorescent moieties, developing chemosensors capable of signaling substrate coordination by changing the physical properties of these functional groups. Thus, this type of chemosensor can be considered as one of the simplest examples in terms of structural design.
One of the first examples of simple open-chain linear polyamine chemosensors was related to the receptors developed by Pina et al., in which different open-chain polyamines were functionalized with naphthalene moieties at the terminal amino groups (Scheme 1, 1–4) [13][14][15].
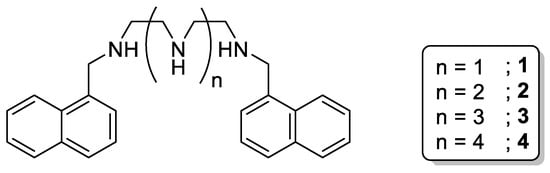
Scheme 1. Compounds 1–4.
Fluorescence emission studies showed the formation of an excimer species whose intensity depended on the length and the protonation degree of the polyamine chains. The authors associated this excimer to the existence of a bending movement that allowed one naphthalene moiety in the excited state and another one in the ground state to approach and interact (see Figure 2). These compounds can be considered as elementary molecular machines, whose movements were driven by light and switched on/off by pH.
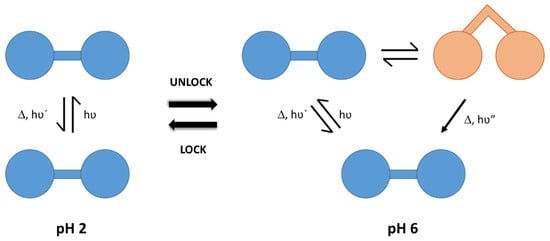
Figure 2. Excimer formation and molecular movement associated to the protonation degree of the polyamine chain acting as spacer between fluorophore groups. Adapted with permission from Ref. [13]. Copyright 2001, The Royal Society of Chemistry.
Using the concept of metal-induced intramolecular excimer formation, Van Arman et al. prepared the first example of a water-soluble ratiometric fluorescent probe for Zn(II), consisting of a linear polyamine that functionalized two anthryl moieties (Scheme 2, 5). When titrating this ligand with Zn(II), a 4-fold increase in the excimer band at 495 nm is observed, together with a smaller increase of the band at 415 nm, which is attributed to the expected chelation enhanced fluorescence (CHEF) effect [16].
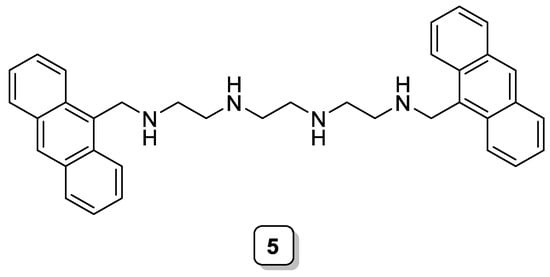
Scheme 2. Compound 5.
An analogous example was described by Shiraishi et al., who reported a chemosensor based on a diethylenetriamine moiety bearing one pyrene fragment at each end (Scheme 3, 6). In this case, a triple-mode fluorescence was observed in water consisting of a monomer emission (acidic-neutral pH), and of short- and long-lived (basic pH) excimer emissions, which were associated to a pH-controlled bending movement of the polyamine chain and the formation of an intramolecular ground-state dimer of the pyrene fragments [17][18]. Substitution of the terminal fluorophores of this polyamine by two quinoline moieties (7) revealed an interesting behavior as a fluorescent Zn2+ water-soluble sensor. In the absence of metal cations, no fluorescence emission was observed at the pH range of study; however, Zn2+ addition promoted a fluorescence emission enhancement, with a linear and stoichiometric response to the Zn2+ concentration [19]. Furthermore, no remarkable emission enhancement was observed for other cations, such as Cd2+, with similar electronic and binding properties [20][21][22][23].
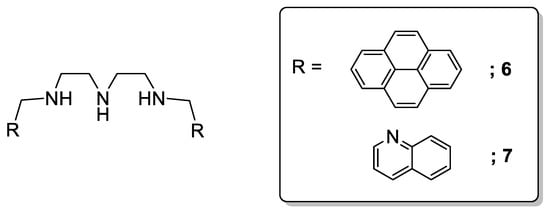
Scheme 3. Compounds 6–7.
The non-symmetrical functionalization of the terminal positions has also been reported by Shiraishi et al. [24]. Molecules 8–10 (Scheme 4) bear anthracene and benzophenone moieties at each end of the polyamine chain. As a result of this configuration, there is a sequential electron transfer and, for this reason, the changes in the emission induced by the pH follow a “gentle slope”, making these molecules suitable pH chemosensors in a wide range of values (pH 2–10).
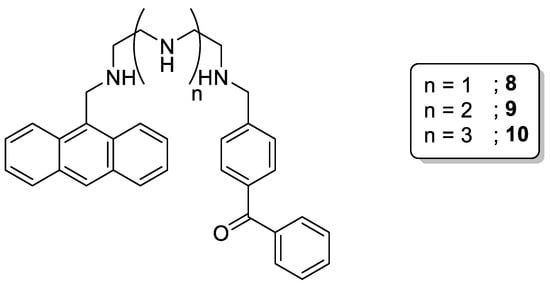
Scheme 4. Compounds 8–10.
2.2. Branched Polyamines
Tripodal tetraamines, containing a single tertiary nitrogen atom and one amino group in each arm, are widely used in supramolecular and coordination chemistry for the development of metal ion and anion receptors [25]. The preorganized structure of tripodal polyamines promotes topological matching with anions of the same symmetry, such as oxoanions, which are a class of anions of great biological and environmental relevance.
An early example is the tripodal anthracene-based chemosensors 16 and 17 (Scheme 5), reported by Czarnik et al. [26], which showed an enhancement of the fluorescence upon the addition of phosphate at pH = 6. The authors ascribed this behavior to the encapsulation of phosphate within the tripodal cavity and the subsequent formation of hydrogen bonds between one OH group of the phosphate with the lone pair of the benzylic nitrogen. This interaction hindered the PET process from the benzylic nitrogen to the excited fluorophore and, hence, the fluorescence enhancement [27].
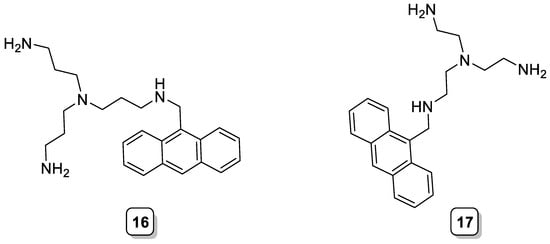
Scheme 5. Compounds 16–17.
The polyamine tris(2-aminoethyl)amine (tren) has been widely used as a scaffold in the preparation of fluorescent sensors. In this line, a recent article by García-España et al. reported on the preparation of the two BODIPY-tren Cu(II) chemosensors, 18 and 19 (Scheme 6) [28]. BODIPY dyes are an important class of fluorophores with excellent photoluminescent characteristics but limited water solubility. By combining them with a polyamine, it was possible to obtain water-soluble conjugates with a high selectivity for Cu(II) ions.
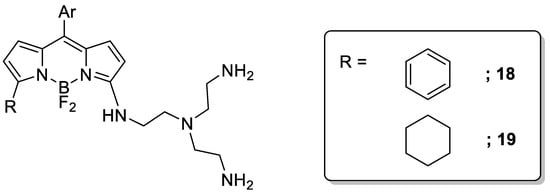
Scheme 6. Compounds 18–19.
Another example of fluorescent receptors based on the polyamine tren was reported by Kataev et al., who used the fluorescent naphthalimide as the chromophoric unit. By different synthetic strategies, they were able to introduce the polyamine in different positions of the naphthalimide ring and prepare chemosensors bearing one or two tren units (20 and 21, Scheme 7). When studied against a panel of different anions, it was found that the ligands bind selectively to pyrophosphate anion. As expected, the ligand with six amino groups (21) and higher overall basicity showed the highest affinity toward this anion [29].
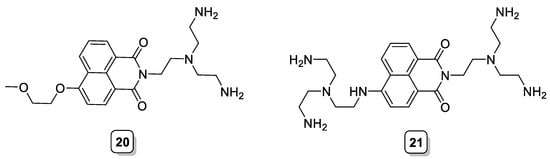
Scheme 7. Compounds 20–21.
Molecule 22 (Scheme 8) was built by functionalizing the polyamine tren with an anthracene and two N,N-dimethylaniline moieties. For the Zn(II) complex of this ligand, it was observed that an intramolecular photoinduced electron transfer takes place, from the N,N-dimethylaniline units to the excited anthracene fluorophore, which are close to each other in the complex, resulting in a low fluorescence emission of [Zn(22)]2+. However, the presence of a bulky anion, such as triphenylacetate, filled the cavity, preventing the occurrence of the intramolecular PET process and, hence, enhancing the fluorescence emission [30].
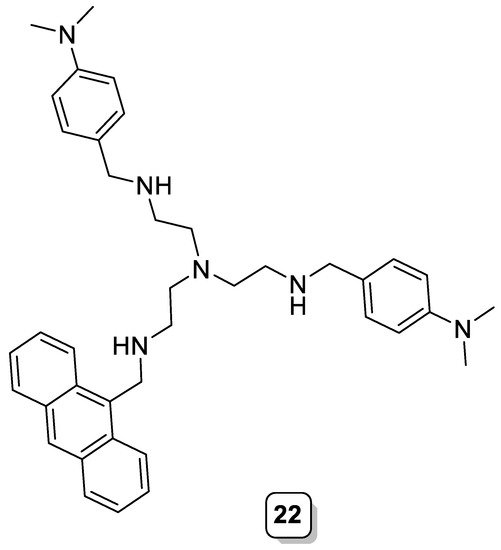
Scheme 8. Compound 22.
Quinn et al. synthesized a tripodal anthracene-based ligand (23, Scheme 9), with an interesting sensing behavior for oxoanions and halides [31]. The ability of the protonated amino groups to form hydrogen bonds coupled with the electron-withdrawing properties of the attached anthracene groups significantly increased the stability of its complexes with anionic species. The addition of anions caused an enhancement of the fluorescence intensity attributed to the formation of a ligand–anion complex that restricts the free rotation of the attached fluorophores.
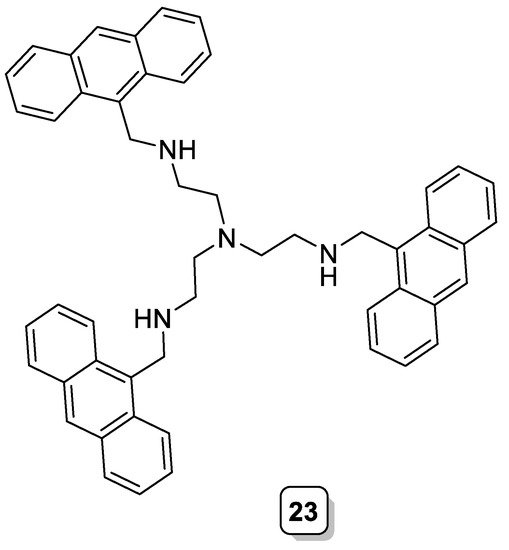
Scheme 9. Compound 23.
3. Macrocyclic Polyamine Derivatives
Macrocyclic polyamines represent a step further in the design of more preorganized receptors. Their cyclic structure allows the formation of polyammonium receptors in aqueous solution with a high positive charge density that are flexible enough to be able to wrap around the anions, stabilizing them through electrostatic interactions and/or hydrogen bonding formation [32]. Functional groups have also been appended to these polyazamacrocyclic receptors in order to increase their ability to bind anionic species or to confer specific functions to the resulting receptors.
3.1. Aza-Crown Macrocycles
Probably the most studied group of polyazamacrocyclic ligands is the aza-crown family, consisting of rings with the formulae (CH2CH2NH)n, where n = 3, 4, 5, and 6. Their wide commercial availability and the possibility of functionalizing the secondary amino groups with fluorophores has made them excellent candidates for the development of fluorescent probes.
An early example can be found in the work of Kimura et al., who prepared the dansylated derivative of 1,4,7,10-tetraazacyclododecane (cyclen) 31 (Scheme 10) [33]. This ligand showed a high affinity toward Zn(II), with a linear response of the fluorescence enhancement until 1:1 ligand to Zn(II) ratio. However, this enhancement of the fluorescence was drastically affected by the presence of Cu(II), which leads to an almost complete quenching of the emission. The presence of other alkaline and alkaline earth cations did not affect the fluorescence response of the Zn(II) complex.
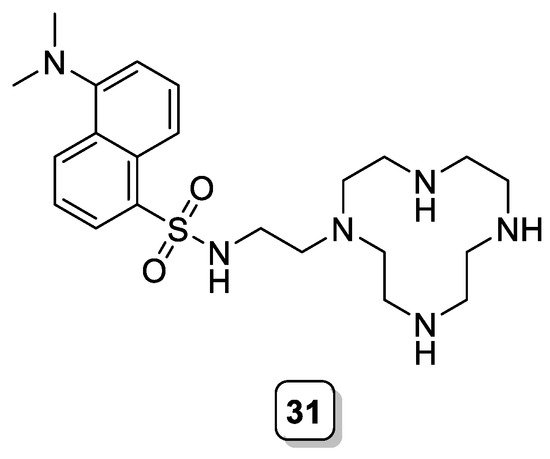
Scheme 10. Compound 31.
A similar approach was investigated by Lippard and Burdette. Using a hybrid fluorescein/rhodamine fluorophore as the signaling unit and cyclen as the Zn(II) binding unit, they prepared ligand 32 (Scheme 11), which acts as a PET sensor for this metal cation with a 50% increase of the emission upon complexation of Zn(II). The competition experiments revealed little interference by other divalent transition metals, with the exception of Cd(II) and, particularly, Cu(II), which induced a strong quenching [34].
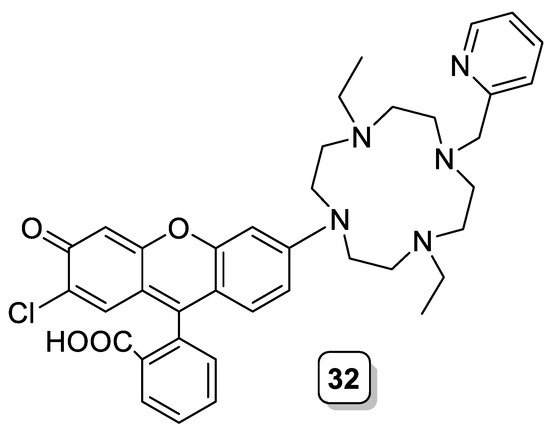
Scheme 11. Compound 32.
In a more recent example of sensors containing cyclen, Li et al. exploited the Zn(II) affinity of this macrocycle and the strong fluorescence of tetraphenylethene (TPE) to prepare fluorescent chemosensors for pyrophosphate (PPi) and adenosine triphosphate (ATP) sensing. Different ligands were prepared, bearing one (33), two (34), or four (35) [Zn(cyclen)]2+ complexes attached to the TPE scaffold (Scheme 12). These ligands showed a pronounced and selective “turn-on” fluorescence response toward PPi and ATP in aqueous media, with detection limits in the nanomolar range [35][36].
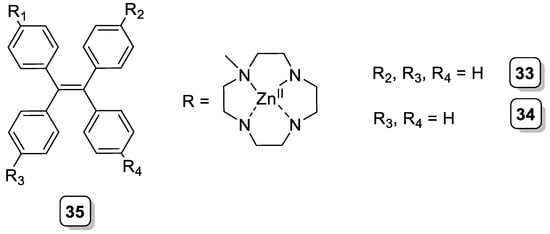
Scheme 12. Compounds 33–35.
3.2. Aza-Scorpiand Ligands
The term “scorpiand” is related to a macrocyclic ligand modified with a pendant arm containing additional donor atoms that exercises an active role in the coordination of metal ions or other substrates inside the macrocyclic cavity [37][38]. The molecular movements generated by the pendant arm as consequence of electrostatic repulsions and binding events can be signalized by the introduction of fluorophores, whose fluorescence changes depend on whether the donor atom in the tail is coordinated or not [39].
Over the last few years, different chemosensors based on aza-scorpiand ligands have been reported. One of the first examples is the phenanthrolinophane (47, Scheme 13) containing an anthracene group in the pendant arm, as reported by Bencini et al. The coordination of Zn2+ in the macrocyclic core and the consequent attachment/detachment of the nitrogen atom present in the pendant arm give rise to on/off switching of the exciplex emission, defining an elementary molecular machine whose movements are driven by both pH and light [40][41]. The coordination of the nitrogen atom in the pending arm to the metal ion brings about the occurrence of π-stacking interactions between the phenanthroline and anthracene rings.
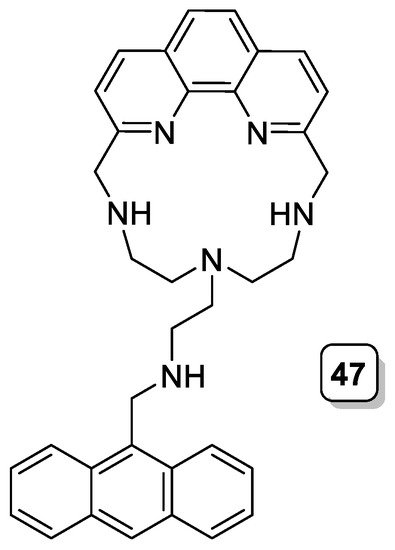
Scheme 13. Compound 47.
Inclán et al. developed anthracene analogous ligands containing a pyridine moiety as an aromatic spacer [42]. 48 and 49 (Scheme 14) exhibited binding selectivity for GTP vs. ATP due to the higher ability of guanine to form π-stacking complexes with the anthracene present in the pendant arm. In addition, 48 and 49 displayed a large fluorescence quenching in the presence of GTP over the whole pH range, while the addition of ATP produced a significant increase in the fluorescence emission. This fluorescence change makes 48 and 49 efficient sensors to effectively and selectively distinguish GTP from ATP in aqueous solution.
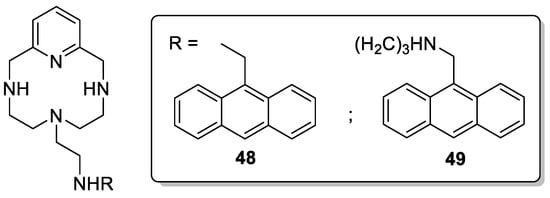
Scheme 14. Compounds 48–49.
4. Grafted Polyamine Derivatives
In order to obtain more efficient, selective, and sensitive chemosensors, an increasing number of papers have recently appeared in which the chemosensor is anchored to different nanosized supports [43][44][45][46]. One of them, boehmite nanoparticles, which is an aluminum oxyhydroxide (γ-AlO(OH)), presents different advantages such as the possibility to make fluorescence emission studies in pure water with little scattering, and the recovering of the sensor system after their use by centrifugation because a change from a sol to a gel state occurs at basic pH. These nanoparticles contain terminal groups that render high reactivity to the surface and provide a hydrophilic environment, improving the homogeneity of the medium. For all these reasons, boehmite is a promising nanosized support.
Delgado-Pinar et al. developed boehmite-silica nanoparticles functionalized with linear polyamines containing terminal anthracene units (61 and 62, Scheme 15) [47]. These grafted polyamines offered the possibility to detect Hg(II) selectively (detection limit = 0.19 ppb) over other metal ions such as Cu(II), Zn(II), Cd(II), and Pb(II). This behavior was attributed to the ability of Hg(II) to form stable complexes with low coordination numbers [48].
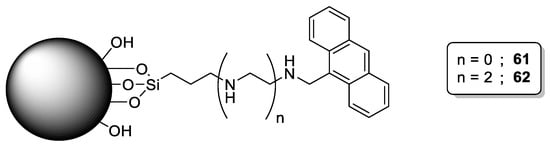
Scheme 15. Compounds 61–62.
Analogously, Carbonell et al. developed boehmite functionalized materials containing pyrene as a fluorophore and different linear or tripodal polyamines (63, 64, and 65, Scheme 16) as coordination sites. A detailed analysis of the interaction with some halides and oxoanions revealed a selective decrease in intensity of the pyrene fluorescence emission only for iodide in aqueous solution with an estimated detection limit of 36 ppb, 65 being the most efficient system [49].
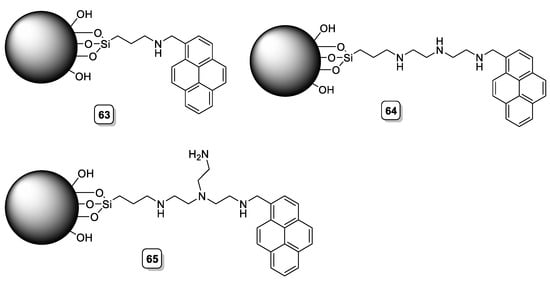
Scheme 16. Compounds 63–65.
On the other hand, Martínez-Mañez et al. reported a pioneering work related to the development of polyamine surface-functionalized mesoporous materials for anion recognition and signaling systems by dye delivery ([Ru(bipy)3]2+) as a function of pH in aqueous solution. [50] Analogously, they reported the design of gated nanodevices based on mesoporous silica materials for the detection of genomic DNA from bacteria (Mycoplasma fermentans) [51][52]. The external surface of these porous systems, loaded with a fluorophore dye, was functionalized with polyamine molecules and oligonucleotides (electrostatically anchored to the protonated amino groups) acting as a cap. Thus, the system remains capped until the presence of the complementary oligonucleotide promoted the hybridization reaction and a displacement of the cap, resulting in pore opening and dye delivery (Figure 5). Based on these examples, different capped mesoporous silica materials for the selective detection of cations, anions, and biomolecules have been described by Martínez-Mañez in a recent review [53].
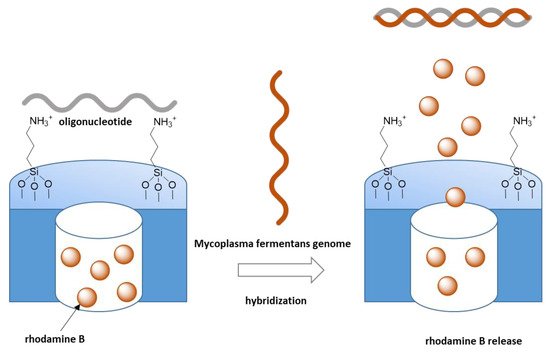
Figure 5. Capped mesoporous silica materials for the selective detection of genomic DNA from bacteria (Mycoplasma fermentans). The presence of complementary oligonucleotide promoted the hybridization reaction and a displacement of the cap resulting in pore opening and dye delivery. Adapted with permission from Ref [53]. Copyright 2019, MDPI, Basel, Switzerland.
Recently, Oshchepkov et al. expanded the work of Kataev to prepare cryopolymers based on a naphthalimide fluorescent ligand that had shown selectivity toward phosphonate (66, Scheme 17). Cryopolymers are super-porous gel structures, prepared via the freeze–thaw method, which show interesting mechanical and chemical properties [54]. Ligand 66 is similar to those described previously in this review (see 20 and 21) and by the reaction shown below. This ligand is similar to those described previously in this review (see 20 and 21). By the reaction shown below, the authors included this fluorescent chemosensor into a cross-linked polymeric material. However, when comparing with the monomer, in the polymeric materials prepared, the selectivity was lost, and a decrease of the fluorescence quantum yield was observed [55].
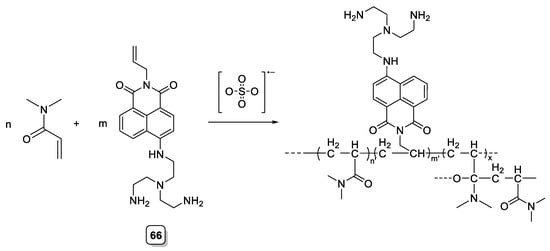
Scheme 17. Compound 66.
References
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40.
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566.
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123.
- Dietrich, B. Design of anion receptors: Applications. Pure Appl. Chem. 1993, 65, 1457–1464.
- Bianchi, A.; Bowman-James, K.; García-España, E. Supramolecular Chemistry of Anions; Wiley-VCH: New York, NY, USA, 1997.
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516.
- Gale, P.A. Anion receptor chemistry: Highlights from 1999. Coord. Chem. Rev. 2001, 213, 79–128.
- Ilioudis, C.A.; Steed, J.W. Organic macrocyclic polyamine-based receptors for anions. J. Supramol. Chem. 2001, 1, 165–187.
- Bianchi, A.; Micheloni, M.; Paoletti, P. Thermodynamic aspects of the polyazacycloalkane complexes with cations and anions. Coord. Chem. Rev. 1991, 110, 17–113.
- Vilar, R. Anion-Templated Synthesis. Angew. Chem. Int. Ed. 2003, 42, 1460–1477.
- Llinares, J.M.; Powell, D.; Bowman-James, K. Ammonium based anion receptors. Coord. Chem. Rev. 2003, 240, 57–75.
- Pina, F.; Bernardo, M.A.; García-España, E. Fluorescent Chemosensors Containing Polyamine Receptors. Eur. J. Inorg. Chem. 2000, 2000, 2143–2157.
- Albelda, M.T.; Bernardo, M.A.; Díaz, P.; García-España, E.; de Melo, J.S.; Pina, F.; Soriano, C.; Luis, S.V. Polyamines containing naphthyl groups as pH-regulated molecular machines driven by light. Chem. Commun. 2001, 1520–1521.
- Seixas de Melo, J.S.; Albelda, M.T.; Díaz, P.; García-España, E.; Lodeiro, C.; Alves, S.P.C.; Lima, J.C.; Pina, F.; Soriano, C. Ground and excited state properties of polyamine chains bearing two terminal naphthalene units. J. Chem. Soc. Perkin Trans. 2 2002, 5, 991–998.
- Bernardo, M.A.; Alves, S.; Pina, F.; de Melo, J.S.; Albelda, M.T.; Garcia-España, E.; Llinares, J.M.; Soriano, C.; Luis, S.V. Polyamine Linear Chains Bearing Two Identical Terminal Aromatic Units. Evidence for a Photo Induced Bending Movement. Supramol. Chem. 2001, 13, 435–445.
- Sclafani, J.A.; Maranto, M.T.; Sisk, T.M.; Van Arman, S.A. An Aqueous Ratiometric Fluorescence Probe for Zn(II). Tetrahedron Lett. 1996, 37, 2193–2196.
- Shiraishi, Y.; Tokitoh, Y.; Hirai, T. pH- and H2O-Driven Triple-Mode Pyrene Fluorescence. Org. Lett. 2006, 8, 3841–3844.
- Shiraishi, Y.; Tokitoh, Y.; Nishimura, G.; Hirai, T. Solvent-Driven Multiply Configurable On/Off Fluorescent Indicator of the pH Window: A Diethylenetriamine Bearing Two End Pyrene Fragments. J. Phys. Chem. B 2007, 111, 5090–5100.
- Shiraishi, Y.; Ichimura, C.; Hirai, T. A quinoline–polyamine conjugate as a fluorescent chemosensor for quantitative detection of Zn(II) in water. Tetrahedron Lett. 2007, 48, 7769–7773.
- Koike, T.; Watanabe, T.; Aoki, S.; Kimura, E.; Shiro, M. A Novel Biomimetic Zinc(II)-Fluorophore, Dansylamidoethyl-Pendant Macrocyclic Tetraamine 1,4,7,10-Tetraazacyclododecane (Cyclen). J. Am. Chem. Soc. 1996, 118, 12696–12703.
- Royzen, M.; Durandin, A.; Young, Y.G., Jr.; Geacintov, N.E.; Canary, J.W. A Sensitive Probe for the Detection of Zn(II) by Time-Resolved Fluorescence. J. Am. Chem. Soc. 2006, 128, 3854–3855.
- Nolan, E.M.; Lippard, S.J. The Zinspy Family of Fluorescent Zinc Sensors: Syntheses and Spectroscopic Investigations. Inorg. Chem. 2004, 43, 8310–8317.
- Nolan, E.M.; Burdette, S.C.; Hervey, J.H.; Hilderbrand, S.A.; Lippard, S.J. Synthesis and characterization of zinc sensors based on a monosubstituted fluorescein platform. Inorg. Chem. 2004, 43, 2624–2635.
- Nishimura, G.; Shiraishi, Y.; Hirai, T. A fluorescent chemosensor for wide-range pH detection. Chem. Commun. 2005, 5313–5315.
- Blakman, A.G. The Coordination Chemistry of Tripodal Tetraamine Ligands. Polyhedron 2005, 24, 1–39.
- Huston, M.E.; Akkaya, E.U.; Czarnik, A.W. Chelation enhanced fluorescence detection of non-metal ions. J. Am. Chem. Soc. 1989, 111, 8735–8737.
- De Santis, G.; Fabbrizzi, L.; Licchelli, M.; Poggi, A.; Taglietti, A. Molecular Recognition of Carboxylate Ions Based on the Metal-Ligand Interaction and Signaled through Fluorescence Quenching. Angew. Chem. Int. Ed. Engl. 1996, 35, 202–204.
- Verbelen, B.; Valckenborgh, M.; Inclán, M.; Nebot, A.; Dehaen, W.; García-España, E. Efficient two-step synthesis of water soluble BODIPY–TREN chemosensors for copper(II) ions. RSC Adv. 2017, 7, 3066–3071.
- Oshchepkov, A.S.; Mittapalli, R.R.; Fedorova, O.A.; Kataev, E.A. Naphthalimide-Based Polyammonium Chemosensors for Anions:Study of Binding Properties and Sensing Mechanisms. Chem. Eur. J. 2017, 23, 9657–9665.
- Bruseghini, I.; Fabbrizzi, L.; Licchelli, M.; Taglietti, A. Coordinative control of photoinduced electron transfer: Bulky carboxylates as molecular curtains. Chem. Commun. 2002, 1348–1349.
- Quinn, W.A.; Saeed, M.A.; Powell, D.R.; Hossain, M. An Anthracene-Based Tripodal Chemosensor for Anion Sensing. Int. J. Environ. Res. Public Health 2010, 7, 2057–2070.
- Bowman-James, K.; Bianchi, A.; García-España, E. Anion Coordination Chemistry; Wiley-VCH, Verlag GmbH & Co.: Weinheim, Germany, 2012.
- Kimura, E.; Koike, T. Recent development of zinc-fluorophores. Chem. Soc. Rev. 1998, 27, 179–184.
- Burdette, S.C.; Lippard, S.J. The Rhodafluor Family. An Initial Study of Potential Ratiometric Fluorescent Sensors for Zn2+. Inorg. Chem. 2002, 41, 6816–6823.
- Xu, H.-R.; Li, K.; Wang, M.-Q.; Wang, B.-L.; Wang, X.; Yu, X.-Q. The dicyclen–TPE zinc complex as a novel fluorescent ensemble for nanomolar pyrophosphate sensing in 100% aqueous solution. Org. Chem. Front. 2014, 1, 1276–1279.
- Xu, H.-R.; Li, K.; Jiao, S.-Y.; Li, L.-L.; Pan, S.-L.; Yu, X.-Q. Tetraphenylethene based zinc complexes as fluorescent chemosensors for pyrophosphate sensing. Chin. Chem. Lett. 2015, 26, 877–880.
- Lotz, T.J.; Kaden, T.A. pH-Induced co-ordination geometry change in a macrocyclic nickel(II) complex. J. Chem. Soc. Chem. Commun. 1977, 15–16.
- Pallavicini, P.S.; Perotti, A.; Poggi, A.; Seghi, B.; Fabbrizzi, L. N-(aminoethyl) cyclam: A tetraaza macrocycle with a coordinating tail (scorpiand). Acidity controlled coordination of the side chain to nickel (II) and nickel (III). J. Am. Chem. Soc. 1987, 109, 5139–5144.
- Amendola, V.; Fabbrizzi, L.; Lichelli, M.; Mangano, C.; Pallavicini, P.; Parodi, L.; Poggi, A. Molecular events switched by transition metals. Coord. Chem. Rev. 1999, 192, 649–669.
- Bencini, A.; Bianchi, A.; Lodeiro, C.; Masotti, A.; Parola, A.J.; Melo, J.S.; Pina, F.; Valtancoli, B. A novel fluorescent chemosensor exhibiting exciplex emission. An example of an elementary molecular machine driven by pH and by light. Chem. Commun. 2000, 1639–1640.
- Bencini, A.; Berni, E.; Bianchi, A.; Fornasari, P.; Giorgi, C.; Lima, J.C.; Lodeiro, C.; Melo, M.J.; de Melo, J.S.; Parola, A.J.; et al. A fluorescent chemosensor for Zn(II). Exciplex formation in solution and the solid state. Dalton Trans. 2004, 2180–2187.
- Inclán, M.; Albelda, M.T.; Carbonell, E.; Blasco, S.; Bauzá, A.; Frontera, A.; García-España, E. Molecular Recognition of Nucleotides in Water by Scorpiand-Type Receptors Based on Nucleobase Discrimination. Chem. Eur. J. 2014, 20, 3730–3741.
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samorì, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304.
- Zhao, Y.-Y.; Yang, J.-M.; Jin, X.-Y.; Cong, H.; Ge, Q.-M.; Liu, M.; Tao, Z. Recent Development of Supramolecular Sensors Constructed by Hybridization of Organic Macrocycles with Nanomaterials. Curr. Org. Chem. 2020, 24, 265–290.
- Mandal, R.; Baranwal, A.; Srivastava, A.; Chandra, P. Evolving trends in bio/chemical sensor fabrication incorporating bimetallic nanoparticles. Biosens. Bioelectron. 2018, 117, 546–561.
- Montalti, M.; Rampazzo, E.; Zaccheroni, N.; Prodi, L. Luminescent Chemosensors Based on Silica Nanoparticles for the Detection of Ionic Species. New J. Chem. 2013, 37, 28–34.
- Delgado-Pinar, E.; Montoya, N.; Galiana, M.; Albelda, M.T.; Frías, J.C.; Jiménez, H.R.; García-España, E.; Alarcón, J. Preparation of Hg2+ selective fluorescent chemosensors based on surface modified core-shell aluminosilicate nanoparticles. New J. Chem. 2010, 34, 567–570.
- Cotton, F.A.; Murillo, C.; Wilkinson, G.; Bochmann, M.; Grimes, R. Advanced Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1999.
- Carbonell, E.; Delgado-Pinar, E.; Pitarch-Jarque, J.; Alarcón, J.; García-España, E. Boehmite Supported Pyrene Polyamine Systems as Probes for Iodide Recognition. J. Phys. Chem. C 2013, 117, 14325–14331.
- Casasús, R.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P. New Methods for Anion Recognition and Signaling Using Nanoscopic Gatelike Scaffoldings. Angew. Chem. Int. Ed. 2006, 45, 6661–6664.
- Climent, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Maquieira, A.; Amorós, P. Controlled Delivery Using Oligonucleotide-Capped Mesoporous Silica Nanoparticles. Angew. Chem. Int. Ed. 2010, 122, 7439–7441.
- Climent, E.; Climent, E.; Mondragón, L.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Murguía, J.R.; Amorós, P.; Rurack, K.; Pérez-Payá, E. Selective, highly sensitive, and rapid detection of genomic DNA by using gated materials: Mycoplasma detection. Angew. Chem. Int. Ed. 2013, 52, 8938–8942.
- Pla, L.; Lozano-Torres, B.; Martínez-Máñez, R.; Sancenón, F.; Ros-Lis, J.V. Overview of the Evolution of Silica-Based Chromo-Fluorogenic Nanosensors. Sensors 2019, 19, 5138.
- Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical Applications of Polymeric Cryogels. Appl. Sci. 2019, 9, 553.
- Oshchepkov, A.; Oshchepkov, M.; Kamagurov, S.; Redchuk, A.; Oshchepkova, M.; Popovb, K.; Kataev, E. Fluorescence detection of phosphonates in water by a naphthalimide-based receptor and its derived cryopolymers. New J. Chem. 2020, 44, 12113–12121.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
29 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




