
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Vaneev | + 3104 word(s) | 3104 | 2021-11-26 04:25:29 | | | |
| 2 | Lindsay Dong | Meta information modification | 3104 | 2021-12-24 02:39:34 | | |
Video Upload Options
Topical drug delivery is one of the most challenging aspects of eye therapy. Eye drops are the most prevalent drug form because they are convenient and easy to apply by patients. However, conventional drug formulations are usually characterized by short retention time in the tear film, insufficient contact with epithelium, fast elimination, and difficulties in overcoming ocular tissue barriers. Not more than 5% of the total drug dose administered in eye drops reaches the interior ocular tissues. Drug-loaded nanoparticles/hydrogels do not enter cells via diffusion. The endocytosis pathway is related to the penetration of drug-loaded nanoparticles/hydrogels into the cell. The interactions between the nanoparticles and the cell membrane generate forces of different origins and lead to the membrane wrapping of the nanoparticles followed by cellular uptake.
1. Introduction
Most of the patients with eye diseases receive drug therapy. The bioavailability of the ophthalmic drugs after systemic administration is strongly restricted by the blood-ocular barrier system formed by two main barriers: the blood-aqueous barrier for the anterior segment and the blood-retinal barrier for the posterior eye segment (Figure 1). The former consists of the tight junctions of the non-pigmented epithelium of the ciliary body, iris tissues, and the iris blood vessels. This barrier limits the drug access to the anterior segment of the eye. The blood-retinal barrier is formed by junctions between retinal capillary endothelial cells and the tight junctions between retinal pigment epithelial cells [1].
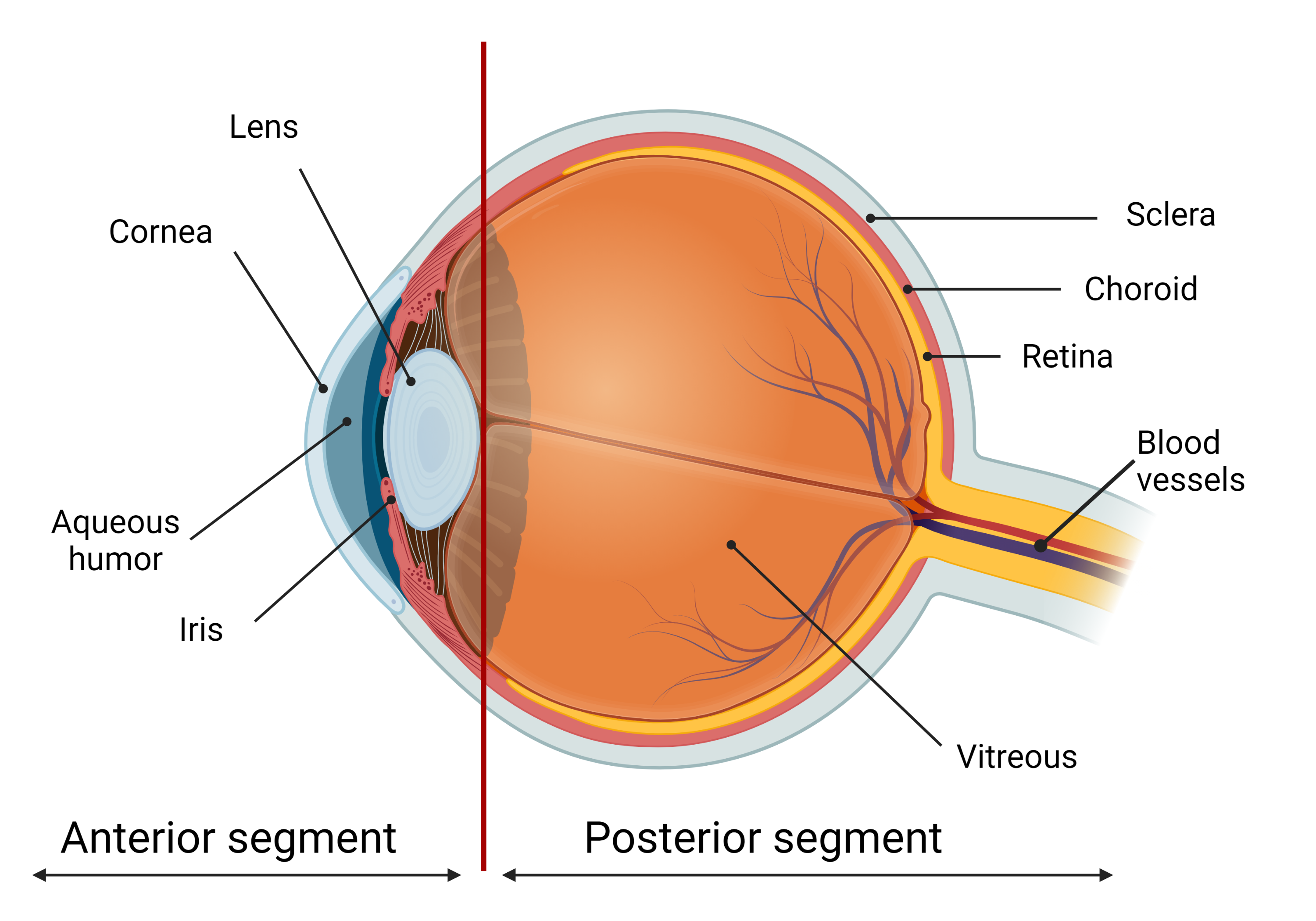
Figure 1. The scheme of the eye. The ocular globe can be conditionally divided into two parts: anterior segment and posterior segment. The anterior eye segment consists of cornea, conjunctiva, iris, ciliary body, lens, and aqueous humor, while sclera, choroid, retina, and vitreous body form the posterior segment. Created with BioRender.com.
The required amount of eye medication by local administration is significantly less than that by systemic administration. Local injections (subconjunctival or intravitreal) of the drugs are traumatic and have to be performed by medical personnel only. Topical administration of eye medications in the form of eye drops or gels is more favorable, as it can be easily performed by patients themselves. Eye drops are the most prevalent way of therapy for anterior eye segment diseases because they are convenient, demand less amount of the drug, and, consequently, are much less likely to cause side effects than after systemic administration. It is the major delivery route used for optimal drug absorption, especially for the treatment of anterior eye segment diseases. Eye drops represent about 90% of the marketed ophthalmic formulations [2][3][4][5][6][7].
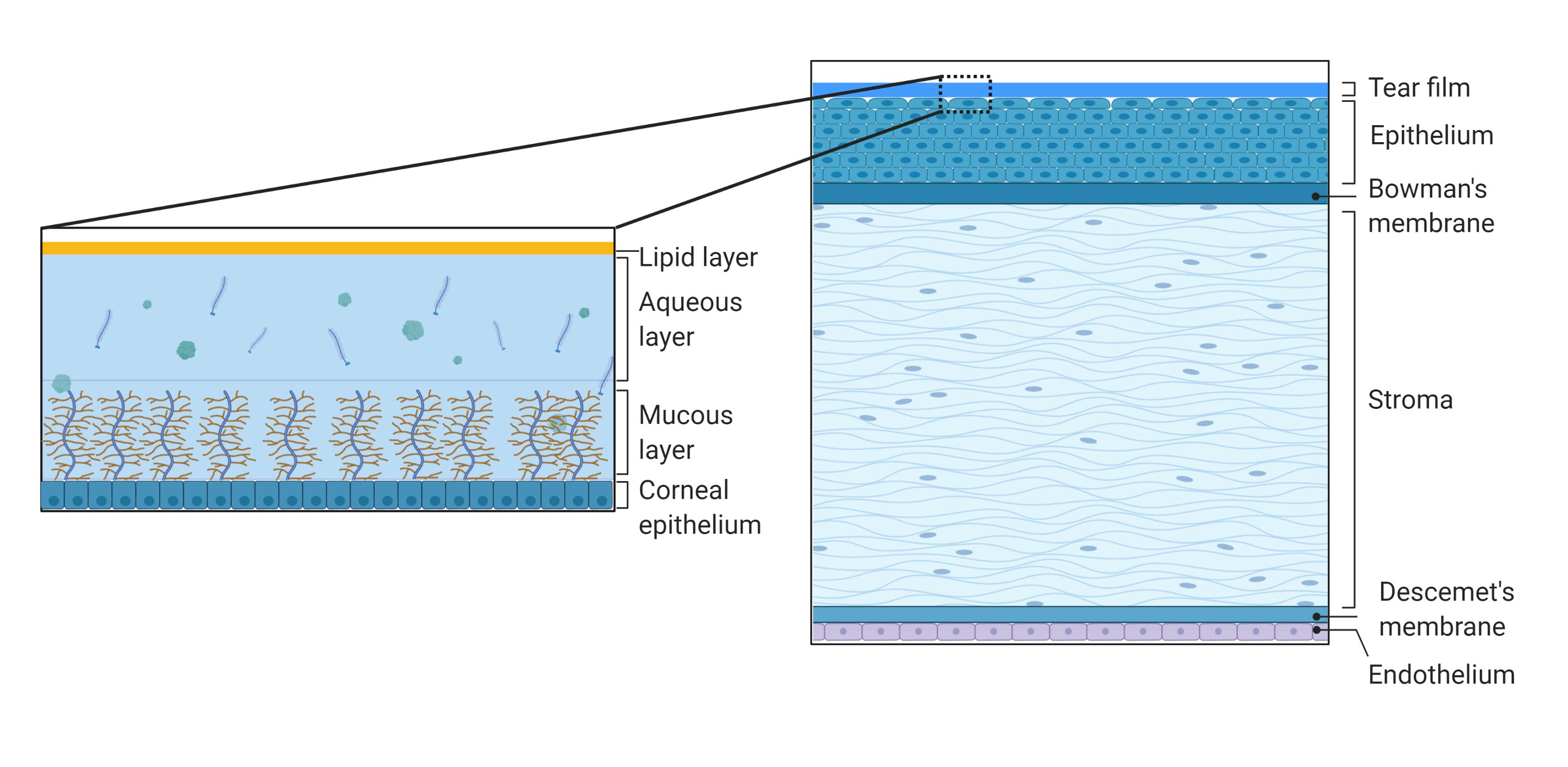
Figure 2. Schematic representation of the structure and composition of the cornea and the tear film. The cornea consists of the tear film, epithelium, Bowman’s membrane, stroma, Descemet’s membrane, and inner endothelium. The tear film consists of outer lipid phase, intermediate aqueous phase, and mucus. Created with BioRender.com.
2. Polymeric Nanoparticles
3. Polymeric Nanomicelles
Nanomicelles are composed of amphiphilic molecules (surfactants or block copolymers) that self-organize in an aqueous solution to form organized supramolecular structures [29][30][31]. During micellization, hydrophobic segments join to form a core region, while hydrophilic segments form a hydrophilic shell of micelles. Micelles of various sizes (10–1000 nm) and shapes can be obtained depending on the molecular weight of the polymer forming the core and crown-forming blocks. Micelle structure can be adapted to obtain unique properties considering delivery requirements, e.g., prolonged stability of micelles in tear fluid to increase the contact time with the cornea. A small particle size, ease of preparation, a high ability to encapsulate a drug made nanomicellar technology popular for ocular drug delivery [32][33]. Recently, nanomicellar approaches have been used with both polymer and surfactant to deliver locally small molecules as well as genes to the anterior segment of the eye [29][34].
In particular, most of the polymeric micelles used in drug delivery consist of amphiphilic di-block (hydrophilic-hydrophobic) polymers, tri-block (hydrophilic-hydrophobic-hydrophilic) polymers, graft (hydrophilic-hydrophobic), and ionic (hydrophilic-ionic) copolymers. For the majority of these systems, PEG is the primary hydrophilic segment. Also, the hydrophilic outer part of polymeric micelles can be composed of poly(ethylene oxide) (PEO), poly (acryloylmorpholine), poly(trimethylene carbonate), or poly(vinylpyrrolidone). Block copolymers such as PEO-poly(L-amino acids) provide an additional advantage to modify the properties of core-forming blocks. For example, the functional groups of the block are capable of chemical conjugation with a drug allowing efficient delivery of therapeutic doses. The hydrophobic core of polymeric micelles is usually made up of Pluronics (poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide)), poly(L-amino acids), such as poly(L-aspartate) and poly(L-glutamate), polyesters, such as poly(glycolic acid, PLA, PLGA, and PCL.
4. Inorganic Particles
5. In Situ Hydrogels as an Additional Carrier of Drug-Loaded Nanoparticles
6. Potential Ocular Nanomedicine
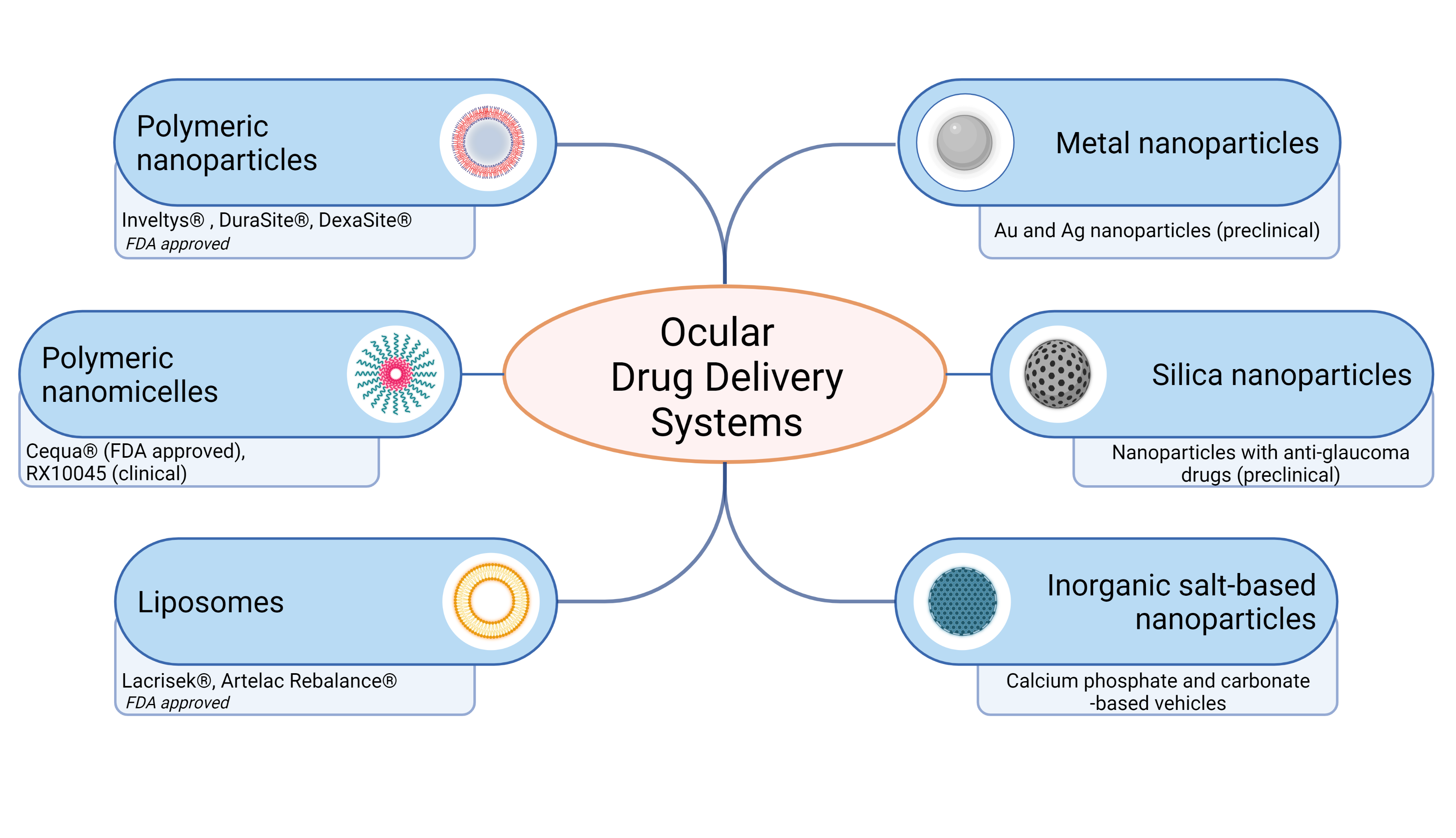
Figure 3. Schematic representation of ocular drug delivery systems for topical administration. Recently FDA approved and developed ocular drug systems presented. Created with BioRender.com
7. Conclusions
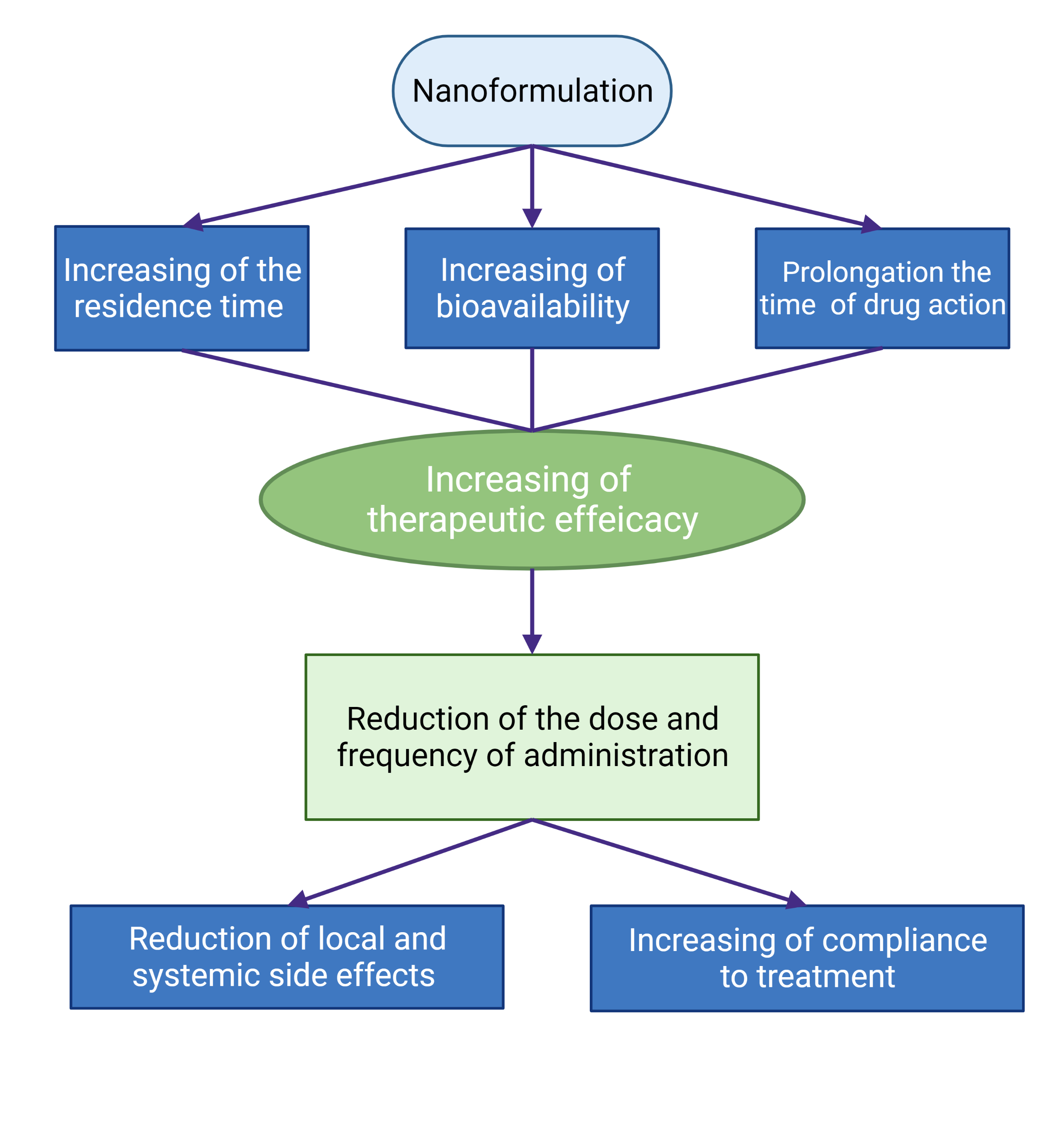
Figure 4. Schematic presentation of the advantages of the use of nanoformulations of ophthalmic drugs.
References
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342.
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287.
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216.
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47.
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223.
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457.
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22.
- Kompella, U.B.; Kadam, R.S.; Lee, V.H. Recent advances in ophthalmic drug delivery. Ther. Deliv. 2010, 1, 435–456.
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754.
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617.
- Watsky, M.A.; Jablonski, M.M.; Edelhauser, H.F. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr. Eye Res. 1988, 7, 483–486.
- Ramsay, E.; Ruponen, M.; Picardat, T.; Tengvall, U.; Tuomainen, M.; Auriola, S.; Toropainen, E.; Urtti, A.; del Amo, E.M. Impact of chemical structure on conjunctival drug permeability: Adopting porcine conjunctiva and cassette dosing for construction of in silico model. J. Pharm. Sci. 2017, 106, 2463–2471.
- Ahmed, I.; Gokhale, R.D.; Shah, M.V.; Patton, T.F. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J. Pharm. Sci. 1987, 76, 583–586.
- Srinivasarao, D.A.; Lohiya, G.; Katti, D.S. Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1548.
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141.
- Lakhani, P.; Patil, A.; Majumdar, S. Recent advances in topical nano drug-delivery systems for the anterior ocular segment. Ther. Deliv. 2018, 9, 137–153.
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent advances in the design of topical ophthalmic delivery systems in the treatment of ocular surface inflammation and their biopharmaceutical evaluation. Pharmaceutics 2020, 12, 570.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403.
- Nagarwal, R.C.; Kant, S.; Singh, P.N.N.; Maiti, P.; Pandit, J.K.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13.
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in biodegradable nano-sized polymer-based ocular drug delivery. Polymers 2019, 11, 1371.
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855.
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830.
- Losa, C.; Calvo, P.; Castro, E.; Vila-Jato, J.L.; Alonso, M.J. Improvement of ocular penetration of amikacin sulphate by association to poly(butylcyanoacrylate) nanoparticles. J. Pharm. Pharmacol. 2011, 43, 548–552.
- Marchal-Heussler, L.; Maincent, P.; Hoffman, M.; Spittler, J.; Couvreur, P. Antiglaucomatous activity of betaxolol chlorhydrate sorbed onto different isobutylcyanoacrylate nanoparticle preparations. Int. J. Pharm. 1990, 58, 115–122.
- Müller, R.H.; Lherm, C.; Herbert, J.; Couvreur, P. In vitro model for the degradation of alkylcyanoacrylate nanoparticles. Biomaterials 1990, 11, 590–595.
- Pignatello, R.; Bucolo, C.; Spedalieri, G.; Maltese, A.; Puglisi, G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials 2002, 23, 3247–3255.
- Giannavola, C.; Bucolo, C.; Maltese, A.; Paolino, D.; Vandelli, M.A.; Puglisi, G.; Lee, V.H.L.; Fresta, M. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm. Res. 2003, 20, 584–590.
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic acid in ocular drug delivery. Carbohydr. Polym. 2021, 264, 118006.
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116.
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268.
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.-I. Recent advances in nanomicelles delivery systems. Nanomaterials 2020, 11, 70.
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the noninvasive delivery of ocular therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413.
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising ocular drug carriers for anterior and posterior segment diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341.
- Özsoy, Y.; Güngör, S.; Kahraman, E.; Durgun, M.E. Polymeric micelles as a novel carrier for ocular drug delivery. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–117.
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327.
- Apaolaza, P.S.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dünker, N.; Göpferich, A. Hyaluronic acid coating of gold nanoparticles for intraocular drug delivery: Evaluation of the surface properties and effect on their distribution. Exp. Eye Res. 2020, 198.
- Masse, F.; Desjardins, P.; Ouellette, M.; Couture, C.; Omar, M.M.; Pernet, V.; Guérin, S.; Boisselier, E. Synthesis of ultrastable gold nanoparticles as a new drug delivery system. Molecules 2019, 24, 2929.
- Salem, H.F.; Ahmed, S.M.; Omar, M.M. Liposomal flucytosine capped with gold nanoparticle formulations for improved ocular delivery. Drug Des. Devel. Ther. 2016, 10, 277–295.
- Pereira, D.V.; Petronilho, F.; Pereira, H.R.S.B.; Vuolo, F.; Mina, F.; Possato, J.C.; Vitto, M.F.; de Souza, D.R.; da Silva, L.; Paula, M.M.d.S.; et al. Effects of gold nanoparticles on endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8036–8041.
- Nguyen, D.D.; Luo, L.J.; Lai, J.Y. Toward understanding the purely geometric effects of silver nanoparticles on potential application as ocular therapeutics via treatment of bacterial keratitis. Mater. Sci. Eng. C 2021, 119, 111497.
- Anbukkarasi, M.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In vitro antioxidant and anticataractogenic potential of silver nanoparticles biosynthesized using an ethanolic extract of Tabernaemontana divaricata leaves. Biomed. Pharmacother. 2017, 91, 467–475.
- MacCarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic applications of cerium oxide nanoparticles. J. Ocul. Pharmacol. Ther. 2020, 36, 376–383.
- Khorrami, M.B.; Sadeghnia, H.R.; Pasdar, A.; Ghayour-Mobarhan, M.; Riahi-Zanjani, B.; Hashemzadeh, A.; Zare, M.; Darroudi, M. Antioxidant and toxicity studies of biosynthesized cerium oxide nanoparticles in rats. Int. J. Nanomed. 2019, 14, 2915–2926.
- Chen, X.; Zhu, S.; Hu, X.; Sun, D.; Yang, J.; Yang, C.; Wu, W.; Li, Y.; Gu, X.; Li, M.; et al. Toxicity and mechanism of mesoporous silica nanoparticles in eyes. Nanoscale 2020, 12, 13637–13653.
- Kim, S.N.; Ko, S.A.; Park, C.G.; Lee, S.H.; Huh, B.K.; Park, Y.H.; Kim, Y.K.; Ha, A.; Park, K.H.; Choy, Y. Bin Amino-functionalized mesoporous silica particles for ocular delivery of brimonidine. Mol. Pharm. 2018, 15, 3143–3152.
- Nikolskaya, I.I.; Beznos, O.V.; Eltsov, A.I.; Gachok, I.V.; Chesnokova, N.B.; Varlamov, V.P.; Kost, O.A. The inclusion of timolol and lisinopril in calcium phosphate particles covered by chitosan: Application in ophthalmology. Vestn. Mosk. Univ. 2018, 59, 170–176.
- Shimanovskaya, E.V.; Nikol’skaya, I.I.; Binevskii, P.V.; Klyachko, N.L.; Kost, O.A.; Beznos, O.V.; Pavlenko, T.A.; Chesnokova, N.B. Lisinopril in the composition of calcium phosphate nanoparticles as a promising antiglaucoma agent. Nanotechnol. Russ. 2014, 9, 219–226.
- Binevski, P.V.; Balabushevich, N.G.; Uvarova, V.I.; Vikulina, A.S. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids Surf. B Biointerfaces 2019, 181, 437–449.
- Bruschi, M.L.; de Toledo, L.d.A.S. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry 2019, 5, 50.
- Wang, Y.; Xia, R.; Hu, H.; Peng, T. Biosynthesis, characterization and cytotoxicity of gold nanoparticles and their loading with N-acetylcarnosine for cataract treatment. J. Photochem. Photobiol. B Biol. 2018, 187, 180–183.
- Li, Y.-J.; Luo, L.-J.; Harroun, S.G.; Wei, S.-C.; Unnikrishnan, B.; Chang, H.-T.; Huang, Y.-F.; Lai, J.-Y.; Huang, C.-C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale 2019, 11, 5580–5594.
- Hendiger, E.B.; Padzik, M.; Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Rizo-Liendo, A.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Chiboub, O.; Rodríguez-Expósito, R.L.; et al. Silver nanoparticles as a novel potential preventive agent against acanthamoeba keratitis. Pathogens 2020, 9, 350.
- Mathew, T.V.; Kuriakose, S. Photochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groups. Mater. Sci. Eng. C 2013, 33, 4409–4415.
- Yang, T.; Yao, Q.; Cao, F.; Liu, Q.; Liu, B.; Wang, X.-H. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: Insight into the cytotoxicity and antiangiogenesis. Int. J. Nanomed. 2016, 11, 6679–6692.
- Zhang, Y.J.; Wang, Z.Y.; Zhao, G.; Liu, J.X. Silver nanoparticles affect lens rather than retina development in zebrafish embryos. Ecotoxicol. Environ. Saf. 2018, 163, 279–288.
- Kim, J.S.; Song, K.S.; Sung, J.H.; Ryu, H.R.; Choi, B.G.; Cho, H.S.; Lee, J.K.; Yu, I.J. Genotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticles. Nanotoxicology 2012, 7, 953–960.
- Wong, L.L.; Hirst, S.M.; Pye, Q.N.; Reilly, C.M.; Seal, S.; McGinnis, J.F. Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PLoS ONE 2013, 8, e58431.
- Baldim, V.; Yadav, N.; Bia, N.; Graillot, A.; Loubat, C.; Singh, S.; Karakoti, A.S.; Berret, J.-F. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42056–42066.
- Cai, X.; Seal, S.; McGinnis, J.F. Non-toxic retention of nanoceria in murine eyes. Mol. Vis. 2016, 22, 1176.
- Liao, Y.-T.; Lee, C.-H.; Chen, S.-T.; Lai, J.-Y.; Wu, K.C.-W. Gelatin-functionalized mesoporous silica nanoparticles with sustained release properties for intracameral pharmacotherapy of glaucoma. J. Mater. Chem. B 2017, 5, 7008–7013.
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2014, 45, 644–658.
- Zhao, Y.; Du, W.; Sun, L.; Yu, L.; Jiao, J.; Wang, R. Facile synthesis of calcium carbonate with an absolutely pure crystal form using 1-butyl-3-methylimidazolium dodecyl sulfate as the modifier. Colloid Polym. Sci. 2013, 291, 2191–2202.
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 167.
- Borodina, T.N.; Trushina, D.B.; Marchenko, I.V.; Bukreeva, T.V. Calcium carbonate-based mucoadhesive microcontainers for intranasal delivery of drugs bypassing the blood–brain barrier. Bionanoscience 2016, 6, 261–268.
- Balabushevich, N.G.; Kovalenko, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Mucin adsorption on vaterite CaCO3 microcrystals for the prediction of mucoadhesive properties. J. Colloid Interface Sci. 2019, 545, 330–339.
- Pitorre, M.; Gondé, H.; Haury, C.; Messous, M.; Poilane, J.; Boudaud, D.; Kanber, E.; Rossemond Ndombina, G.A.; Benoit, J.P.; Bastiat, G. Recent advances in nanocarrier-loaded gels: Which drug delivery technologies against which diseases? J. Control. Release 2017, 266, 140–155.
- Upadhayay, P.; Kumar, M.; Pathak, K. Norfloxacin loaded pH triggered nanoparticulate in-situ gel for extraocular bacterial infections: Optimization, ocular irritancy and corneal toxicity. Iran. J. Pharm. Res. 2016, 15, 3–22.
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15.
- Singh, J.; Chhabra, G.; Pathak, K. Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: In vitro, ex vivo evaluation and pharmacodynamic study. Drug Dev. Ind. Pharm. 2014, 40, 1223–1232.
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as “smart” carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402.
- Patil, P.R.; Shaikh, S.S.; Shivsharan, K.J.; Shahi, S.R. In situ: A novel drug delivery system. Indo Am. J. Pharm. Res. 2014, 4, 5406–5414.
- Deka, M.; Ahmed, A.B.; Chakraborty, J. Development, evaluation and characteristics of ophthalmic in situ gel system: A Review. Int. J. Curr. Pharm. Res. 2019, 11, 47–53.
- Qian, Y.; Wang, F.; Li, R.; Zhang, Q.; Xu, Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev. Ind. Pharm. 2010, 36, 1340–1347.
- Asasutjarit, R.; Thanasanchokpibull, S.; Fuongfuchat, A.; Veeranondha, S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int. J. Pharm. 2011, 411, 128–135.
- Li, J.; Liu, H.; Liu, L.L.; Cai, C.N.; Xin, H.X.; Liu, W. Design and evaluation of a brinzolamide drug-resin in situ thermosensitive gelling system for sustained ophthalmic drug delivery. Chem. Pharm. Bull. 2014, 62, 1000–1008.
- Maddiboyina, B.; Jhawat, V.; Desu, P.K.; Gandhi, S.; Nakkala, R.K.; Singh, S. Formulation and evaluation of thermosensitive flurbiprofen in situ nano gel for the ocular delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 1584–1597.
- Schopf, L.; Enlow, E.; Popov, A.; Bourassa, J.; Chen, H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol. Ther. 2014, 3, 63–72.
- Kim, T.; Sall, K.; Holland, E.; Brazzell, R.K.; Coultas, S.; Gupta, P.K. Safety and efficacy of twice daily administration of KPI-121 1% for ocular inflammation and pain following cataract surgery. Clin. Ophthalmol. 2018, 13, 69–86.




