
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Indra Prasad Subedi | + 1917 word(s) | 1917 | 2021-12-17 07:18:37 | | | |
| 2 | Beatrix Zheng | + 130 word(s) | 2047 | 2021-12-23 02:13:16 | | |
Video Upload Options
Seventy ant species from thirty-six genera and six subfamilies were recorded from eastern, central, and western regions of Nepal using vegetation beating, sweeping, and hand collection methods. The research also discovered five genera and nine species new for the country, as well as eight tramp species, four of which are major ecological, agricultural, and/or household pests. Ant diversity was found to decrease with increasing elevation. The assessment of ant diversity using multiple sampling methods that cover all seasons and forest types may be useful in obtaining complete ant diversity data. Early intervention through sustainable forest management initiatives would aid in preventing invasive ant incursions in the forests of Nepal.
1. Introduction
2. Current Insights
2.1. Ant Species Diversity
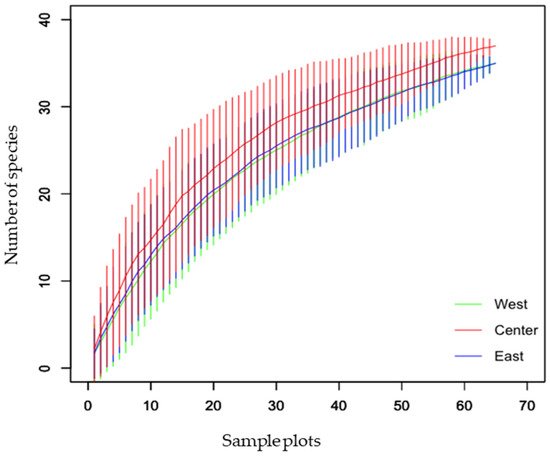
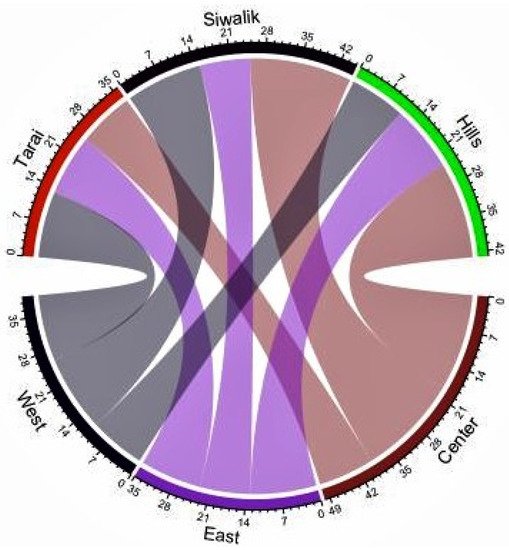
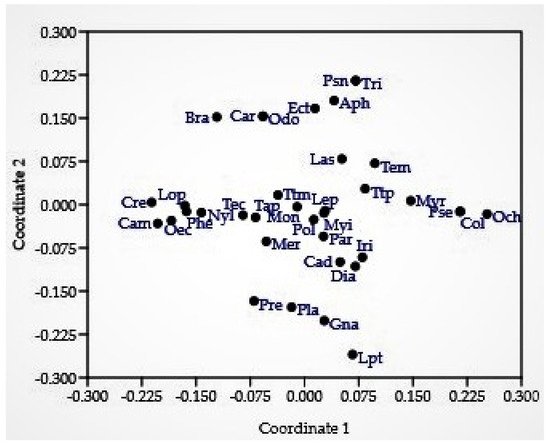
2.2. Distribution of Ants in Nepal
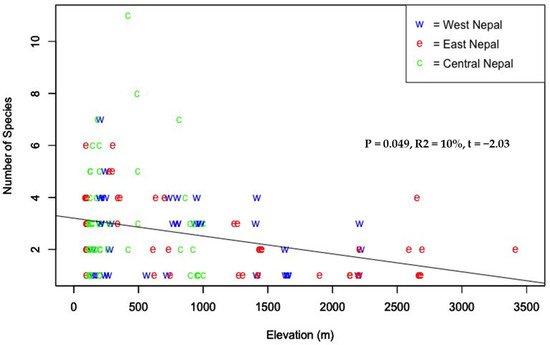
2.3. Ants and Forest Health
References
- Guénard, B. An overview of the species and ecological diversity of ants. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2013.
- Thurman, J.H.; Northfield, T.D.; Snyder, W.E. Weaver ants provide ecosystem services to tropical tree crops. Front. Ecol. Evol. 2019, 7, 120.
- Ohyama, L.; King, J.R.; Jenkins, D.G. Are tiny subterranean ants top predators affecting aboveground ant communities? Ecology 2020, 101, e03084.
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244.
- Frouz, J.; Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199.
- Kovář, P.; Vojtíšek, P.; Zentsová, I. Ants as ecosystem engineers in natural restoration of human made habitats. J. Landsc. Ecol. 2013, 6, 18–31.
- Warren, R.J.; Giladi, I. Ant-mediated seed dispersal: A few ant species (Hymenoptera: Formicidae) benefit many plants. Myrmecol. News 2014, 20, 129–140.
- Anjos, D.V.; Andersen, A.N.; Carvalho, R.L.; Sousa, R.M.; Del-Claro, K. Switching roles from antagonist to mutualist: A harvester ant as a key seed disperser of a myrmecochorous plant. Ecol. Entomol. 2020, 45, 1063–1070.
- Offenberg, J.; Cuc, N.T.T.; Wiwatwitaya, D. The effectiveness of weaver ant (Oecophylla smaragdina) biocontrol in Southeast Asian citrus and mango. Asian Myrmecol. 2013, 5, 139–149.
- Wetterer, J.K. Geographic distribution of the weaver ant Oecophylla smaragdina. Asian Myrmecol. 2017, 9, e009004.
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12.
- Wilkie, K.T.R.; Mertl, A.L.; Traniello, J.F. Diversity of ground-dwelling ants (Hymenoptera: Formicidae) in primary and secondary forests in Amazonian Ecuador. Myrmecol. News 2009, 12, 139–147.
- Stephens, S.S.; Wagner, M.R. Using ground foraging ant (Hymenoptera: Formicidae) functional groups as bioindicators of forest health in northern Arizona ponderosa pine forests. Environ. Entomol. 2006, 35, 937–949.
- Majer, J.D.; Orabi, G.; Bisevac, L. Ants (Hymenoptera: Formicidae) pass the bioindicator scorecard. Myrmecol. News 2007, 10, 69–76.
- Marathe, A.; Priyadarsanan, D.R.; Krishnaswamy, J.; Shanker, K. Spatial and climatic variables independently drive elevational gradients in ant species richness in the Eastern Himalaya. PLoS ONE 2020, 15, e0227628.
- Bharti, H.; Sharma, Y.P.; Bharti, M.; Pfeiffer, M. Ant species richness, endemicity and functional groups, along an elevational gradient in the Himalayas. Asian Myrmecol. 2013, 5, 79–101.
- Subedi, I.P.; Budha, P.B.; Bharti, H.; Alonso, L. An updated checklist of Nepalese ants (Hymenoptera, Formicidae). ZooKeys 2020, 1006, 99–136.
- Subedi, I.P.; Budha, P.B.; Bharti, H.; Alonso, L.; Yamane, S. First Record of the Ant Subgenus Orthonotomyrmex of the Genus Camponotus from Nepal (Hymenoptera, Formicidae). Zoodiversity 2021, 55, 279–284.
- Forel, A. Les fourmis de l’Himalaya. Bull. De La Société Vaud. Des. Sci. Nat. 1906, 42, 79–94.
- Collingwood, C. Formicidae (Hymenoptera: Aculeata) from Nepal. Khumbu Himal 1970, 3, 371–387.
- Lindenmayer, D.; Franklin, J.; Fischer, J. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol. Conserv. 2006, 131, 433–445.
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D.B. Indicators of biodiversity for ecologically sustainable forest management. Conserv. Biol. 2000, 14, 941–950.
- Junninen, K.; Penttilä, R.; Martikainen, P. Fallen retention aspen trees on clear-cuts can be important habitats for red-listed polypores: A case study in Finland. Biodivers. Conserv. 2007, 16, 475–490.
- Ohsawa, M. Species richness and composition of Curculionidae (Coleoptera) in a conifer plantation, secondary forest, and old-growth forest in the central mountainous region of Japan. Ecol. Res. 2005, 20, 632–645.
- Hartley, M.J. Rationale and methods for conserving biodiversity in plantation forests. For. Ecol. Manag. 2002, 155, 81–95.
- Ferris, R.; Humphrey, J. A review of potential biodiversity indicators for application in British forests. Forestry 1999, 72, 313–328.
- Majer, J. Ants: Bio-indicators of minesite rehabilitation, land-use, and land conservation. Environ. Manage 1983, 7, 375–383.
- Hoffmann, B.D.; Griffiths, A.D.; Andersen, A.N. Responses of ant communities to dry sulfur deposition from mining emissions in semi-arid tropical Australia, with implications for the use of functional groups. Austral Ecol. 2000, 25, 653–663.
- Agosti, D.; Alonso, L.E. The ALL protocol: A standard protocol for the collection of ground-dwelling ants. In Ants. Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L.E., Schultz, T.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 204–206.
- Alonso, L.E. Ants as indicators of diversity. In Ants. Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L.E., Schultz, T.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 80–88.
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182.
- Ribas, C.R.; Schoereder, J.H. Ant communities, environmental characteristics and their implications for conservation in the Brazilian Pantanal. Biodivers.Conserv. 2007, 16, 1511–1520.
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233.
- Pfeiffer, M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E.; Kohout, R.J. The Formicidae of Borneo (Insecta: Hymenoptera): A preliminary species list. Asian Myrmecol. 2011, 4, 9–58.
- Fellowes, J.R. Ant (Hymenoptera: Formicidae) genera in southern China: Observations on the Oriental-Palaearctic boundary. Myrmecol. Nachr. 2006, 8, 239–249.
- Liu, C.; Guénard, B.; Hita Garcia, F.; Yamane, S.; Blanchard, B.; Yang, D.; Economo, E. New records of ant species from Yunnan, China. ZooKeys 2015, 477, 17–78.
- Bharti, H.; Wachkoo, A.A.; Kumar, R. First inventory of ants (Hymenoptera: Formicidae) in northwestern Shivalik, India. Halteres 2017, 8, 33–68.
- Fontanilla, A.M.; Nakamura, A.; Xu, Z.; Cao, M.; Kitching, R.L.; Tang, Y.; Burwell, C.J. Taxonomic and functional ant diversity along tropical, subtropical, and subalpine elevational transects in Southwest China. Insects 2019, 10, 128.
- Wachkoo, A.A.; Akbar, S.A.; Jan, U.; Shah, G.M. Taxonomic inventory of ants (Hymenoptera: Formicidae) in Jammu and Kashmir state. In Biodiversity of the Himalaya: Jammu and Kashmir State; Springer Nature: Basingstoke, UK, 2020; pp. 733–747.
- Wilson, E.O. Which are the most prevalent ant genera. Studia Entomol. 1976, 19, 187–200.
- Offenberg, J. Ants as tools in sustainable agriculture. J. Appl. Ecol. 2015, 52, 1197–1205.
- Subedi, I.P.; Budha, P.B. Diversity and distribution patterns of ants along elevational gradients. Nepal. J. Zool. 2020, 4, 44–49.
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239.
- Brühl, C.A.; Mohamed, M.; Linsenmair, K.E. Altitudinal distribution of leaf litter ants along a transect in primary forests on Mount Kinabalu, Sabah, Malaysia. J. Trop. Ecol. 1999, 15, 265–277.
- Karaman, M.G. Zoogeography, diversity and altitudinal distribution of ants (Hymenoptera: Formicidae) in the Mediterranean and the oro-Mediterranean parts of Montenegro. North West J. Zool. 2011, 7, 26–34.
- Burwell, C.J.; Nakamura, A. Distribution of ant species along an altitudinal transect in continuous rainforest in subtropical Queensland, Australia. Mem. Qld. Mus. 2011, 55, 391–411.
- Longino, J.T.; Colwell, R.K. Density compensation, species composition, and richness of ants on a neotropical elevational gradient. Ecosphere 2011, 2, 1–20.
- Yusah, K.M.; Turner, E.C.; Yahya, B.E.; Fayle, T.M. An elevational gradient in litter-dwelling ant communities in Imbak Canyon, Sabah, Malaysia. J. Trop. Biol. Conserv. JTBC 2012, 9, 192–199.
- Liu, C.; Dudley, K.L.; Xu, Z.H.; Economo, E.P. Mountain metacommunities: Climate and spatial connectivity shape ant diversity in a complex landscape. Ecography 2018, 41, 101–112.
- Bhattarai, K.R.; Vetaas, O.R. Can Rapoport’s rule explain tree species richness along the Himalayan elevation gradient, Nepal? Diver. Distrib. 2006, 12, 373–378.
- Acharya, K.P.; Vetaas, O.R.; Birks, H. Orchid species richness along Himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833.
- Manish, K.; Pandit, M.K. Phylogenetic diversity, structure and diversification patterns of endemic plants along the elevational gradient in the Eastern Himalaya. Plant Ecol. Divers. 2018, 11, 501–513.
- Grau, O.; Grytnes, J.A.; Birks, H. A comparison of altitudinal species richness patterns of bryophytes with other plant groups in Nepal, Central Himalaya. J. Biogeogr. 2007, 34, 1907–1915.
- Baniya, C.B.; Solhøy, T.; Gauslaa, Y.; Palmer, M.W. The elevation gradient of lichen species richness in Nepal. Lichenologist 2010, 42, 83–96.
- Wetterer, J.K. Geographic origin and spread of cosmopolitan ants (Hymenoptera: Formicidae). Halteres 2015, 6, 66–78.
- Wetterer, J.K. Worldwide spread of the longhorn crazy ant, Paratrechina longicornis (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 137–149.
- McGlynn, T.P. The worldwide transfer of ants: Geographical distribution and ecological invasions. J. Biogeogr. 1999, 26, 535–548.
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146.
- Gorb, S.N.; Gorb, E.V. Effects of ant species composition on seed removal in deciduous forest in eastern Europe. Oikos 1999, 84, 110–118.
- Wike, L.D.; Martin, F.D.; Paller, M.H.; Nelson, E.A. Impact of forest seral stage on use of ant communities for rapid assessment of terrestrial ecosystem health. J. Insect Sci. 2010, 10, 77.
- Brener, A.G.F.; Silva, J.F. Leaf-cutting ant nests and soil fertility in a well-drained savanna in western Venezuela. Biotropica 1995, 17, 250–254.
- Knoepp, J.D.; Coleman, D.C.; Crossley, D., Jr.; Clark, J.S. Biological indices of soil quality: An ecosystem case study of their use. For. Ecol. Manag. 2000, 138, 357–368.
- Roychoudhury, N. New record of natural enemies of sal heartwood borer, Hoplocerambyx spinicornis newman (Coleoptera: Cerambycidae). Pestology 2017, 41, 33–38.
- Lach, L. Invasive ants: Unwanted partners in ant-plant interactions? Ann. Mo. Bot. Gard. 2003, 90, 91–108.
- Verdinelli, M.; Yakhlef, S.E.B.; Cossu, C.S.; Pilia, O.; Mannu, R. Variability of ant community composition in cork oak woodlands across the Mediterranean region: Implications for forest management. iForest 2017, 10, 707.
- Hoffmann, B.D.; Luque, G.M.; Bellard, C.; Holmes, N.D.; Donlan, C.J. Improving invasive ant eradication as a conservation tool: A review. Biol. Conserv. 2016, 198, 37–49.
- MoFE Nepal National REDD+ Strategy (2018–2022); Ministry of Forests and Environment, Government of Nepal, Singh Durbar: Kathmandu, Nepal, 2018.
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. Insect Ecology: Behavior, Populations and Communities; Cambridge University Press: Cambridge, UK, 2011; p. 828.
- Rossi, N.; Feldhaar, H. Carpenter ants. In Encyclopedia of Social Insects; Springer: Cham, Switzerland, 2020; pp. 973–978.
- Malla, R.; Pokharel, K.K. Forest Pests and Pathogens Problem in Different Forest Types of Nepal; Department of Forest Research and Survey: Kathmandu, Nepal, 2018.
- Finley, K.; Chhin, S. Forest health management and detection of invasive forest insects. Resources 2016, 5, 18.




