Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Przemysław Piech | + 1577 word(s) | 1577 | 2021-12-13 03:58:37 | | | |
| 2 | Amina Yu | + 141 word(s) | 1718 | 2021-12-23 02:16:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Piech, P. Bitterling (Rhodeus amarus) Larvae and Fry. Encyclopedia. Available online: https://encyclopedia.pub/entry/17455 (accessed on 07 February 2026).
Piech P. Bitterling (Rhodeus amarus) Larvae and Fry. Encyclopedia. Available at: https://encyclopedia.pub/entry/17455. Accessed February 07, 2026.
Piech, Przemysław. "Bitterling (Rhodeus amarus) Larvae and Fry" Encyclopedia, https://encyclopedia.pub/entry/17455 (accessed February 07, 2026).
Piech, P. (2021, December 22). Bitterling (Rhodeus amarus) Larvae and Fry. In Encyclopedia. https://encyclopedia.pub/entry/17455
Piech, Przemysław. "Bitterling (Rhodeus amarus) Larvae and Fry." Encyclopedia. Web. 22 December, 2021.
Copy Citation
European bitterling (Rhodeus amarus Bloch) and Rhodeus meridionalis belong to the group of ostrakophilous fish. The embryonic and larval development of the fish in this reproductive group until the time of the yolk sac resorption takes place in the gill cavity of river mussels (Anodonta sp. or Unio sp.)
bitterling
controlled conditions
fry
larvae
rearing
1. Introduction
The majority of over two dozen species of the genus Rhodeus described in the literature occur in Asia. There are only two bitterling species living in Europe: Rhodeus meridionalis found only in the Balkans, and Rhodeus amarus. Rhodeus amarus, inhabiting the majority of freshwaters of Europe, including Poland [1][2][3][4]. Currently, the bitterling is regarded as a native species in a large part of its distribution range in Europe. However, it had only occurred in the Ponto-Caspian region before the year 1100. The earliest mention of the bitterling in Western and Central Europe come from regions where carp breeding was common and bitterling spread along with the gradual carp breeding expansion. After the initial expansion period, the species practically disappeared from Europe during the coldest part of the Little Ice Age. Bitterlings reappeared in the late 18th century. Their presence was recorded in historical carp breeding centres. The bitterling became a common species in Europe after the mid-19th century. It did not reach the current range until the 20th century. This was associated with intensive bivalve expansion, temperature increase and anthropogenic changes in the environment, which all favoured bitterlings. Bitterlings are found most frequently in the littoral zone of lakes, shallow oxbow lakes, and in slow-flowing rivers, where they can be found in groups of several or even several hundred individuals. However, their presence depends on the occurrence of mussels: Unio sp. and Anodonta sp. [5][6][7]. Bivalves are indispensable for bitterling reproduction because the fish spawn can be incubated only in the gill cavities of mussels [8][9][10][11][12]. Bitterlings become sexually mature in second year of life under natural conditions [2][6]. One female bitterling with a mean length of 5–6 cm can lay several dozen to several hundred grains of large, non-sticky spawn into the gill cavities of several mussels. The number of eggs is closely related to the female size [7][12]. Even several dozen eggs can be laid into one mussel. According to literature reports, as many as 200 bitterling embryos can be found in one bivalve [9]. The water temperature ranges from 17 °C to 26 °C during the spawning period. Bitterlings develop inside a bivalve after fertilization. Larvae swim out of their incubators after 3–4 weeks (depending on the water temperature) band form shoals of several to several hundred larvae very soon thereafter [13][14]. They prefer shallow places, with dense vegetation, where they are not exposed to attacks of predators, i.e., other fish and aquatic insects, larvae and adults, such as great diving beetle Dytiscus marginalis, the beetle Cybister lateralimarginalis [15], or dragonfly larvae Anax sp. of the family Aeshnidae [16]. The bitterling population size dropped in 1960–1980 in Western and Central Europe, mainly due to water pollution. The decrease was so significant that strict national and international regulations aimed at the species conservation were implemented. The bitterling was entered on the list of endangered species and is now covered by strict all-year protection [17]. Currently, the bitterling existence in Polish waters is not endangered, but the species is protected all the same. This is due to the unsatisfactory status of bitterling populations in other European countries. A decrease in the bivalve populations (their natural incubators) is the greatest threat to bitterlings. Bivalves are susceptible to natural aquatic environment pollution, and since they live a sedentary lifestyle, they cannot “swim away” from polluted places.
2. Rearing of Bitterling (Rhodeus amarus) Larvae and Fry under Controlled Conditions for the Restitution of Endangered Populations
Bitterling larvae at the onset of active swimming were 8.6 mm (±0.1) long on average. During the first stage of rearing, which lasted for 45 days, very good results were obtained, in terms of both the growth and survivability of bitterling larvae (Figure 1 and Figure 2).
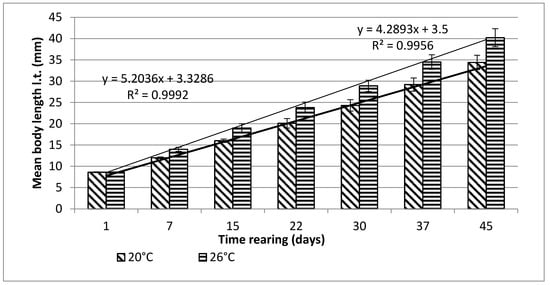
Figure 1. Stage I—Increase in the body length of bitterling larvae during rearing (45 days) in water at different temperatures. Data are shown as means ± SD.
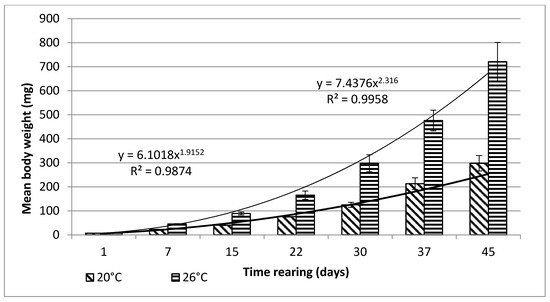
Figure 2. Stage I—Increase in the body weight of bitterling larvae during rearing (45 days) in water at different temperatures. Data are shown as means ± SD.
From day 7 of the rearing on, both mean body weights and mean body lengths of larvae kept in the water of 20 °C in temperature were statistically significantly different from those of the larvae maintained in the warmer water. From day 22 of the rearing, the differences in the mean body length between the individuals reared in the water of the temperature 20 °C and 26 °C remained basically on the same level, whereas differences in the body weight between these two groups increased exponentially. As soon as four weeks into the rearing, juvenile bitterlings kept in the warmer water resembled adult specimens in body shape. As the end of the first stage, bitterlings reared in the water of the temperature 26 °C reached the average body weight of 720.8 mg (±80.4) and the average body length of 40.2 mg (±2.1). Meanwhile, those kept in the warmer water, of the temperature of 20 °C, reached 298.5 mg (±32.2) of average weight and 34.4 mg (±1.67) of average length at the end of the rearing period. This meant they weighed nearly 2.5-fold less than their peers reared in the warmer water. Likewise, their rearing parameters, such as ITL, RGR, or the Fulton’s index, calculated on the basis of the measured body weights and lengths, were lower than the ones achieved for the bitterlings reared in the water of the temperature of 26 °C (Table 1). The increase in the total body length per unit of time (ITL) calculated for the bitterling fry from the water of the temperature of 20 °C and 26°C was 0.57 and 0.7 mm·d−1, respectively. The biomass of the fish reared in the warmer water at the end of the 1.5-month-long rearing period was 7.21 g·dm−3. Under the analysed thermal conditions, the RGR and RBR were higher for the fry maintained in the warmer water. During the second stage of rearing, which lasted for 150 days, the growth of bitterling fry in the water of the temperature of 26 °C was observed to gradually slow down. This was most probably caused by the fact that the fish achieved sexual maturity, which was manifested by the appearance of the mating robe. The growth of the bitterling fry in the colder water proceeded more rapidly, which is reflected by the computed values of rearing indices. At the end of the rearing period, bitterlings kept in the colder water decelerated their growth when reaching sexual maturity. Despite the initial differences in the body weight and length growth, no statistically significant differences were determined at the end of rearing between the fish originating from the two temperature regimes. At the end of the 6.5-month-long rearing period, the bitterlings from the water of the temperature of 20 °C reached the average body weight of 3242 mg (±427) at the average body length of 64.48 mm (±3.4). In the warmer water, they reached the average body weight of 3389 mg (±548) and the average body length of 66.2 mm (±3.0) (Figure 3 and Figure 4).
Table 1. The results of rearing bitterling Rhodeus amarus larvae and fry in two water temperature.
| Parameter | I STAGE (Time Rearing 1.5 Months) |
II STAGE (Time Rearing 5 Months) |
||
|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | |||
| 20 | 26 | 20 | 26 | |
| Initial mean body weight (mg) | 7 ± 0.23 a | 7 ± 0.24 a | 298 ± 45.8 a | 721 ± 97.55 b |
| Final mean body weight (mg) | 298 ± 32.2 a | 721 ± 80.4 b | 3242 ± 427 a | 3389 ± 548 a |
| Initial mean body length (mm) | 8.6 ± 0.11 a | 8.6 ± 0.12 a | 34.4 ± 0.63 a | 40.2 ± 1.98 b |
| Final mean body length (mm) | 34.4 ± 1.67 a | 40.2 ± 2.1 b | 64.48 ± 3.4 a | 66.2 ± 3.0 a |
| Initial stock (indiv.) | 200 | 200 | 200 | 200 |
| Final stock (indiv.) | 200 | 200 | 200 | 200 |
| Survival (%) | 100 | 100 | 100 | 100 |
| Time rearing (days) | 45 | 45 | 150 | 150 |
| Increase in total lenght (ITL) (mm d−1) | 0.57 ± 0.02 a | 0.70 ± 0.04 a | 0.20 ± 0.01 a | 0.17 ± 0.02 b |
| Relative growth rate (RGR) for weight (% d−1) | 8.69 ± 0.4 a | 10.85 ± 0.6 b | 1.60 ± 0.17 a | 1.03 ± 0.24 b |
| Relative growth rate (RGR) for length (% d−1) | 3.13 ± 0.1 a | 3.47 ± 0.2 b | 0.42 ± 0.02 a | 0.33 ± 0.04 b |
| Relative growth rate (RBR) for biomass (% d−1) | 8.69 ± 0.4 a | 10.85 ± 0.6 b | 1.60 ± 0.05 a | 1.03 ± 0.03 b |
| Biomass (g dm−3) | 2.98 ± 0.3 a | 7.21 ± 0.51 b | 10.81 ± 0.62 a | 11.3 ± 0.53 a |
| Fultona K | 0.73 ± 0.06 a | 1.11 ± 0.09 b | 1.21 ±0.3 a | 1.2 ± 0.2 b |
Mean value ± S.D. Results in rows from the same stage with the same index of letters are not statistically significantly different (p ≤ 0.05).
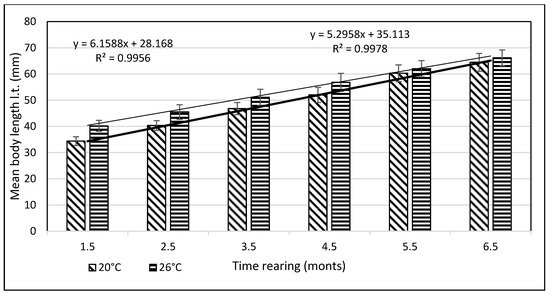
Figure 3. Stage II—Increase in the body length of bitterling fry during rearing (5 months) in water at different temperatures. Data are shown as means ± SD.
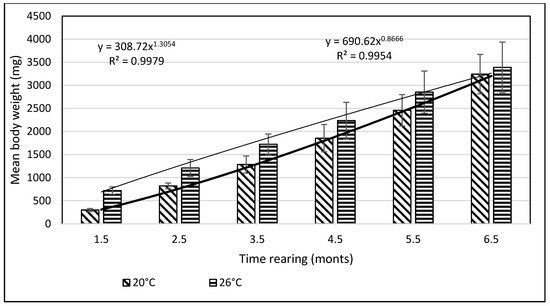
Figure 4. Stage II—Increase in the body weight of bitterling fry during rearing (5 months) in water at different temperatures. Data are shown as means ± SD.
The values of the rearing indices calculated at the end of the rearing period were approximately the same for both water temperature regimes. At that time, the body length increase per unit of time (ITL) calculated for the bitterling fry kept in water at 20 °C and 26 °C was 0.20 and 0.17 mm·d−1, respectively. The biomass of fish reared in the warmer water at the end of an over six-month rearing period was 11.3 g·dm−3. The values of such indicators as the RGR and RBR were higher for the bitterling reared in the cooler water. Throughout the entire rearing period, no fish losses were recorded in either temperature regime, despite the fact that the fry were periodically subjected to manipulations when taking the measurements of their body weight and length. Based on the results of these measurements during the first and second stage of the rearing period, a curve was plotted illustrating the dependence between the length and the weight of the fry. This curve is shown in Figure 5.
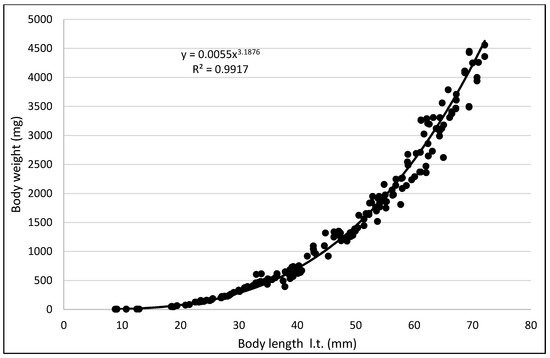
Figure 5. Relationship between length and weight of bitterling.
3. Conclusions
It is demonstrated that it is possible to rear the bitterling larvae and fry under controlled conditions. During the first stage of the experiment, the bitterling larvae reared for 45 days in the warmer water grew more rapidly. However, during the second stage, when the bitterling fry was reared for 150 days, the rate at which their weight and body length increased was much lower. At the end of rearing, both in the cooler and warmer water, the bitterling fish obtained were ready for reproduction, which was confirmed by the appearance of the mating robe. Throughout the entire rearing period, no losses were observed among the fry, despite the fact that they were periodically submitted to manipulations in order to take measurements of their body weight and length. The bitterling specimens reared during the experiment (during a 6.5-month long rearing period) can be an excellent stocking material in sites in which, for various reasons, populations of this fish have diminished drastically.
References
- Rhodeus Sericeus (Pallas 1776). Available online: https://www.fishbase.se/summary/2948 (accessed on 10 August 2021).
- Przybylski, M. Różanka. In Ryby Słodkowodne Polski; Brylińska, M., Ed.; Wyd. PWN: Warszawa, Poland, 2000; Volume 285, pp. 233–237.
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Berlin, Germany, 2007; p. 287.
- Konečná, M.; Smith, C.; Reichard, M. Population and individual consequences of breeding resource availability in the European bitterling (Rhodeus amarus). Behav. Ecol. Sociobiol. 2010, 64, 1069–1079.
- Wiepkema, P.R. An ethological analysis of the reproductive behaviour of the bitterling (Rhodeus amarus Bloch). Arch. Neerl. De Zoologie. 1961, 14, 103–199.
- Przybylski, M.; Reichard, M.; Spence, R.; Smith, C. Spatial distribution of oviposition sites determines variance in the reproductive rate of European bitterling (Rhodeus amarus). Behaviour 2007, 144, 1403–1417.
- Kujawa, R. Rozwój zarodków oraz larw różanki (Rhodeus sericeus) uzyskanych w efekcie rozrodu kontrolowanego. In Podchowy Organizmów Wodnych—Osiagnięcia, Wyzwania, Perspektywy; Zakęś, Z., Demska Zakęś, K., Eds.; Wyd. IRS: Olsztyn, Poland, 2015; pp. 179–188.
- Reynolds, J.D.; Debuse, V.J.; Aldridge, D.C. Host specialisation in an unusual symbiosis: European bitterlings spawning in freshwater mussels. Oikos 1997, 78, 539–545.
- Smith, C.; Reichard, M.; Jurajda, P.; Przybylski, M. The reproductive ecology of the European bitterling (Rhodeus sericeus). J. Zool. 2004, 262, 107–124.
- Reichard, M.; Przybylski, M.; Kaniewska, P.; Liu, H.; Smith, C. A possible evolutionary lag in the relationship between freshwater mussels and European bitterling. J. Fish Biol. 2007, 70, 709–725.
- Reichard, M.; Liu, H.; Smith, C. The co-evolutionary relationship between bitterling fishes and freshwater mussels: Insights from interspecific comparisons. Evol. Ecol. Res. 2007, 9, 239–259.
- Aldridge, D.C. Development of European bitterling in the gills of freshwater mussels. J. Fish Biol. 1999, 54, 138–151.
- Smith, C.; Reynolds, J.D.; Sutherland, W.J. The population consequences of reproductive decisions. Proc. R. Soc. Lond. Ser. B 2000, 267, 1327–1334.
- Smith, C.; Reynolds, J.D.; Sutherland, W.J.; Jurajda, P. Adaptive host choice and avoidance of super parasitism in the spawning decisions of bitterling (Rhodeus sericeus). Behav. Ecol. Sociobiol. 2000, 48, 29–35.
- Frelik, A. Predation of adult large diving beetles Dytiscus marginalis (Linnaeus, 1758), Dytiscus circumcinctus (Ahrens, 1811) and Cybister lateralimarginalis (De Geer, 1774) (Coleoptera: Dytiscidae) on fish fry. Int. J. Oceanogr. Hydrobiol. 2014, 43, 360–365.
- Folsom, T.C.; Collins, N.C. Food availability in nature for the larval dragonfly Anax junius (Odonata: Aeshnidae). Freshw. Invertebr. Biol. 1982, 1, 33–40.
- Witkowski, A.; Kotusz, J.; Przybylski, M. Stopień zagrożenia słodkowodnej ichtiofauny Polski: Czerwona lista minogów i ryb—Stan 2009. Chrońmy Przyr. Ojcz. 2009, 65, 33–52.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
23 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




