Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishnu D. Rajput | + 2332 word(s) | 2332 | 2021-11-24 07:58:25 | | | |
| 2 | Vivi Li | Meta information modification | 2332 | 2021-12-22 08:59:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rajput, V.D. ZnO Nanoparticles and Wheat and Maize. Encyclopedia. Available online: https://encyclopedia.pub/entry/17374 (accessed on 07 February 2026).
Rajput VD. ZnO Nanoparticles and Wheat and Maize. Encyclopedia. Available at: https://encyclopedia.pub/entry/17374. Accessed February 07, 2026.
Rajput, Vishnu D.. "ZnO Nanoparticles and Wheat and Maize" Encyclopedia, https://encyclopedia.pub/entry/17374 (accessed February 07, 2026).
Rajput, V.D. (2021, December 21). ZnO Nanoparticles and Wheat and Maize. In Encyclopedia. https://encyclopedia.pub/entry/17374
Rajput, Vishnu D.. "ZnO Nanoparticles and Wheat and Maize." Encyclopedia. Web. 21 December, 2021.
Copy Citation
The zinc oxide nanoparticle (ZnO NP) is a commonly used metal oxide ENPs finding application as sunscreens and cosmetics, biosensors, and in solar cells, etc. Presently, many kinds of metal oxide NPs have been applied in agriculture, specifically in fertilization and plant protection in abiotic and biotic stress conditions.
antioxidant enzymes
α-amylase
dehydrogenase
lipid peroxidation
zinc accumulation
1. Introduction
Nanotechnology is an emerging field of science, whose potential is seen in almost every facet of life. The field of nanotechnology uses various engineered nanoparticles (NPs) and encompasses various fields of science under one umbrella, including biology, physics, chemistry, and engineering. The areas starting from drug delivery to wastewater cleanup, soil remediation, and controlled fertilizers come under the purview of nanotechnology. The use of a variety of NPs is increasing day by day in these fields, including agriculture, where NPs have found uses as fertilizers and stress-counteractive agents [1]. An engineered nanoparticle refers that particle having their size less than 100 nm, with at least one dimension, e.g., titanium dioxide (TiO2), zinc oxide (ZnO), etc. However, as the use of NPs is increasing, the chances of their accidental spillage to unwanted locations, such as ponds or groundwater, are also increasing [2].
Zinc (Zn) is an essential micronutrient for both plants and animals owing to its involvement in several enzyme activities and metabolic functions. Zinc deficiency in plants leads to poor yield levels, while, in humans, it malnutrition and several ailments [3][4]. However, it also poses serious threats to soil and water ecosystem, if present in quantities more than permissible limits. The critical soil value of Zn is 70 to 400 mg/kg [5]. There are several sources through which Zn is released into the environment, such as mining, smelting operations, and agriculture. The excess of Zn in soil and water reaches plants and ultimately biomagnifies through the food chain to accumulate in higher then optimal levels in humans [4]. The presence of Zn in the system can function as a stress that causes physiological and biochemical changes that leads to plant growth inhibition [6].
The zinc oxide nanoparticle (ZnO NP) is a commonly used metal oxide ENPs finding application as sunscreens and cosmetics, biosensors, and in solar cells, etc. [7]. Presently, many kinds of metal oxide NPs have been applied in agriculture, specifically in fertilization and plant protection in abiotic and biotic stress conditions [8][9]. The effects of NPs on plant germination, growth, and biochemical responses have been studied widely, in the recent past, with the goal of sustaining agricultural production. Nanoparticles are released through deliberate application and accidentally, due to increasing use in consumer products and other fields, reaching to aquatic, terrestrial, and atmospheric environments. The unique properties of NPs and their inadvertent contamination could potentially lead to unexpected health or environmental hazards [10]. Therefore, organisms, such as algae, plants, and fungi, are expected to be affected, as a result of their exposure to NPs. Zinc oxide nanoparticles solubility decreases if the soil pH increases, and that is the main reason ZnO NPs are less available to plants [11]. Transport of these NPs into different plant parts may be limited, due to the presence of different kind of barriers found in roots [12]. However, increased production and release of ZnO NPs may cause adverse effects on terrestrial and aquatic ecosystems.
2. Seed Germination and Enzyme Activities
Result on α–amylase and dehydrogenase activities, in the germinated seedlings of maize and wheat, are presented in Figure 1A–D. All treatments led to 100% germination of seeds. The activity of α–amylase was increased, as the concentration of ZnO NPs increased in wheat and maize seedlings; however, at 150 and 200 mg/L, α–amylase activity decreased in wheat plants, as compared to the control. The dehydrogenase activity showed a consistently declining trend, in both wheat and maize, in response to ZnO NPs, as compared to the control (Figure 1). Seed germination and early seedling establishment constitute the most important phase of plant development. Successful early growth of seedlings strengthens them and enables them to tolerate stresses effectively [13]. In this study, seeds showed 100% germination in all treatments, suggesting no negative impact of ZnO NPs. This is validated by earlier findings of Lin and Xing [14], who reported seed germination was affected by ZnO NPs in corn only at 2000 mg/L level. Amylase is an enzyme found in the germinating seeds, while dehydrogenase is important, as it catalyzes the stored products in anaerobic phase of seed germination. This will lead to the release of energy in the early growth of seeds in germination [15]. Amylase activity is affected by many factors, which include temperature, enzyme concentration, pH, and substrate concentrations.
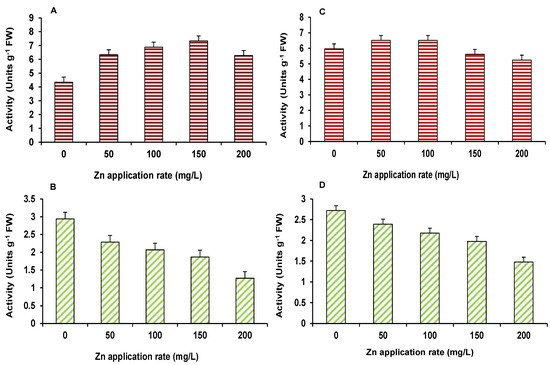
Figure 1. Effect of ZnO NPs treatment on germination related alpha-amylase (A,C) and dehydrogenase (B,D) activities of maize (A,B) and wheat (C,D). Values are means of four replicates. Error bars indicate the least significant value (LSD) at p ≤ 0.05 among the treatments.
3. Accumulation of Zinc in Different Plant Parts and Effect on Growth of Plants
Data on root and shoot length and dry biomass of maize and wheat are presented in Figure 2A–D. For maize, root and shoot length and biomass increased up to 100 mg/L ZnO NPs treated plants (Figure 2A,B). However, higher ZnO NPs level induced some decline in growth parameters with the root and shoot length being lower than the control, only at 200 mg/L (Figure 2A,B). Similarly, in wheat, the length and biomass of plants showed higher control values, up to 150 mg/L ZnO NPs. At the maximum dose of 200 mg/L ZnO NPs, both length and biomass of plants were found to be lower than the control (Figure 2C,D).
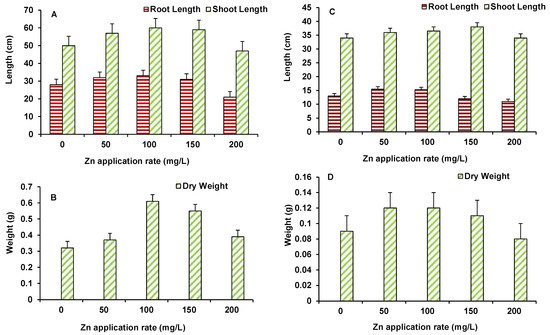
Figure 2. Effect of ZnO NPs treatment on root and shoot length (A,C), and dry weight (B,D) of maize (A,B) and wheat (C,D). Values are means of four replicates. Error bars indicate the least significant value (LSD) at p ≤ 0.05 among the treatments.
Accumulation of Zn in roots was higher, as compared to shoots in both wheat and maize (Figure 3A,B). The maximum Zn accumulation was observed at 200 mg/L ZnO NPs treated wheat (121 ppm in roots and 66 ppm in shoots) and maize (95 ppm in roots and 37 ppm in shoots) plants. Initially up to 100 mg/L ZnO NPs, enhanced length and biomass of plants. Zinc is an essential element for plants and has a role in protein synthesis and in activity of several enzymes, as well as in membrane integrity, metabolic reactions, water uptake and transport, and gene expressions [16]. Hence, an increase in Zn level was beneficial for the plants, leading to improvement in growth. However, toxicity occurred at higher ZnO NPs treatment (150–200 mg/L) level to some extent that might be due to perturbed homeostasis of Zn and indirect effects on other element uptake and mutual elemental interactions. Zinc application, in different forms, has been found to important protection against a number of abiotic stresses and improve growth of plants. However, only a few studies have demonstrated the effect of ZnO NPs on plant growth and productivity [14][17] and observed that ZnO NPs affect the growth and yield of plants, and Zn accumulates in various tissues, including grains/produce. ZnO-NPs shows enhanced plant growth, up to a certain concentration [18], where Zn2+ has been provided as micronutrient [19].
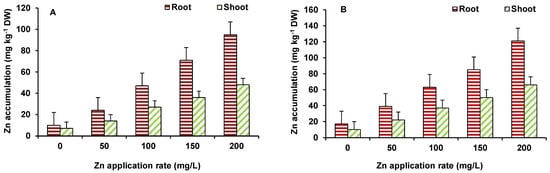
Figure 3. Effect of ZnO NP treatment on Zn accumulation in maize (A) and wheat (B). Values are means of four replicates. Error bars indicate the least significant value (LSD) at p ≤ 0.05 among the treatments.
4. Effect of Photosynthetic Pigments
ZnO NPs exhibited similar responses on photosynthetic pigments (Chl a, Chl b, total Chl, and carotenoid) upon Zn exposure in maize and wheat plants. The level of Chl a, Chl b, and total Chl was increased as the concentration of ZnO NPs increased (Figure 4A,B). The maximum increase in Chl a (19% in maize and 25% in wheat), Chl b (30% in maize and 24% in wheat), and total Chl (50% in maize and 50% in wheat) was observed at 200 mg/L ZnO NPs. Similar to chlorophylls, carotenoids were also increased as the concentration of Zn increased for both the plants. The maximum increase in carotenoids in maize and wheat occurred at 200 mg/L ZnO NPs (Figure 4A,B). Photosynthesis is one of the primary reactions affected by stresses and by any disturbance to metabolism of the plants. Therefore, Zn accumulation was found to be positive stimulator of chlorophyll and carotenoid synthesis that would have led to improved photosynthetic efficiency of plants evident in increased biomass. Further, Zn is crucial for the activity of carbonic anhydrase that mediate hydration of CO2 to bicarbonate for delivery to the chloroplasts [20] Carbonic anhydrase also plays role in stomata regulation [21]. Thus, optimum Zn supply helps plants improve photosynthesis.
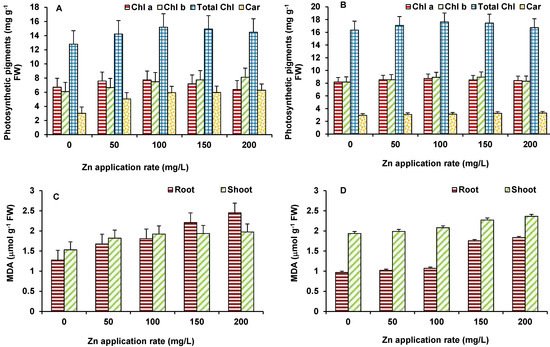
Figure 4. Effect of ZnO NPs treatment on photosynthetic pigments and MDA content of maize (A,C) and wheat (B,D). Values are means of four replicates. Error bars indicate the least significant value (LSD) at p ≤ 0.05 among the treatments.
5. Effect on Lipid Peroxidation and Antioxidant Enzymes
In order to evaluate the membrane damage imposed by ZnO NPs, MDA content (Figure 4C,D) was measured to analyze lipid peroxidation. MDA increased gradually with increasing concentration of Zn in both the plants. The maximum increase in MDA in shoots (12% in maize and 18% in wheat) and roots (47% in both maize and in wheat) was observed at 200 mg/L ZnO NPs (Figure 4C,D).
In this study antioxidant enzymes showed varying response with increase in ZnO NPs concentrations. The used NPs have the capability to stimulate reactive oxygen species (ROS) generation in the system, which leads to cell death when the antioxidative capacity of the cell increases [22]. The activity of SOD in maize (Figure 5A) increased at 50 mg/L ZnO NPs but decreased afterwards; however, it was higher than the control at all ZnO NPs treated treatments. The activity of APX in both root and shoot of maize was higher than the control up to 100 mg/L ZnO NPs but declined at higher ZnO NPs treatment, as compared to the control (Figure 5B). A similar response was seen for CAT activity (Figure 5C). GPX activity (Figure 5D) showed decline, in comparison to the control, at 100 mg/L onwards in roots and shoots.
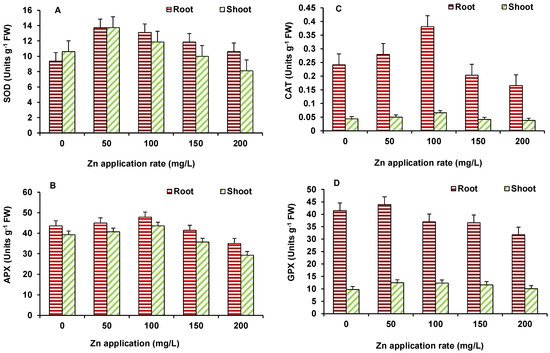
Figure 5. Effect of ZnO NP treatment on activity of SOD (A), APX (B), CAT (C), and GPX (D) of maize. Values are means of four replicates. Error bars indicate the least significant value (LSD) at p ≤ 0.05 among the treatments.
In case of wheat also, the activity of SOD (Figure 6A), APX (Figure 6B) and CAT (Figure 6C) showed significant increase mostly up to 100 mg/L ZnO NPs and then after decline occurred in the activity. However, significant decline, in comparison to the control, occurred only in APX at 200 mg/L ZnO NPs treated treatments. GPX activity (Figure 6D) depicted decline at all concentrations in shoot and 100 mg/L ZnO onwards in roots, as compared to the control.
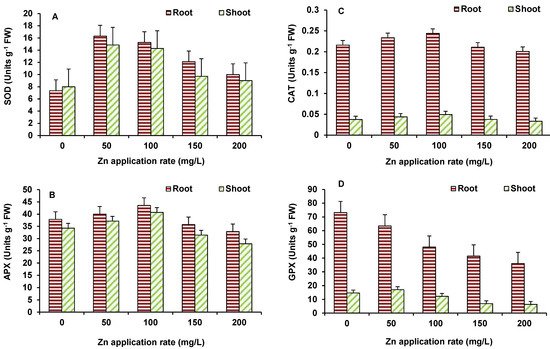
Figure 6. Effect of ZnO NPs treatment on activity of SOD (A), APX (B), CAT (C), and GPX (D) of wheat. Values are means of four replicates. Error bars indicate the least significant value (LSD) at p ≤ 0.05 among the treatments.
Zinc is well-known for its role as a cofactor of SOD and, therefore, it performs as an antioxidant and helps plants in quenching of ROS [23]. In the present study, an increase in Zn levels upon ZnO NPs supply, the activity of various antioxidant enzymes mostly depicted an increase, particularly up to 100 mg/L ZnO NPs. This would have helped plants in the regulation of ROS and assisted in achieving improved growth. At higher ZnO NPs levels, although Zn accumulation increased, it presumably overburdened plants and disturbed Zn homeostasis. This resulted in higher stress that was evident in increased MDA levels and decreasing trend of antioxidant enzymes and growth, as well. Hence, lower concentrations of ZnO NPs were stimulatory to plants. In an earlier study by Faizan et al. [24], ZnO NPs were found to act as natural regulator for plants under stressed and non-stressed conditions by modulating key physiological parameters and thereby enhancing plant growth and development. The changes in lipid peroxidation and antioxidant metabolism of plants have been recorded upon the ZnO NPs supply, which could reduce the oxidative stress and protect plants from damaging effects of ROS. The small size of ZnO-NPs allows its entry in plant cells, assisting in seed germination and growth [25].
SOD is a key antioxidant enzyme [26], in the chloroplast, cytosol, and mitochondria catalyzing the dismutation of superoxide radicals (O2−) to H2O2 and O2, thus playing a major role in counteracting oxidative stress [27]. SOD activity was higher in the highest dose in maize [28]. Increased activity of SOD and POX in Gossypium and subsequent decrease in lipid peroxidation was reported [29]. Apart from SOD, other enzymes of antioxidant system, such as CAT, GPX, and APX, reduce H2O2 into water and oxygen in cytoplasm and various cellular organelles [30]. In conditions of stress, induced by NPs [31], oxidative stress has been observed, when the equilibrium between ROS production and the defense system in plant is impaired [32].
Zinc is present in the cystolic and chloroplastic Cu/Zn-SOD enzymes [33][34], which play a critical role against the oxidative stress. Du et al. [35] highlighted the potential benefits of supplementing the Zn NPs as a fertilizer because Zn deficiency in plants is the major concern globally [36]. Dimpka et al. [37] showed that higher concentration of ZnO NPs may also cause the toxicity to the plants. The effects of ZnO NPs in crop plants, such as rice and barley, have been studied and positive influences on cellular redox state, antioxidant defense and growth of plants have been observed [38][39][40]. Prasad et al. [41] reported increased seed germination, along with enhanced seedling vigour in peanut seeds, when treated with 1000 mg/kg of ZnO NPs (average size ~25 nm). The positive effects of ZnO NPs were also seen in early flowering, increased leaf chlorophyll content, increased stem and root growth, and higher pod yield per plant. In addition, Dimpka et al. [42] showed the beneficial effect of ZnO NPs on shoot and root length, plant height, biomass, chlorophyll, grain yield, and uptake in wheat crops, with 40% field moisture capacity. ZnO NPs showed greater positive effects, as compared to ZnO bulk treatment [42].
References
- Nowack, B. The behaviour and effects of nanoparticles in the environment. Environ. Pollut. 2009, 157, 1063–1185.
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 14963.
- Srivastava, P.C.; Rawat, D.; Pachauri, S.P.; Shrivastava, M. Strategies for enhancing zinc efficiency in crop plants. In Nutrient Use Efficiency: From Basics to Advances; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: New Delhi, India, 2015; pp. 87–101.
- Stietiya, M.H.; Wang, J.J. Zinc and cadmium adsorption to aluminum oxide nanoparticles affected by naturally occurring ligands. J. Environ. Qual. 2014, 43, 498–506.
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010.
- Subin, M.P.; Steffy, F. Phytotoxic effects of cadmium on seed germination, early seedling growth and antioxidant enzyme activities in Cucurbita maxima Duchesne. Int. Res. J. Biol. Sci. 2013, 2, 40–47.
- Kumar, S.A.; Chen, S.M. Nanostructured zinc oxide particles in chemically modified electrodes for biosensor applications. Anal. Lett. 2008, 41, 141–158.
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792.
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221.
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Warheit, D.B. Safe handling of nanotechnology. Nature 2006, 444, 267–269.
- Waalewijn-Kool, P.L.; Ortiz, M.D.; van Straalen, N.M.; van Gestel, C.A.M. Sorption, dissolution and pH determine the long-term equilibration and toxicity of coated and uncoated ZnO nanoparticles in soil. Environ. Pollut. 2013, 178, 59–64.
- Kořenková, L.; Šebesta, M.; Urík, M.; Kolenčík, M.; Kratošová, G.; Bujdoš, M.; Dobročka, E. Physiological response of culture media-grown barley (Hordeum vulgare L.) to titanium oxide nanoparticles. Acta Agric. Scand. Sect. B 2017, 67, 285–291.
- Srivastava, S.; Akkarakaran, J.J.; Suprasanna, P.; D’Souza, S.F. Response of adenine and pyridine metabolism during germination and early seedling growth under arsenic stress in Brassica juncea. Acta Physiol. Plant. 2013, 35, 1081–1091.
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250.
- Oaikhena, E.E.; Ajibade, G.A.; Appah, J.; Bello, M. Dehydrogenase enzyme activities in germinating cowpea (Vigna unguiculata (L) Walp). J. Biol. Agric. Healthc. 2013, 3, 32–36.
- Hafeez, B.M.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition-a review. Am. J. Exp. Agric. 2013, 3, 374–391.
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479.
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686.
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139.
- Zabaleta, E.; Martin, M.V.; Braun, H.P. A basal carbon concentrating mechanism in plants? Plant Sci. 2012, 187, 97–104.
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO 2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93.
- Bedi, P.; Kaur, A. An overview on uses of zinc oxide nanoparticles. World J. Pharm. Pharm. Sci. 2015, 4, 1177–1196.
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205.
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. Sustain. Agric. Rev. 2020, 41, 83–99.
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53.
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228.
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ. Pollut. 2018, 240, 802–816.
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants–a soil microcosm experiment. Chemosphere 2016, 147, 88–97.
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Sahi, S.V. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127.
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front. Plant Sci. 2016, 7, 301.
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 6, 1242.
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018, 62, 801–808.
- Singh, A.; Singh, N.Á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201.
- Yruela, I. A Handbook of Plant Nutrition, 2nd ed.; Estación Experimental de Aula Dei, Consejo Superior de Investigaciones Científicas (EEAD-CSIC): Zaragoza, Spain, 2015.
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116.
- Impa, S.M.; Morete, M.J.; Ismail, A.M.; Schulin, R.; Johnson-Beebout, S.E. Zn uptake, translocation and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J. Exp. Bot. 2013, 64, 2739–2751.
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 2015, 24, 119–129.
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 2021, 10, 2254.
- Azarin, K.; Usatov, A.; Minkina, T.; Plotnikov, A.; Kasyanova, A.; Fedorenko, A.; Duplii, N.; Vechkanov, E.; Rajput, V.D.; Mandzhieva, S. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 2022, 287, 132167.
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Chernikova, N.; Hassan, T.; Mandzhieva, S.; Sushkova, S.; Lysenko, V.; Soldatov, M.A.; Burachevskaya, M. Effects of zinc oxide nanoparticles on physiological and anatomical indices in spring barley tissues. Nanomaterials 2021, 30, 1722.
- Prasad, T.N.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927.
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020, 722, 137808.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
22 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




