| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | DANIELA DE OLIVEIRA | + 3183 word(s) | 3183 | 2020-08-18 05:05:02 | | | |
| 2 | Bruce Ren | Meta information modification | 3183 | 2020-08-25 11:29:43 | | |
Video Upload Options
Plants represent the main source of molecules for the development of new drugs, which intensifies the interest of transnational industries in searching for substances obtained from plant sources, especially since the vast majority of species have not yet been studied chemically or biologically, particularly concerning anti-inflammatory action. Anti-inflammatory drugs can interfere in the pathophysiological process of inflammation, to minimize tissue damage and provide greater comfort to the patient. Therefore, it is important to note that due to the existence of a large number of species available for research, the successful development of new naturally occurring anti-inflammatory drugs depends mainly on a multidisciplinary effort to find new molecules. Although many review articles have been published in this regard, the majority presented the subject from a limited regional perspective.
1. Introduction
The magnitude of global plant diversity is estimated at more than 500,000 species [1][2], and the variety and complexity of plant metabolites represent a challenge when considering exploration of the chemical repertoire offered. From this point of view, the Plant Kingdom has been pragmatic, especially when these molecules are reported as substances with the high medicinal potential to treat diseases that affect living beings [3].
Medicinal plants continue to be an interesting source of natural products for treating various health conditions. It is estimated that more than 150,000 plant species have been studied, many of which contain valuable therapeutic agents, and the applications of novel compounds from plants for pharmaceutical purposes have been gradually increasing in recent years [4][5].
Plants have played an important role in human health care since ancient times. In an adaptation against attacking pathogen and environmental stress, plants produce several substances that exert biological activities. These small organic molecules come from secondary metabolism and have several biological activities. Among the diverse functions, anti-inflammatory actions are highlighted [6][7].
It is known that inflammation is an evolutionarily conserved process of protection and a critical survival mechanism [8]. It is composed of complex sequential changes in the tissue to eliminate the initial cause of the cell injury, which may have been caused by infectious agents or substances from their metabolism (microorganisms and toxins), as well as by physical agents (radiation, burn, and trauma), or chemicals (caustic substances) [9][10]. The signs of inflammation are local redness, swelling, pain, heat, and loss of function [7].
In general, this complex biological response leads to the restoration of homeostasis. However, in cases of prolonged release of inflammatory mediators and the activation of harmful signal-transduction pathways, the inflammatory process persists, and a mild but chronic proinflammatory state may arise [8]. A low-grade inflammatory state is correlated with various disorders and chronic health conditions, such as obesity, diabetes, cancer, and cardiovascular diseases, among others [11][12][13][14][15][16][17][18].
Therefore, the discovery of a new generation of therapeutic agents to use in the resolution of inflammation is desirable. The treatment of inflammation involves some mechanisms that can be used as therapeutic targets [8]. Due to the production of secondary metabolites with clinically curative effects, medicinal plants play an important role in the development of new and potent drugs [19][20].
Another motivating scientific investigation related to drugs and medicines made from plants is their interaction with gut microbiota. Certain gut bacteria intensively metabolize drugs rich in the low-molecular-mass products of secondary metabolisms, such as tannins and anthocyanins. Metabolites derived from bacterial metabolization are small, bioavailable, and potentially bioactive metabolites. They also have potential modulatory effects on the gut microbiome, which is interesting to prevent metabolic disorders [21].
2. Anti-Inflammatory Drugs
Anti-inflammatory drugs can interfere in the pathophysiology of inflammation, seeking to minimize tissue damage and provide greater patient comfort. The major classes of anti-inflammatory agents are the glucocorticoids and non steroidal anti-inflammatory drugs (NSAIDs). Fundamentally these differ in their mode of action. In short, glucocorticoids act by inhibiting prostaglandins and proteins involved in inflammatory processes, such as corticosteroids, which among other indications are used in treatment for asthma and autoimmune inflammatory response. Non-steroidal drugs, on the other hand, have an inhibitory action through the enzyme cyclooxygenase and are indicated for moderate and mild pain and body temperature control. An example of a non-steroidal drug is acetylsalicylic acid [22].
NSAIDs are the most commonly used drugs worldwide , utilized to treat acute and chronic pain resulting from an inflammatory process [22]. NSAIDs encompass a range of agents and, in general, all their effects are related to the inhibition of COX action in the production of prostaglandins and thromboxanes [23][24][25][26].
The main mechanism of action of NSAIDs is the inhibition of COX, both central and peripheral, interfering in the conversion of arachidonic acid to prostaglandins E2, prostacyclins, and thromboxanes. Enzymes related to the action of NSAIDs can be divided into COX-1 and COX-2, acting in different regions. COX-1 appears in most cells, even fetal and amniotic fluid, and participates in physiological effects, such as regulatory and protective effects. On the other hand, COX-2 is activated by inflammation and proinflammatory cytokines [27][28].
There are several ways to classify NSAIDs; according to COX-2 inhibitory potency over COX-1, concentration to achieve clinical effects, among others. NSAIDs can be classified into non-selective NSAIDs (ketoprofen, aspirin, naproxen, flunixin, meglumine, and others), preferential COX-2 inhibitors (meloxican, etodolac, nimesulide), and highly selective COX-2 inhibitors (coxib). Most of the side effects are related to the inhibition of COX-1 due to its performance in several systems related to cell cleansing. Besides, NSAIDs can also be classified according to their chemical structure (Table 1).
Table 1. Classification of NSAIDs [29]
|
Salicylates |
Indoleacetic acid Derivatives |
Aryl Acetic Derivatives |
Enolic Acids |
||||||||
|
Acetylsalicylic acid Lysine clonixinate Benorilate Diflunisal Salicylamide Etersalate Salsalate or salicylic acid |
Acemethacin Glucamethacin Indomethacin Proglumethacin Oxamethacin Sulindac Tolmetin Difenpiramide |
Aceclofenac Diclofenac Etodolac Fentiazac Ketorolac Bufexamac Lonazolac Alclofenac Zomepirac |
Oxicans: Droxicam |
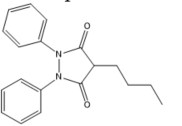
Pyrazolones: Phenylbutazone Mofebutazone Oxyphenbutazone Kebuzone Metamizole (Dipyrone) Feprazone Nifenazone Suxibuzone
Aminophenazone |
|||||||
|
|
|
|
|
|||||||
|
Aspirin |
Sulindac |
Etodolac |
Piroxicam |
Phenylbutazone |
|||||||
|
Arylpropionic Derivatives |
Phenemates |
Others |
|||||||||
|
Butibufen Phenoprofen Phenobufen Flurbiprofen Benoxaprofen Ibuprofen Ibuproxam |
Ketoprofen Dexetoprofen Pyprophene Indoprofen Naproxen Tiaprofen Dexibuprofen Phenoprofen Flunoxaprofen Alminoprofen |
Meclofenamic acid Mefenamic acid Flufenamic acid Tolipanic acid Etofenamate |
Nabumetone Glucosamine Diacerhein Nimesulide Proquazone Azapropazone Benzidamine Orgotein Feprazone Morniflumato Tenidap Glucosaminoglycan |
Coxibs: Celecoxib Rofecoxib Parecoxib Valdecoxib Etoricoxib 4-Aminophenol Paracetamol (Acetaminophen) |
|||||||
|
|
|
|
|
|
|||||||
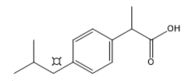
(S)-Ibuprofen |
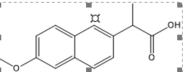
Naproxen |
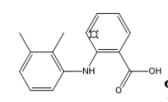
Mefenamic acid |
Nimesulide |
Valdecoxib |
|||||||
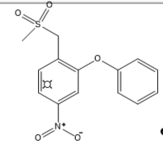
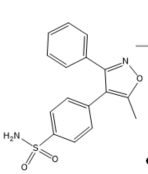
Structurally, COX-2 selective drugs contain sulfonamide groups or sulfones, responsible for the selectivity of the enzyme and do not have a carboxylic group and, therefore, they can selectively target the COX-2 enzyme. They have little water solubility, which hinders parenteral administration [30].
Acetylsalicylic acid (ASA) is one of the most widely used drugs in the world. It is used as an analgesic, antipyretic, and anti-inflammatory [31][32]. This drug also has antiplatelet or anticoagulant effects and is used to prevent heart attacks, strokes, and blood clots [33]. However, its use can also lead to exacerbated respiratory tract disease and cancer [34].
Historically, non-steroidal anti-inflammatory drugs, such as acetylsalicylic acid (Aspirin®), indomethacin, ibuprofen, and piroxicam have been used clinically for the treatment of inflammation due to their suppression of the effects of COX activity .
However, these traditional NSAIDs act in a non-selective manner inhibiting both forms of COX and have also demonstrated side effects. Specific modalities of anti-inflammatory effects and side effects are associated with the existence of two COX isoforms [35].
Inhibitory actions of aspirin or other non-steroidal anti-inflammatory drugs against COX-1 may present crucial problems in pharmacotherapy. Some anti-inflammatory drugs that act only to inhibit COX-1 are ibuprofen, naproxen, fenoprofen, ketoprofen, flurbiprofen, and oxaprozin (derivatives of propionic acid); indomethacin, sulindac, and etodolac (indoleacetic acids); piroxicam (derived from oxyanas); mefenamic acid and meclofenamate (phanamates); and diclofenac .
Thus, the scientific community has focused its efforts on the search for selective COX-2 inhibitors, with lower adverse side effects, since highly selective COX-2 inhibitors are required for the treatment of inflammatory diseases [35]. The first selective COX-2 inhibitor was celecoxib (Celebrex®), followed by rofecoxib (Vioxx®). In a short time, the coxibs (celecoxib and rofecoxib) have achieved wide dissemination [36]. Other drugs are also more selective for COX-2 than for COX-1, such as nimesulide and etodolac [37].
Despite initial success, shortly after the launch of selective COX-2 inhibitors, adverse cardiovascular and renal effects have been reported and, in high-doses, gastrointestinal effects. These adverse effects occur due to inhibition of the constitutive production of COX-2 in some tissues. Thus, in recent years, the safety of the use of NSAIDs in clinical practice has been questioned, due to the emergence of evidence that suggests a high risk of acute myocardial infarction, stroke, heart failure, renal failure, and arterial hypertension [38].
There is no absolute selectivity in COXs. Even a selective COX-2 inhibitor will also inhibit COX-1 when in high concentrations. Therefore, in all NSAIDs selective for COX-1 or COX-2, to different degrees, there is a risk of adverse cardiovascular side effects .
In September 2004, Merck removed Vioxx® from the world market and in April 2005, the coxib study led the American Committee to conclude about the Cardiovascular Risk and suspension of Pfizer’s Bextra® (valdecoxib). Celecoxib is marketed only with a black stripe indication and the adverse cardiovascular effects are explained in the package leaflet .
A derivative of celecoxib based a benzo[b]furan moiety was reported to demonstrate selective activity against COX-2. Besides, new molecules containing rhodanine and benzofuran scaffolds were designed, synthesized, and reported to exhibit dual COX-2, and 5-LOX inhibitory potential [39][40]. A recent patent survey reported a review focused on benzofuran inhibitors [41][42].
Since a large proportion of NSAIDs available on the market have significant undesirable effects, the need for new anti-inflammatory drugs contributes to the advancement of research for newer, safer, effective molecules with fewer side effects and from vegetal sources. Therefore, it can be observed that a significant number of substances of vegetal origin form part of the therapeutic arsenal of modern medicine. It is important to emphasize that due to the existence of a large number of species available for research, the success of the development of new naturally occurring anti-inflammatory drugs depends fundamentally on a multidisciplinary effort in the discovery of new leading molecules.
3. Plant Use and the Development of Drugs
The World Health Organization (WHO) estimates that approximately 65% of the world’s population incorporates traditional medicine (ethnobotanical uses) into medical care. Ethnobotanical studies over the years have allowed the association of highly diversified plants with biological activities, from observation, description, and experimental research, which has greatly contributed to the discovery of natural products with biological action. The use of medicinal plant-based natural compounds to treat many illnesses has become a great trend in clinical research. Polyphenolic compounds have drawn significant attention due to their modulation effects on inflammasomes [43]. These multi-protein complexes are associated with the initiation and progression of metabolic disorders and chronic diseases, such as cancer and neurodegenerative diseases [44].
Thus, plants have become the first source of substances for the development of new drugs, and a considerable part of the drugs prescribed in the world are derived from them[45][46][47]. Plants contain reservoirs of potential secondary metabolites that are the major sources of drugs, which intensifies the interest of transnational industries in the search for substances obtained from plant sources, particularly since the great majority of species have not been studied chemically or biologically [48].
The use of plants or plant products for medicinal purposes is mostly documented in books and, lately, on an enormous number of websites (the reliability of some of which must be examined carefully). In recent decades, hundreds of research and review articles have been published regarding the anti-inflammatory activities of plants (Table 2) [49][50][51][52].
Table 2. Anti-inflammatory activity of some medicinal plants.
|
Number |
Botanical |
Plant/Family |
Parts Used |
Constituent Compounds |
||
|
01 |
Acacia catechu |
Mimosaceae |
Bark, wood, |
Tannin, gum, catechuic acid |
||
|
02 |
Azadirachta indica |
Meliaceae |
Leaf, root, oil, seed, gum, fruit, flower. |
Margosine, bitter oil, azadirachtin. |
||
|
03 |
Caesalpinia crista |
Caesalpiniaceae |
Seeds, root, leaf, root bark. |
Oleic, linoleic, palmitic, stearic acid, phytosterols. |
||
|
04 |
Cassia angustifolia |
Caeasalpinaceae |
Pods, dried leaves. |
Emodin, eatharitin, mucilage, |
||
|
05 |
Coriandrum sativum |
Umbelliferae |
Leaf, bark, flower |
Tannin, cathartin, malic acid, |
||
|
06 |
Cuscuta reflexa |
Convolvulaceae |
Plant, seed, fruit, stem. |
Cuscutine, flavonoid, glucoside, |
||
|
07 |
Enicostema littorale |
Gentianaceae |
Whole plant. |
Alkaloids, gentiocrucine |
||
|
08 |
Erythrina variegate |
Papilionaceae |
Leaves, bark, roots, flower. |
2-Hydroxygenistein, genistein. |
||
|
09 |
Euphorbia hirta |
Euphorbiaceae |
Plant, roots, leaves |
Ascorbic acid, β-amyrin, choline, inositol, linoleic acid, β-sitosterol. |
||
|
10 |
Euphorbia tirucalli |
Euphorbiaceae |
Root, plant (milk, juice). |
β-sitosterol, ellagic acid, citric acid, |
||
|
11 |
Fagonia cretica |
Zygophyllaceae |
Leaves, twigs, bark. |
Betulin |
||
|
12 |
Ficus benghalensis |
Moraceae |
Aerial roots, bark, seeds, leaves, buds, |
Skin, fruits contain 10% tannin. |
||
|
13 |
Ficus carica |
Moraceae |
Fruit, root. |
Alkaloids, ascorbic acid, caffeic acid, niacin, linoleic acid, lutein, b-carotene, |
||
|
14 |
Ficus religiosa |
Moraceae |
Bark, leaves, fruits, tender shoots, seeds. |
The bark contains tannins, rubber, wax. |
||
|
15 |
Foeniculum vulgare |
Apiaceae |
Fruit, root, seeds, leaves. |
Ascorbic acid, estragole, coumaric acid, |
||
|
16 |
Gentiana kuroo |
Gentianaceae |
Rhizomes (roots) |
Gentiopicrine, gentianic acid |
||
|
17 |
Gloriosa superba |
Liliaceae |
Rhizome, tuber, leaves, flower |
Choline, colchicine, stigmasterol, |
||
|
18 |
Glycyrrhiza glabra |
Papilionaceae |
Roots, leaves. |
Genistein, eugenol, bergapten, glycyrrhizin, acetophenone, estragole, camphor, |
||
|
19 |
Gmelina arbórea Roxb |
Verbenaceae |
Whole plant. |
Betulin |
||
|
20 |
Grewia asiatica |
Tiliaceae |
Leaves, roots,fruits, bark. |
Betulin |
||
|
21 |
Hibiscus rosa-Sinensis |
Malvaceae |
Buds, roots, leaves, flower |
Quercetin, ascorbic acid. |
||
|
22 |
Hygrophila auriculata |
Acanthaceae |
Roots, leaves, seeds. |
Oleic and linoleic acids in seed oil, |
||
|
23 |
Manihot esculenta |
Euphorbiaceae |
Tuberous roots. |
Ascorbic acid, palmitic acid, lauric acid, |
||
|
24 |
Martynia annua |
Pedaliaceae |
Fruits, leaves. |
Pelargonidin-3,5-diglucoside, |
||
|
25 |
Momordica charantia |
Cucurbitaceae |
Whole plant |
5-Hydroxytryptamine, alkaloids, ascorbic acid, |
||
|
26 |
Moringa oleifera |
Moringaceae |
Roots, bark, leaves, seeds. |
Choline, moringinine, myristic, ascorbic acid, |
||
|
27 |
Nelumbo nucifera |
Nymphaeaceae |
Whole plant. |
Anonaine, ascorbic acid, β-carotene, copper, |
||
|
28 |
Nicotiana tobacum |
Solanaceae |
Leaves. |
1,8-Cineole, 4-vinylguaiacol, acetaldehyde, acetophenone, alkaloids, anabasine, nicotinic acid, nicotine, scopoletin, quercitrin, sorbitol, |
||
|
29 |
Nigella sativa |
Ranunculaceae |
Seeds. |
α-spinasterol, ascorbic acid, β-sitosterol, carvone, |
||
|
30 |
Ocimum basilicum |
Laminaceae |
Whole plant |
Acetic acid, ascorbic acid, aspartic acid, |
||
|
31 |
Plumbago zeylanica |
Plumbaginaceae |
Root, leaves, root, bark. |
Plumbagin, droserone, 3-chloroplumbagin, chitranone, zeylinone, elliptione, isozeylinone. |
||
|
32 |
Portulaca oleraceae |
Portulaceae |
Stem, leaves, seeds. |
Oleracins I and II, acylated betacyanins, |
||
|
33 |
Pterocarpus marsupium |
Fabaceae |
leaves, flower, gum Heartwood, |
Alkaloids, gum, essential oil, semi-drying fixed oil. |
||
|
34 |
Solanum melongena |
Solanaceae |
Roots, leaves, tender fruits. |
Ascorbic acid, alanine, arginine, caffeic acid. |
||
|
35 |
Solanum nigrum |
Solanaceae |
Whole plant. |
Solenin, solasodine. |
||
|
36 |
Stereopermum |
Bignoniaceae |
Roots, flower |
Mucilage, albumin, sugar, wax, lapachol, |
||
|
37 |
Tephrosia purpurea |
Fabaceae |
Whole plant |
Tephrosin, betulinic acid, lupeol, rutin. |
||
|
38 |
Terminalia chebula |
Combretaceae |
Mature, immature fruits. |
Ascorbic acid, gallic acid, ellagic acid, chebulic acid. |
||
|
39 |
Thespesia populnea |
Malvaceae |
Whole plant |
Gossypol, herbacetin, kaempferol. |
||
|
40 |
Thespesia populneoides |
Malvaceae |
Whole plant |
Populneol, gossypol, kaempferol, |
||
|
41 |
Tinospora cordifolia |
Menispemaceae |
Stem |
Alkaloids, starch. |
||
|
42 |
Vernonia cinerea |
Asteraceae |
Whole plant |
Linoleic acid, lupeol, vernolic acid. |
||
Other plant species with anti-inflammatory properties have already been described in the literature. However, the parts of the plants used and the compounds responsible for the anti-inflammatory activity have not yet been fully elucidated.
Phytochemical studies carried out with the species Myracroduo nurundeuva Allemão, Schinus terebinthifolius Raddi, Spondias mombin L., Spondias purpurea L. and Spondias tuberosa Arruda, belonging to the Anacardiaceae family, detected the presence of several secondary metabolites. The most abundant are phenols, triterpenes, flavonoids, and cinnamic acid, which are responsible for their anti-inflammatory action [53][54][55][56][57].
The plants that make up the Euphorbiaceae family, such as the species Euphorbiaceae Acalypha hispida Burm. f., Acalypha indica L., Phyllanthus niruri L., are rich mainly in phenolic compounds, saponins, tannins, and triterpenes, which are responsible for their anti-inflammatory action [58][59][60].
Research with the species Ruellia asperula (Mart. Ex Ness) Lindau (family Acanthaceae), Achyranthes aspera L., Alternanthera brasiliana (L.) Kuntze (family Amaranthaceae), Himatanthus drasticus (Mart.) Plumel (family Apocynaceae), Matricaria chamomilla L. (family Asteraceae), Heliotropium indicum L. (family Boraginaceae), Momordica charantia L. (family Cucurbitaceae), Mimosa tenuiflora (Willd.) Poir (family Leguminosae), Borreria verticillata (L.) G.Mey. (family Rubiaceae), Solanum paniculatum L. (family Solanaceae), and Zingiber officinale Roscoe (family Zingiberaceae) also indicates the existence of compounds with anti-inflammatory activity [61][62][63].
It is important to note that the extraction of plant materials is the first major step to test biological activities, presenting many advantages and some disadvantages compared to the isolation of pure active compounds [50]. When an entire extract is used, there is a good chance of synergism between active components that can be lost when each of these components is isolated. This synergism was discovered in several medicinal tests, including those for anti-inflammatory activity. On the contrary, the mixture of different compounds together may also lead to inhibitory effects, namely, that one component may reduce the biological activity of the other. In line with this assumption, some studies have shown that the anti-inflammatory activity of pure compounds (such as amentoflavone, pseudohypericin, and hyperforin) is higher than that of the extracts [64][65].
Medicinal plants are used instead of Non-steroidal anti-inflammatory drugs (NSAIDs) as the use of non-steroidal anti-inflammatory drugs is associated with several side effects, among which are unwanted effects on the gastrointestinal tract and the renal system. The biggest disadvantage of recently available potent synthetic drugs is concerning their toxicity and the reappearance of symptoms after discontinuation. Therefore, the screening and development of drugs with anti-inflammatory activity are necessary and there are many efforts to find anti-inflammatory drugs from medicinal plants .
Inflammation is a huge challenge for human kind. Although many anti-inflammatory drugs are available, it is believed that these drugs, such as opioids and analgesia inducing drugs like NSAIDs, are not useful in all cases and these drugs also produce side effects, so to overcome these problems, new drug molecules need to be discovered from plants. Plants have many phytoconstituents helpful in reducing inflammation and fewer side effects .
The objectives of the use of plants as therapeutic agents are: to concentrate and/or isolate bioactive substances for direct use as drugs; to produce bioactive compounds of novel or already known structures for semi synthesis to produce patentable entities of higher activity and/or lower toxicity; to use agents as pharmacological tools; and to use the whole plant or part of it as a herbal remedy [66].
It is worth mentioning that for the acquisition of new drugs, molecular diversity and biological function distinguish products of natural origin from synthetic products. The molecular diversity of natural products is far superior to that derived from synthesis processes, which, despite technological advances, are still restricted. This fact makes it possible for the chemical compounds present in plants to become potential drugs for different diseases .
An example of a phytotherapeutic anti-inflammatory agent is Acheflan®, indicated for the local treatment of inflammatory processes, and Daflon 500 mg®, a drug composed of a purified flavonoid fraction that presents venotonic and vasoprotective action . Therefore, the study of the immunopharmacological activities of plant species has provided evidence on different extracts/fractions and chemical classes with high therapeutic potential, which represents a promising alternative to the inflammatory processes and diseases related to them, as well as a form of validation of their ethnobotanical use. Besides, data from the scientific literature have shown that molecules of plant origin present important anti-inflammatory activities and that many of their actions are related to the ability to inhibit the synthesis or action of cytokines, chemokines, and adhesion molecules, and arachidonic acid and nitric oxide pathways [67][68].
References
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant. Divers. 2016, 38, 10–16, doi:10.1016/j.pld.2016.01.001.
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Álvarez, P.; et al. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294, doi:10.1038/nature11318.
- Souza, R.K.D.; Mendonça, A.C.A.M.; Silva, M.A.P. Ethnobotanical, phytochemical and pharmacological aspects Rubiaceae species in Brazil. Rev. Cubana Plant Med. 2013, 18, 140–156.
- Shazhni, J.A.; Renu, A.; Vijayaraghavan, P. Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium x hortulanum. Saudi J. Boil. Sci. 2018, 25, 1755–1761, doi:10.1016/j.sjbs.2018.03.013.
- Cao, Z.; Deng, Z. De Novo Assembly, Annotation, and Characterization of Root Transcriptomes of Three Caladium Cultivars with a Focus on Necrotrophic Pathogen Resistance/Defense-Related Genes. Int. J. Mol. Sci. 2017, 18, 712, doi:10.3390/ijms18040712.
- Locatelli, C.; Nardi, G.M.; anuário, A.d.F.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.A.; Nascimento, S.R.D.; Gon, A.C.; Mariano, L.N.B.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016, 8, S42–S49, doi:10.4103/0974-8490.178642.
- Virshette, S.J.; Patil, M.K.; Somkuwar, A.P. A review on medicinal plants used as anti inflammatory agents. J. Pharmacogn. Phytochem. 2019, 8, 1641–1646.
- Liu, C.H.; Abrams, N.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; Prabhudas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180, doi:10.1038/ni.3828.
- Fialho, L.; Cunha-E-Silva, J.A.; Santa-Maria, A.F.; Madureira, F.A.; Iglesias, A.C. Comparative study of systemic early postoperative inflammatory response among elderly and non-elderly patients undergoing laparoscopic cholecystectomy. Rev. Col. Bras. Cir. 2018, 45, e1586, doi:10.1590/0100-6991e-20181586.
- Jang, C.H.; Kim, Y.Y.; Seong, J.Y.; Kang, S.H.; Jung, E.K.; Sung, C.M.; Kim, S.B.; Cho, Y.B.; Sung, J.Y. Clinical characteristics of pediatric external auditory canal cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 5–10, doi:10.1016/j.ijporl.2016.05.029.
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.-J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 1–14, doi:10.3389/fimmu.2018.00696.
- Purohit, S.; Sharma, A.; Zhi, W.; Bai, S.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; et al. Proteins of TNF-α and IL6 Pathways Are Elevated in Serum of Type-1 Diabetes Patients with Microalbuminuria. Front. Immunol. 2018, 9, 154, doi:10.3389/fimmu.2018.00154.
- Clark, M.; Kroger, C.J.; Tisch, R.M. Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response. Front. Immunol. 2017, 8, 1–10, doi:10.3389/fimmu.2017.01898.
- Espigol-Frigole, G.; Planas-Rigol, E.; Lozano, E.; Corbera-Bellalta, M.; Terrades-García, N.; Prieto-González, S.; García-Martínez, A.; Hernández-Rodríguez, J.; Grau, J.M.; Cid, M.C. Expression and Function of IL12/23 Related Cytokine Subunits (p35, p40, and p19) in Giant-Cell Arteritis Lesions: Contribution of p40 to Th1- and Th17-Mediated Inflammatory Pathways. Front. Immunol. 2018, 9, 1–11, doi:10.3389/fimmu.2018.00809.
- Donninelli, G.; Del Cornò, M.; Pierdominici, M.; Scazzocchio, B.; Varì, R.; Varano, B.; Pacella, I.; Piconese, S.; Barnaba, V.; D’Archivio, M.; et al. Distinct Blood and Visceral Adipose Tissue Regulatory T Cell and Innate Lymphocyte Profiles Characterize Obesity and Colorectal Cancer. Front. Immunol. 2017, 8, 643, doi:10.3389/fimmu.2017.00643.
- Katare, P.B.; Bagul, P.K.; Dinda, A.K.; Banerjee, S.K. Toll-Like Receptor 4 Inhibition Improves Oxidative Stress and Mitochondrial Health in Isoproterenol-Induced Cardiac Hypertrophy in Rats. Front. Immunol. 2017, 8, 719, doi:10.3389/fimmu.2017.00719.
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front. Immunol. 2017, 8, 1058, doi:10.3389/fimmu.2017.01058.
- Qi, H.; Yang, S.; Zhang, L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front. Immunol. 2017, 8, 928, doi:10.3389/fimmu.2017.00928.
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020, 148, 80–89, doi:10.1016/j.plaphy.2020.01.006.
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202, doi:10.1016/j.micpath.2018.08.034.
- Thumann, T.A.; Pferschy-Wenzig, E.-M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153, doi:10.1016/j.jep.2019.112153.
- Lima, A.S.; Alvim, H.G.O. Review on non-steroid antiinflammatory: Acetylsalicylic acid. Rev. Inic. Ciente. Ext. 2018, 1, 169–174.
- Pereira-Leite, C.; Nunes, C.; Jamal, S.K.; Cuccovia, I.M.; Reis, S. Nonsteroidal Anti-Inflammatory Therapy: A Journey Toward Safety. Med. Res. Rev. 2016, 37, 802–859, doi:10.1002/med.21424.
- Sandoval, A.C.; Fernandes, D.R.; Silva, E.A.; Terra Júnior, A.T. The indiscriminated use of non-steroid anti-inflammatory (NSAID). Rev. Cient. FAEMA 2017, 8, 165–176.
- Sostres, C.; Lanas, Á. Appropriate prescription, adherence and safety of non-steroidal anti-inflammatory drugs. Med. Clin. 2016, 146, 267–272, doi:10.1016/j.medcli.2015.09.022.
- Patel, D.P.; Schenk, J.M.; Darke, A.K.; Myers, J.B.; Brant, W.O.; Hotaling, J.M. Non-steroidal anti-inflammatory drug (NSAID) use is not associated with erectile dysfunction risk: Results from the Prostate Cancer Prevention Trial. BJU Int. 2015, 117, 500–506, doi:10.1111/bju.13264.
- Inotai, A.; Hanko, B.; Meszaro, A. Trends in the non-steroidal anti-inflammatory drug market in six central-eastern european countries based on retail information. Pharmacoepidemiol. Drug Saf. 2010, 19, 183–190, doi:10.1002/pds.1893.
- Golden, J.M.; Escobar, O.H.; Nguyen, M.V.L.; Mallicote, M.U.; Kavarian, P.; Frey, M.R.; Gayer, C.P. Ursodeoxycholic acid protects against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2-dependent mechanisms. Am. J. Physiol. Liver Physiol. 2018, 315, G259–G271, doi:10.1152/ajpgi.00354.2017.
- Suleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–58.
- Oliveira, M.M.C.; Silva, M.M.; Moreira, T.L.M.; Couto, V.F.; Coelho, Y.N.; Nunes, C.P. The Chronic Use of Non-Steroid Anti-Inflammatory and Their Adverse Effects. Rev. Cad. Medicina 2019, 2, 90–100.
- Ayyadevara, S.; Bharill, P.; Dandapat, A.; Hu, C.; Khaidakov, M.; Mitra, S.; Reis, R.J.S.; Mehta, J.L. Aspirin Inhibits Oxidant Stress, Reduces Age-Associated Functional Declines, and Extends Lifespan of Caenorhabditis elegans. Antioxid. Redox Signal 2013, 18, 481–490, doi:10.1089/ars.2011.4151.
- Moon, H.-G.; Kim, Y.-S.; Choi, J.-P.; Choi, D.-S.; Yoon, C.M.; Jeon, S.G.; Gho, Y.S.; Kim, Y.-K. Aspirin attenuates the anti-inflammatory effects of theophylline via inhibition of cAMP production in mice with non-eosinophilic asthma. Exp. Mol. Med. 2009, 42, 47–60, doi:10.3858/emm.2010.42.1.005.
- Desborough, M.J.R.; Keeling, D.M. The aspirin story—from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683.
- Bibbins-Domingo, K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836, doi:10.7326/m16-0577.
- Hayashi, S.; Sumi, Y.; Ueno, N.; Murase, A.; Takada, J. Discovery of a novel COX-2 inhibitor as an orally potent anti-pyretic and anti-inflammatory drug: Design, synthesis, and structure-activity relationship. Biochem. Pharmacol. 2011, 82, 755–768, doi:10.1016/j.bcp.2011.06.036.
- Yaghoobi, R.; Kazerouni, A.; Kazerouni, O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 100–104, doi:10.17795/jjnpp-9487.
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; Júnior, G.B.D.S.; Da Silva, G.B. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. Braz. J. Nephrol. 2019, 41, 124–130, doi:10.1590/2175-8239-jbn-2018-0107.
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–47.
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2-p53 interaction. Bioorg. Chem. 2019, 86, 598–608, doi:10.1016/j.bioorg.2019.01.053.
- Abdellatif, K.R.A.; Fadaly, W.A.; Elshaier, Y.A.M.M.; Ali, W.A.; Kamel, G.M. Non-acidic 1,3,4-trisubstituted-pyrazole derivatives as lonazolac analogs with promising COX-2 selectivity, anti-inflammatory activity and gastric safety profile. Bioorg. Chem. 2018, 77, 568–578, doi:10.1016/j.bioorg.2018.02.018.
- El-miligy, M.M.; Hazzaa, A.A.; El-messmary, H.; Nassra, R.A.; E-hawash, S.A. New hybrid molecules combining benzothiophene or benzofuran with rhodanine as dual COX-1/2 and 5-LOX inhibitors: Synthesis, biological evaluation and docking study. Bioorg. Chem. 2017, 72, 102–115.
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346, doi:10.1039/c8ra02358a.
- Owona, B.A.; Abia, W.A.; Moundipa, P.F. Natural compounds flavonoids as modulators of inflammasomes in chronic diseases. Int. Immunopharmacol. 2020, 84, 1–9.
- Anand, P.K. Lipids, inflammasomes, metabolism, and disease. Immunol. Rev. 2020, 00, 1–15, doi:10.1111/imr.12891.
- Newman, D.J. Developing natural product drugs: Supply problems and how they have been overcome. Pharmacol. Therapeut. 2015, 162, 1–9.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661, doi:10.1021/acs.jnatprod.5b01055.
- Arya, V.; Arya, M.L. A review on anti-inflammatory plant barks. Int. J. Pharm. Tech. Res. 2011, 3, 899–908.
- Shah, B.; Seth, A.; Maheshwari, K. A Review on Medicinal Plants as a Source of Anti-inflammatory Agents. Res. J. Med. Plant. 2011, 5, 101–115, doi:10.3923/rjmp.2011.101.115.
- Oguntibeju, O.O. Hypoglycaemic and anti-diabetic activity of selected African medicinal plants. Int J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 224–237.
- Azab, A.N.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321, doi:10.3390/molecules21101321.
- Sharopov, F.; Braun, M.S.; Gulmurodov, I.; Khalifaev, D.; Isupov, S.; Wink, M. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils of selected aromatic plants from Tajikistan. Foods 2015, 4, 645–653.
- Bauri, R.K.; Tigga,M. N.; Saleebkullu, S. A review on use of medicinal plants to control parasites. J. Nat. Prod. Resour. 2015, 6, 268–277.
- Cabral, B.; Siqueira, E.M.S.; Bitencourt, M.A.O.; Lima, M.C.J.S.; Lima, A.K.; Ortmannd, C.F.; Chaves, V.C.; Fernandes-Pedrosa, M.F.; Rochac, H.A.O.; Scortecci, K.C.; et al. Phytochemical study and anti-inflammatory and antioxidant potential of Spondias mombin leaves. Rev. Bras. Farmacog. 2016, 26, 304–311.
- Barbosa, H.M.; Nascimento, J.N.D.; Araújo, T.A.; Duarte, F.S.; Albuquerque, U.P.; Vieira, J.R.; De Santana, E.R.; Yara, R.; Lima, C.S.; Lira, E.C.; et al. Acute Toxicity and Cytotoxicity Effect of Ethanolic Extract of Spondias tuberosa Arruda Bark: Hematological, Biochemical and Histopathological Evaluation. An. Acad. Bras. Ciênc. 2016, 88, 1993–2004, doi:10.1590/0001-3765201620160041.
- Rosas, E.; Correa, L.B.; Pádua, T.D.A.; Costa, T.E.M.M.; Mazzei, J.L.; Heringer, A.P.; Bizarro, C.A.; Kaplan, M.A.C.; Figueiredo, M.R.; Henriques, M.D.G.M.O. Anti-inflammatory effect of Schinus terebinthifolius Raddi hydroalcoholic extract on neutrophil migration in zymosan-induced arthritis. J. Ethnopharmacol. 2015, 175, 490–498, doi:10.1016/j.jep.2015.10.014.
- Rios, R.; Silva, H.B.F.; Carneiro, N.V.Q.; Costa, R.S.; Carneiro, T.C.B.; Marques, C.R.; Machado, M.S.S.; Velozo, E.S.; Silva, T.M.; Silva, T.M.S.; et al. Anti-inflammatory Activity of Jurubeba (Solanum paniculatum L.) Through Reducing the T-bet and GATA3 Gene Expression, In Vitro. J. Allergy Clin. Immunol. 2017, 139, AB268, doi:10.1016/j.jaci.2016.12.865.
- Ortiz, M.I.; Fernández-Martínez, E.; Soria-Jasso, L.E.; Lucas-Gómez, I.; Villagómez-Ibarra, R.; González-García, M.P.; Castañeda-Hernández, G.; Salinas-Caballero, M. Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed. Pharmacother. 2016, 78, 248–256, doi:10.1016/j.biopha.2016.01.029.
- Mostofora, R.; Ahmed, S.; Begum, M.M.; Rahman, M.S.; Begum, T.; Ahmed, S.U.; Tuhin, R.H.; Das, M.; Hossain, A.; Sharma, M.; et al. Evaluation of anti-inflammatory and gastric anti-ulcer activity of Phyllanthus niruri L. (Euphorbiaceae) leaves in experimental rats. BMC Complement Altern. Med. 2017, 17, 267.
- Zahidin, N.S.; Saidin, S.; Zulkifli, R.M.; Muhamad, I.I.; Ya’Akob, H.; Nur, H. A review of Acalypha indica L. (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J. Ethnopharmacol. 2017, 207, 146–173, doi:10.1016/j.jep.2017.06.019.
- Siraj, A.; Shilpi, J.A.; Hossain, G.; Uddin, S.J.; Islam, K.; Jahan, I.A.; Hossain, H. Anti-Inflammatory and Antioxidant Activity of Acalyphahispida Leaf and Analysis of its Major Bioactive Polyphenols by HPLC. Adv. Pharm. Bull. 2016, 6, 275–283, doi:10.15171/apb.2016.039.
- Kyei, S.; Koffuor, G.; Ramkissoon, P.; Ameyaw, E.O.; Asiamah, E.A. Anti-inflammatory effect of Heliotropium indicum Linn on lipopolysaccharide-induced uveitis in New Zealand white rabbits. Int. J. Ophthalmol. 2016, 9, 528–535, doi:10.18240/ijo.2016.04.08.
- Cruz, M.P.; Andrade, C.M.F.; Silva, K.O.; De Souza, E.P.; Yatsuda, R.; Marques, L.M.; David, J.P.; David, J.M.; Napimoga, M.H.; Clemente-Napimoga, J.T. Antinoceptive and Anti-inflammatory Activities of the Ethanolic Extract, Fractions and Flavones Isolated from Mimosa tenuiflora (Willd.) Poir (Leguminosae). PLoS ONE 2016, 11, e0150839, doi:10.1371/journal.pone.0150839.
- Funk, J. L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-Inflammatory Effects of the Essential Oils of Ginger (Zingiber officinale Roscoe) in Experimental Rheumatoid Arthritis. PharmaNutrition 2016, 4, 123–131.
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2015, 30, 1343–1352, doi:10.1080/14786419.2015.1062761.
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; Mamadou, G.; Limas-Nzouzi, N.; Sebai, H.; El-Benna, J.; Marzouki, L.; Eto, B.; Amri, M. Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Adv. 2016, 6, 44345–44353, doi:10.1039/c6ra03457h.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614, doi:10.1016/j.biotechadv.2015.08.001.
- Walker, J.; Reichelt, K.V.; Obst, K.; Widder, S.; Hans, J.; Krammer, G.E.; Ley, J.; Somoza, V. Identification of an anti-inflammatory potential of Eriodictyon angustifolium compounds in human gingival fibroblasts. Food Funct. 2016, 7, 3046–3055, doi:10.1039/C6FO00482B.
- Asadi-Samani, M.; Kafash-Farkhad, N.; Azimi, N.; Fasihi, A.; Alinia-Ahandani, E.; Rafieian-Kopaei, M. Medicinal plants with hepatoprotective activity in Iranian folk medicine. Asian Pac. J. Trop. Biomed. 2015, 5, 146–157, doi:10.1016/s2221-1691(15)30159-3.