Video Upload Options
Transcription factor 21 (TCF21) could promote chicken preadipocytes differentiation at least in part via activating MAPK/JNK pathway.
1. Introduction
2. Overexpression of TCF21 Leads to Enhanced Lipid Droplets Accumulation
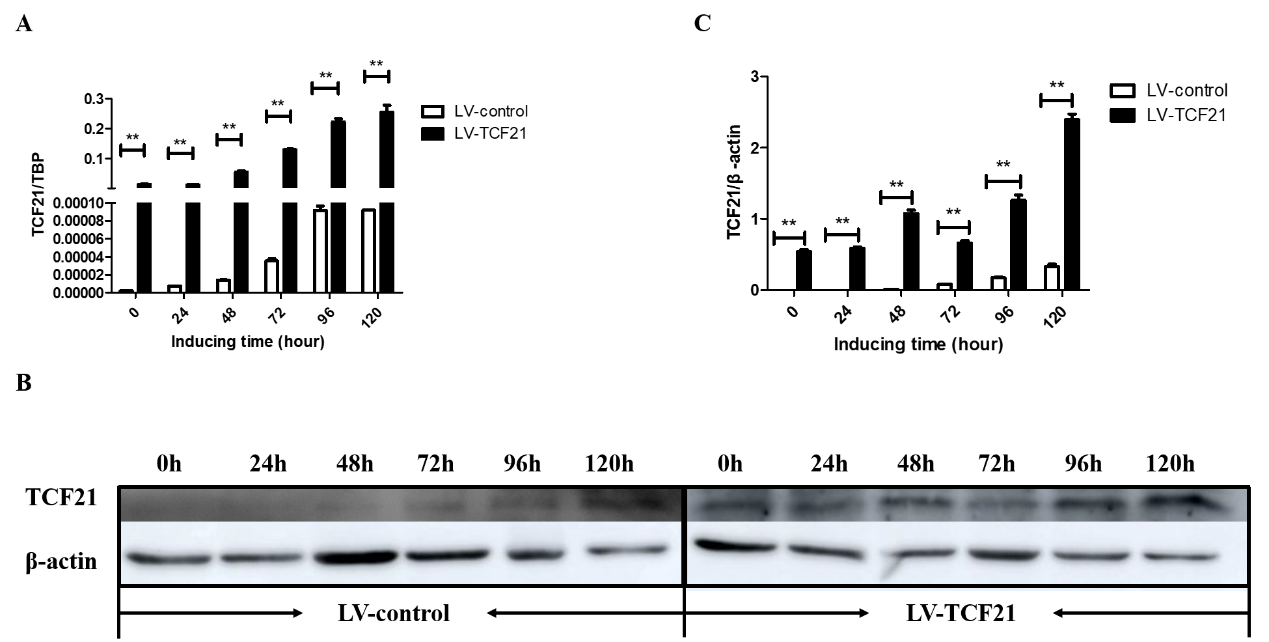
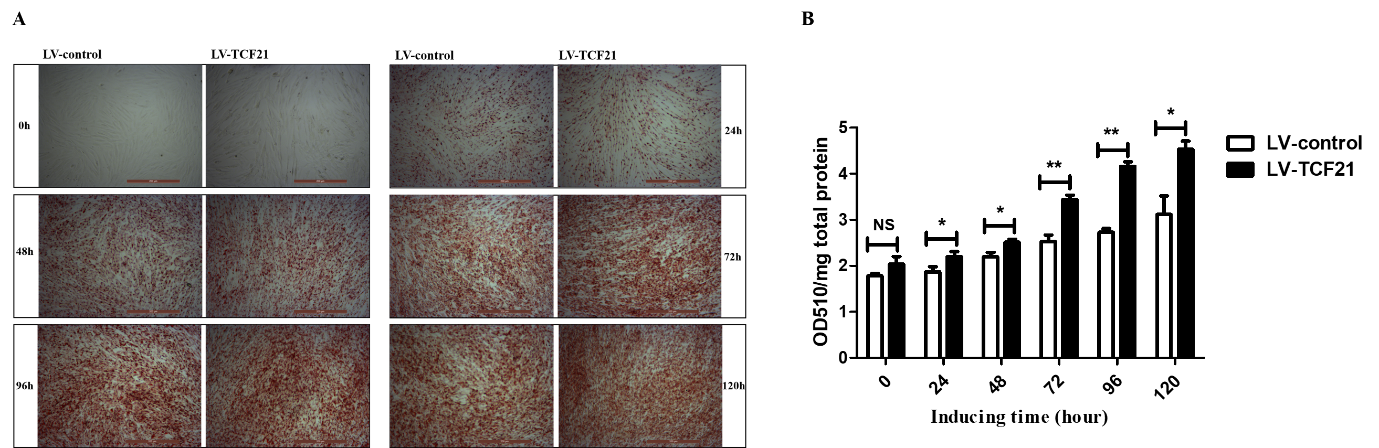
Supplementary Figure S1. Detection of TCF21 over-expression efficiency and its effect on lipid droplets accumulation. Oleic acid was used to induce the differentiation of LV-control and LV-TCF21 preadipocytes for 0 - 120 h. (A) The mRNA expression of TCF21 in LV-control and LV-TCF21 detected by real-time PCR. (B) Images for the protein expression of TCF21 in LV-control and LV-TCF21 detected by western blot (representative of three independent experiments). (C) The quantification of protein bands by Image J. (D) Images of the accumulation of lipid droplets in LV-control and LV-TCF21 cells by Oil red-O staining (representative of three independent
experiments). (E) The Oil red-O dye was extracted from stained LV-control and LV-TCF21 preadipocytes at the indicated time points in order to quantify staining intensity. Graphs are plotted as mean ±SE from three independent experiments. NS, no significance, *p<0.05, ** p< 0.01
3. MAPK/JNK Signaling Pathway Was Activated by TCF21 Overexpression
| Pathway |
LV-control(mean±SE) |
LV-TCF21(mean±SE) |
P-value |
|
Amino acid deprivation |
0.41 ± 0.37 |
0.062 ± 0.043 |
0.44 |
|
Androgen |
/ |
/ |
/ |
|
Antioxidant response |
/ |
/ |
/ |
|
ATF6 |
0.015 ± 0.0048 |
0.013 ± 0.0027 |
0.72 |
|
C/EBP |
/ |
/ |
/ |
|
cAMP/PKA |
/ |
/ |
/ |
|
Cell cycle |
/ |
/ |
/ |
|
DNA damage |
/ |
/ |
/ |
|
EGR1 |
/ |
/ |
/ |
|
ER stress |
0.95 ± 0.72 |
0.90 ± 0.60 |
0.96 |
|
Estrogen |
/ |
/ |
/ |
|
GATA |
/ |
/ |
/ |
|
Glucocorticoid |
/ |
/ |
/ |
|
Heat shock |
/ |
/ |
/ |
|
Heavy metal |
0.090 ± 0.065 |
0.11 ± 0.078 |
0.87 |
|
Hedgehog |
/ |
/ |
/ |
|
HNF4 |
/ |
/ |
/ |
|
Hypoxia |
/ |
/ |
/ |
|
Interferon regulation |
/ |
/ |
/ |
|
Type 1 interferon |
/ |
/ |
/ |
|
Interferon-r |
/ |
/ |
/ |
|
KLF4 |
/ |
/ |
/ |
|
Liver X |
/ |
/ |
/ |
|
MAPK/Erk |
0.12 ± 0.043 |
0.11 ± 0.044 |
0.88 |
|
MAPK/Jnk |
0.028 ± 0.0060 |
0.19 ± 0.014 |
0.000423 |
|
MEF2 |
/ |
/ |
/ |
|
Myc |
/ |
/ |
/ |
|
Nanog |
/ |
/ |
/ |
|
Notch |
/ |
/ |
/ |
|
NFκB |
0.081 ± 0.037 |
0.20 ± 0.11 |
0.36 |
|
Oct4 |
/ |
/ |
/ |
|
Pax6 |
/ |
/ |
/ |
|
PI3K/Akt |
/ |
/ |
/ |
|
PKC/Ca+2 |
/ |
/ |
/ |
|
PPAR |
/ |
/ |
/ |
|
Progesterone |
/ |
/ |
/ |
|
Retinoic acid |
/ |
/ |
/ |
|
Retinoid X |
/ |
/ |
/ |
|
Sox2 |
/ |
/ |
/ |
|
SP1 |
0.3 ± 0.28 |
0.097 ± 0.067 |
0.52 |
|
STAT3 |
/ |
/ |
/ |
|
TGF-β |
/ |
/ |
/ |
|
Vitamin D |
/ |
/ |
/ |
|
Wnt |
/ |
/ |
/ |
|
Xenobiotic |
/ |
/ |
/ |
|
Negative control |
0.00057 ± 0.000067 |
0.000625 ± 0.000062 |
0.59 |

Figure 1. MAPK/JNK signaling pathway was activated by TCF21 overexpression. At 24 h post-induction of differentiation, lysates from LV-control and LV-TCF21 cells were collected. (A) A schematic overview of the constructs used for the Cignal Finder 45-Pathway Reporter Array. A. The inducible transcription factor-responsive construct expressing firefly luciferase. B. The constitutively expressing Renilla luciferase construct. C. The non-inducible firefly luciferase reporter construct. D. The constitutively expressing GFP construct. E. The constitutively expressing firefly luciferase construct. The negative control is a mixture of C. and B. (20:1). The positive control is a mixture of D., E. and B. Each reporter is a mixture of A. and B. (20:1). (B) A Luciferase activity-based array was used in order to identify those signaling pathways that were responsive to overexpression of TCF21. Graphs are plotted as mean ± SE relative to luciferase activity in LV-control cells from three independent experiments; (C) images for TCF21, JNK1, JNK2, p-JNK1, p-JNK2, and β-actin expressions in cells by Western blotting; (D) bands intensities were quantified by Image J software. Graphs are plotted as mean ± SE from three independent experiments. ** p < 0.01.
4. MAPK/JNK Signaling and Lipid Droplets Accumulation Are Inhibited by SP600125 in a Dose-Dependent Manner
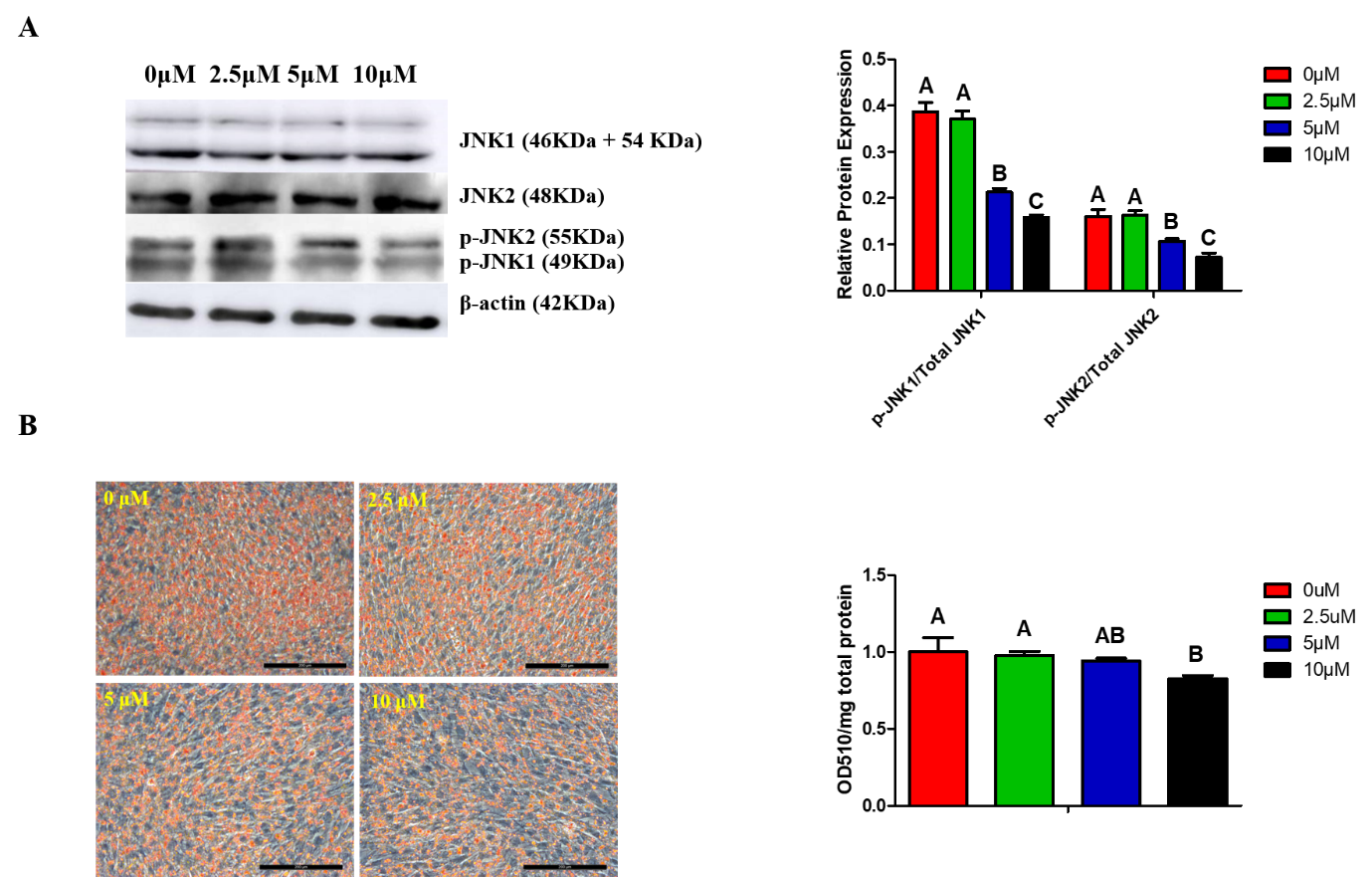
Figure 2. MAPK/JNK signaling and lipid droplets accumulation were inhibited by SP600125 in a dose-dependent manner. At 24 h post-induction of differentiation, ICP cells were then incubated for an additional 24 h in differentiation medium containing 0, 2.5, 5, or 10 μM SP600125. (A) Images for JNK1, JNK2, p-JNK1, p-JNK2, and β-actin expressions in cells treated with different concentrations of SP600126 by Western blotting (representative of three independent experiments). Then, the bands intensities were quantified by Image J software. Graphs are plotted as mean ± SE from three independent experiments. Different uppercase letters above columns denote significant differences; (B) images for oil-red O staining of lipid droplets in preadipocytes treated with different concentrations of SP600125 (representative of three independent experiments). Then, oil-red O dye was extracted from the cells treated with different concentrations of SP600125 in order to quantify staining intensity. Graphs are plotted as mean ± SE from three independent experiments. Different uppercase letters above columns denote significant differences.
5. Inhibition of MAPK/JNK Signaling Attenuates TCF21-Mediated Promotion of Preadipocyte Differentiation
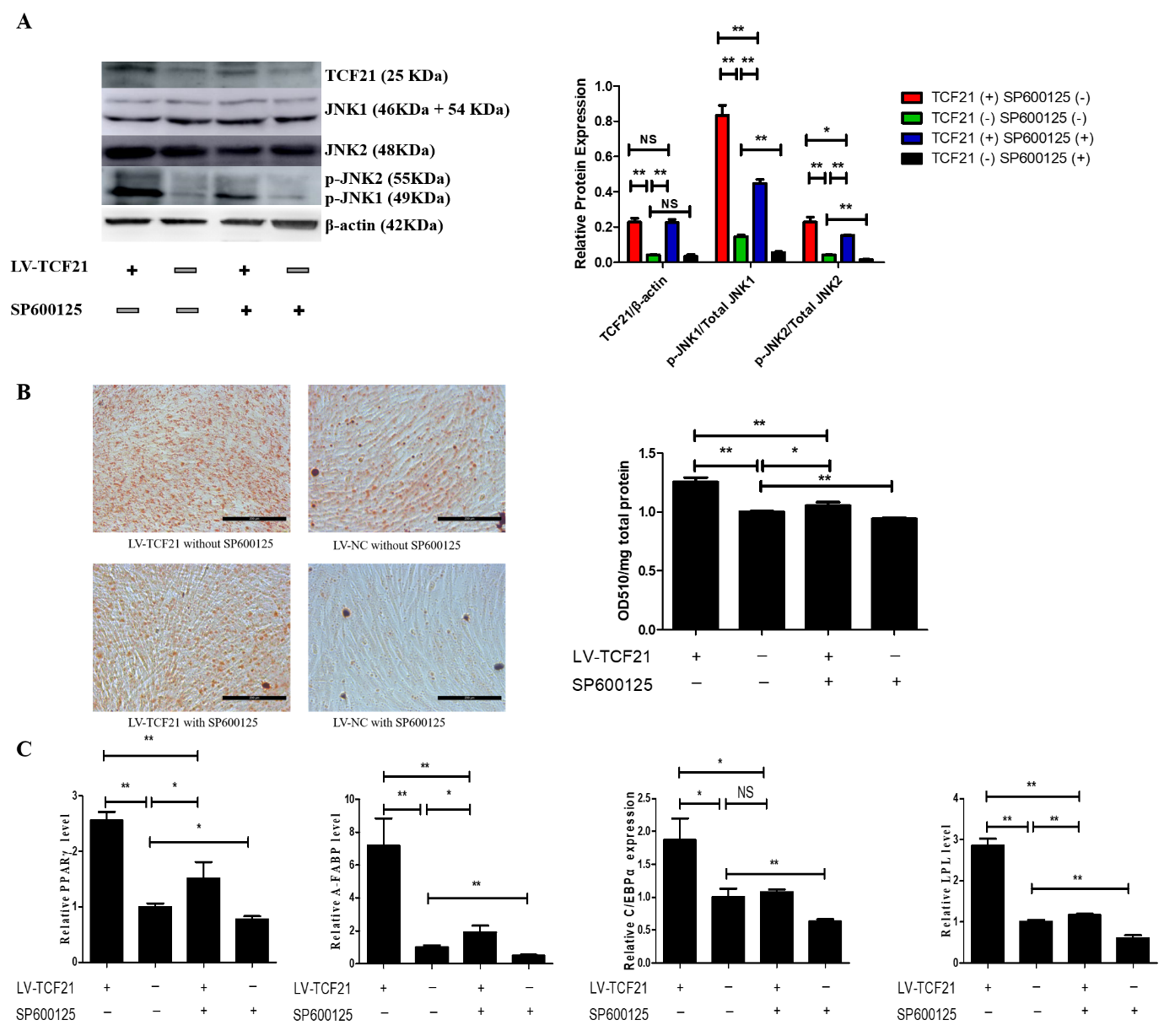
Figure 3. Inhibition of MAPK/JNK signaling attenuates TCF21-mediated enhancement of preadipocyte differentiation. At 24 h post-induction of differentiation, LV-TCF21 and LV-control preadipocytes were then incubated for an additional 24 h in differentiation medium containing either 0 or 10 μM SP600125. (A) Images for JNK1, JNK2, p-JNK1, p-JNK2, and β-actin expressions in LV-control or LV-TCF21 cells treated with 0 or 10 μM SP600126 by Western blotting (representative of three independent experiments). Then, the bands intensities were quantified by Image J software. Graphs are plotted as mean ± SE from three independent experiments. NS, no significance, * p < 0.05, ** p < 0.01; (B) images for oil-red O staining of lipid droplets in differentiated LV-control or LV-TCF21 preadipocytes treated with 0 or 10 μM SP600125 (representative of three independent experiments). Then, oil-red O dye was extracted from the cells in order to quantify staining intensity. Graphs are plotted as mean ± SE from three independent experiments relative to staining intensity of LV-control treated with 0 μM SP600125. * p < 0.05, ** p < 0.01; (C) expressions of pro-adipogenic genes in differentiated LV-control or LV-TCF21 preadipocytes treated with 0 or 10 μM SP600125 by real-time PCR. Graphs are plotted as mean ± SE from three independent experiments relative to the gene expression in LV-control treated with 0 μM SP600125. NS, no significance, * p < 0.05, ** p < 0.01.
References
- Mallard, J.; Douaire, M. Strategies of selection for leanness in meat production. In Leanness in Domestic Birds: Genetic, Metabolic and Hormonal Aspects; Leclerq, B., Whitehead, C.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 3–23.
- Julibert, A.; Bibiloni, M.D.M.; Mateos, D.; Angullo, E.; Tur, J.A. Dietary fat intake and metabolic syndrome in older adults. Nutrients 2019, 11, 1901.
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16.
- Wu, G.Q.; Deng, X.M.; Li, J.Y.; Li, N.; Yang, N. A potential molecular marker for selection against abdominal fatness in chickens. Poult. Sci. 2006, 85, 1896–1899.
- Sahraei, M. Feed restriction in broiler chickens production: A review. Glob. Vet. 2012, 8, 449–458.
- Julian, R.J. Production and growth related disorders and other metabolic diseases of poultry—A review. Vet. J. 2015, 169, 350–369.
- Chen, C.Y.; Huang, Y.F.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Development of metabolic cardiomyopathy. Poult. Sci. 2017, 96, 2438–2446.
- Chen, C.Y.; Lin, H.Y.; Chen, Y.W.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Pathological adaption of cardiac hypertrophy. Poult. Sci. 2017, 96, 2428–2437.
- Zhang, X.Y.; Wu, M.Q.; Wang, S.Z.; Zhang, H.; Du, Z.Q.; Li, Y.M.; Cao, Z.P.; Luan, P.; Leng, L.; Li, H. Genetic selection on abdominal fat content alters the reproductive performance of broilers. Animal 2018, 12, 1232–1241.
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736.
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108.
- Rosen, E.D.; Macdougald, O.A. Adipocyte differentiation from inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896.
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734.
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236.
- Zhang, X.; Cheng, B.; Liu, C.; Du, Z.; Zhang, H.; Wang, N.; Wu, M.; Li, Y.; Cao, Z.; Li, H. A novel regulator of preadipocyte differentiation, transcription factor TCF21, functions partially through promoting LPL expression. Front. Physiol. 2019, 10, 458.