Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrii Vasin | + 1994 word(s) | 1994 | 2021-12-10 01:46:28 | | | |
| 2 | Conner Chen | Meta information modification | 1994 | 2021-12-20 02:15:53 | | | | |
| 3 | Conner Chen | Meta information modification | 1994 | 2021-12-20 02:17:18 | | | | |
| 4 | Conner Chen | Meta information modification | 1994 | 2021-12-20 02:18:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vasin, A. Luminescent Carbon Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/17310 (accessed on 07 February 2026).
Vasin A. Luminescent Carbon Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/17310. Accessed February 07, 2026.
Vasin, Andrii. "Luminescent Carbon Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/17310 (accessed February 07, 2026).
Vasin, A. (2021, December 20). Luminescent Carbon Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/17310
Vasin, Andrii. "Luminescent Carbon Nanoparticles." Encyclopedia. Web. 20 December, 2021.
Copy Citation
Among the newest nanostructured luminescent materials, carbon nanoparticles, commonly known as carbon nanodots (CNDs), are of special interest. The term CNDs is commonly used to refer to carbonaceous particles with a size of less than 10 nm. However, the structure and morphology of these particles can be quite diverse. They can include nanoparticles of diamond and graphite, as well as amorphous nanoparticles with a diamond-like, polymer-like, or graphite-like structure. Nanoflakes of graphene and graphene oxide are also often referred to as CNDs. This manuscript referes to luminescent carbon nanoprecipitates synthesised by pyrolysis/thermolysis of organic precursors and despersed in nanoporous silicas.
luminescent materials
luminescent carbon nanoparticles
1. Introduction
Luminescent materials take an important place in many areas of human activity and in daily life, including various optoelectronic devices, artificial lighting, and visualization systems. In recent decades, new luminescent materials based on nanosized particles have been actively developed. The luminescent properties of such materials have many advantages compared to those of the bulk material, due to their large specific surface areas and quantum confinement effects [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16]. Among the newest nanostructured luminescent materials, carbon nanoparticles, commonly known as carbon nanodots (CNDs), are of special interest. The term CNDs is commonly used to refer to carbonaceous particles with a size of less than 10 nm. However, the structure and morphology of these particles can be quite diverse. They can include nanoparticles of diamond and graphite, as well as amorphous nanoparticles with a diamond-like, polymer-like, or graphite-like structure [10][11][13][17][18][19]. Nanoflakes of graphene and graphene oxide are also often referred to as CNDs [10][11][17][19].
Unlike the well-defined carbon atom arrangements in fullerenes, nanotubes, graphenes, and diamond nanoparticles, the structural configurations of CNDs are quite uncertain and cannot be definitely specified. Usually, they are represented as combinations of molecular-like polycyclic clusters with sp2-coordinated carbon atoms, as well as aggregates of these clusters and amorphous sp3-coordinated networks. Due to their lack of a clear shape, CNDs have a developed defective surface with a variety of functional groups and dangling bonds on it. The variety of properties of luminescent centers in CNDs determines the characteristic features of the photoluminescence (PL) spectra of this material, such as a broad emission band, as well as the dependence of the emission spectrum on the wavelength of the exciting radiation, i.e., the so called “excitation dependent photoluminescence” [10][11][17][20][21].
Unlike luminescent semiconductor quantum dots, CNDs are quite a friendly material for the human body; therefore, this material is under intensive development as an effective nanoscale luminescent label for the visualization of biological objects in biomedical experiments. The excitation-dependent emission of CNDs used as fluorescent nanomarkers in biological experiments makes it possible to identify a useful CND signal from the interfering fluorescence of cells or contaminants. It should be noted, though, that excitation dependence is not a “universal” feature and, in some research studies, it has not been observed [17][22][23][24]. Compared to semiconductor nanoparticles and organic fluorophores, CNDs are not subject to the effect of blinking (fluorescence intermittency) [17][19][25]. Due to their relatively high synthesis temperature, CNDs are stable in the temperature range of biomedical experiments and can be stored for a long time at room temperature (without refrigeration). They are also stable in a wide range of pH environments (high ion strength) [26][27]. At present, CNDs can be considered as a separate segment of luminescent materials for the visualization of biological objects [28].
There are many methods of synthesizing CNDs. They can, in general, be classified into two types: (1) “from-top-to-bottom” methods, i.e., the fragmentation of bulk material into nanoparticles, and (2) “from-bottom-to-top” methods, i.e., synthesis from organic molecular precursors along with surface fictionalization and passivation [11][17]. The “from-bottom-to-top” methods have key advantages over the “from-top-to-bottom” methods, including being more environmentally friendly, less time-consuming, and allowing for the easy modification of the surface state and composition of the CNDs [17]. A huge number of the processes related to the “from-bottom-to-top” concept and corresponding carbon precursors have been reported. The impressive variety of methods and materials indicates that the formation of luminescent CNDs over the course of spontaneous carbon precipitation is a rather general property.
2. Oxidized Porous Si:C
Thin layers of porous SIO2:C were formed on the silicon wafer using the following sequence of procedures: electrochemical etching of the Si wafer (formation of porous Si layer) -> carbonization in the flow of acetylene at a high temperature (formation of porous Si:C layer) -> selective oxidation of the porous Si network using water vapor (formation of porous SiO2:C layer) [29]. The specific surface area of the as-prepared porous silicon layer was 200–300 m2/g [30], and this sample exhibited red PL, which is typical for such kinds of materials. The carbonization procedure led to the quenching of the red emission and no PL was observed after this step. After the wet oxidation of the carbonized layer, a strong visible PL, which could be observed by the eye as a white light (Figure 1a,b), was observed under 351 nm excitation.
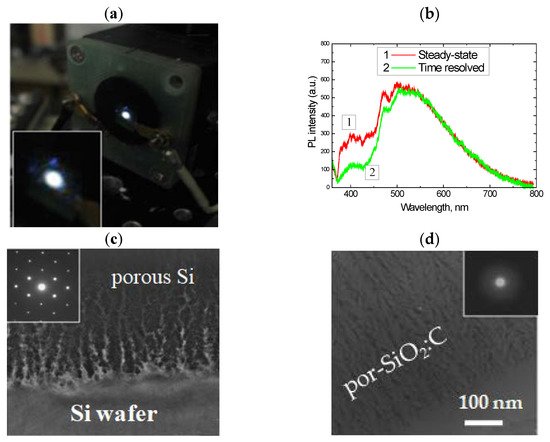
Figure 1. (a) Emission spot of por-SiO2:C layer under 351 nm excitation (Ar+ laser); (b) PL spectra of por-SiO2:C layer measured in steady-state mode and in time-resolved mode; (c,d) TEM image of morphology of the carbonized porous silicon layer before and after wet oxidation. Insets in (c) and (b) represent electron diffraction patterns.
The electron diffraction pattern of the samples subjected to wet oxidation at 800 °C showed diffused halos, indicating the full conversion of the crystalline porous Si into amorphous silicon oxide. However, the morphology of the layer remained porous after oxidation treatment (Figure 1c,d). The oxidation rate of nanostructured silicon in water vapor at such temperatures was high, while the pyrolytical carbon on the silicon surface was quite inert to the water molecules. Therefore, the oxidation of carbonized porous silicon resulted in the formation of a porous silica network filled with carbon nanoprecipitates located on the silica surface and/or encapsulated in the silica.
The spectral properties and intensity of PL in the por-SiO2:C layers strongly depended on the synthesis conditions. A common feature of light emission is a broad spectral distribution spread from near UV to near IR, with a maximum-intensity variable from blue to green, depending on the synthesis procedure [30]. Time-resolved PL measurements using an N2 laser (pulse duration 10 ns) and a stroboscopic registration system (cutting PL signal within 0.1 ns gate and with 0.7–1.0 ns delay from the start of the laser pulsing) demonstrated that such a broad emission band is not homogeneous, but rather, is composed of a “blue” component with a slow response and a basic “green” band with a faster response (Figure 1b).
The fast component was attributed to the emission centers associated with the carbon that was incorporated into the porous silica layer, and the slow one was attributed to the triplete states of the point defects in the silicon oxide matrix. This assumption was approved by evolution of the PL after annealing in oxygen at 600 °C [31]. After the removal of carbon from the porous layer (Figure 2a), the low-energy PL band was quenched and only the blue component remained detectable (Figure 2b). The inset in the figure shows the result of subtracting the spectrum “after annealing” from the spectrum “before annealing”. This spectrum can be identified as “carbon-related”.
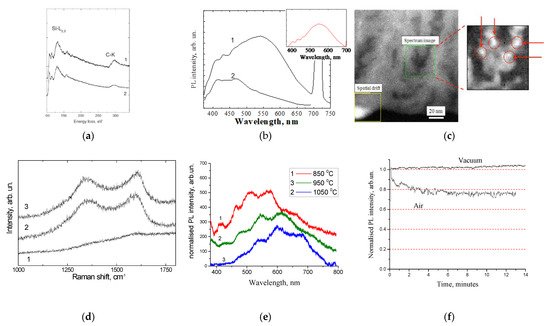
Figure 2. (a) Electron energy loss spectroscopy and (b) PL spectra of por-SiO2:C layer before and after annealing in oxygen at 600 °C (reprinted from ref. [31]); (c) HRSTEN/EELS image of distribution of carbon inside pore (reprinted from ref. [32]); (d) Raman scattering spectra of porous silicon carbonized at different temperature: 850 °C (1), 950 °C (2), 1050 °C (3) (reprinted from ref. [33]); (e) PL spectra of por-SiO2:C samples synthesized using different carbonization temperatures; (f) evolution of PL intensity in por-SiO2:C in time.
The analysis of the distribution of carbon in the porous layers, using high-resolution scanning transmission electron microscopy (HRSTEM) in combination with energy filtered electron energy loss spectroscopy (Figure 2c), showed that this distribution was not uniform, and that it was characterized by evident precipitates of a few nanometers in size [32].
The effect of the amount of carbon in the porous layer was investigated using samples that were synthesized using different carbonization temperatures [33]. The Raman scattering spectra indicated that the increasing of the carbonization temperature resulted in the growth of the amount of carbon inside the porous layer (Figure 2d), which was accompanied by red shifting of the carbon-related emission (Figure 2e). This spectral shift was preliminary assigned to the growth of the size of the carbon precipitates. As will be shown in the next sections, this effect was quite common for all kinds of luminescent CNDs. The periodic features on the PL spectra (Figure 2e) were caused by the interference of the emission light in the thin porous layers.
The analysis of the time-dependent evolution of the PL intensity under the UV radiation showed significant photo-bleaching in the por-SiO2:C layers [33]. It is important to note that photo-bleaching was observed only under atmospheric conditions, while under vacuum conditions, the dynamics of changes in the PL intensity were the opposite, i.e., a slight increase in the PL intensity was observed during the period of the experiment (Figure 2f). Such a behavior implies that the mechanism of photo-bleaching in the por-SiO2:C layers was similar to that of the organic fluorophores, i.e., light stimulated the oxidation of the emitters via the oxygen contents in the air.
3. Luminescent a-SiOC:H Thin Films
A similar methodological concept to the oxidation of carbonized porous silicon layers was adopted for the amorphous silicon-carbon alloy thin films deposited using the magnetron sputtering technique. It is well known that crystalline silicon carbide (SiC) is a chemically inert and corrosion-resistant material. However, low density amorphous hydrogenated silicon-carbon alloy films (a-Si1−xCx:H) deposited at low substrate temperatures can be easily oxidized by oxygen or water vapor at temperatures as low as 700 °C, or even lower [34][35][36]. As was found earlier, a-Si1−xCx:H enriched by carbon (x > 0.5) exhibiteda higher oxidation rate than that of the stochiometric films (x = 0.5). Figure 3a represents the evolution of the PL of the as-deposited carbon-rich a-SiC:H thin films after annealing in dry Ar and wet Ar at 450 °C for 30 min. The PL spectrum of the as-deposited film is a broad emission band covering the entire visible range.
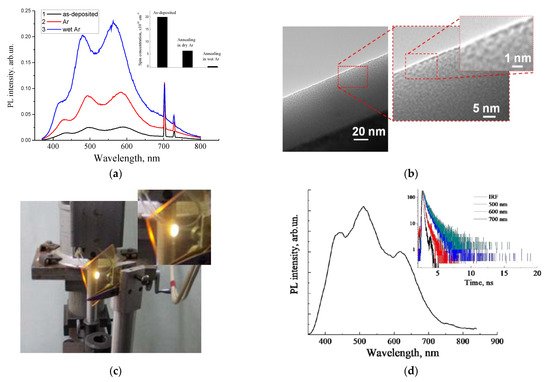
Figure 3. (a) PL spectra of the as-deposited and annealed carbon-rich a-SiC:H thin film (inset shows the corresponding evolutions of concentrations of carbon-related paramagnetic centers); (b) high-resolution transmission electron microscopy image of the nanoscale morphology of the oxidized a-SiC:H thin film;(c) photo image of the emission spot on the as-deposited a-SiOC:H film (excitation by 408 nm), deposited on the glass substrate; (d) corresponding PL spectrum and decay curves, measured at different emission wavelengths.
Periodic modulation occurred due to the effect of interference [33][35][37], which was similar to that observed in por-SiO2:C thin layers. It can be seen that the PL intensity significantly increased after low-temperature annealing in an inert medium, which was associated with the passivation of paramagnetic dangling bonds by hydrogen, which were released from metastable bound states at low temperature, or with oxidation with residual oxygen [35][38].
A further increase in the PL intensity was observed after oxidation with water vapor; that was correlated with the further decreasing of the concentration of the paramagnetic centers (inset in Figure 3d). FTIR measurements revealed a partial oxidation of the amorphous SiC network. HRTEM examination of the carbon-rich film showed an inhomogeneous density distribution (Figure 3b), which can be interpreted as the nanoporous morphology of the amorphous SiC/SiOx:C film.
An alternative process of formation of luminescent silica-carbon layers without the oxidation annealing procedure, performedthrough the deposition of carbon-enriched a-SiOC:H films using excess carbon precursor (methane) in the magnetron deposition process, was suggested [39]. Strong photoluminescence was observed only in a-SiOC:H films with high carbon incorporation (Figure 3c). The emission bands of such a-SiOC:H films were quite similar to those observed in the por-SiO2:C layers and oxidized carbon-rich a-SiC:H thin films, with similar regular interference features in the spectrum distribution (Figure 3d).
References
- Gao, P.; Wang, G.; Zhou, L. Luminescent sulfur quantum dots: Synthesis, properties and potential applications. ChemPhotoChem 2020, 4, 5235–5244.
- Costa-Fernandez, J.M.; Pereiro, R.; Sanz-Medel, A. The use of luminescent quantum dots for optical sensing. Trends Anal. Chem. 2006, 25, 207–218.
- Ma, Y.; Zhang, Y.; Yu, W.W. Near infrared emitting quantum dots: Synthesis, luminescence properties and applications. J. Mater. Chem. C 2019, 7, 13662–13679.
- Purcell-Milton, F.; Gun’ko, Y.K. Quantum dots for luminescent solar concentrators. J. Mater. Chem. 2012, 22, 16687–16697.
- Sreenivasan, V.K.A.; Zvyagin, A.V.; Goldys, E.M. Luminescent nanoparticles and their applications in the life sciences. J. Phys. Condens. Matter. 2013, 25, 194101.
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592.
- Shi, D.; Guo, Z.; Bedford, N. Basic properties of nanomaterials. In Nanomaterials and Devices; Shi, D., Guo, Z., Bedford, N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Tsinghua University Press: Beijing, China, 2015; pp. 1–23.
- Shi, D.; Guo, Z.; Bedford, N. Semiconductor quantum dots. In Nanomaterials and Devices; Shi, D., Guo, Z., Bedford, N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Tsinghua University Press: Beijing, China, 2015; pp. 83–104.
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T.; et al. Carbon nanodots: Towards a comprehensive understanding of their photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316.
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcarce, L.M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326.
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381.
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807.
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671.
- Tuerhong, M.; Yang, X.; Yin, X.-B. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150.
- Li, J.-L.; Tang, B.; Yuan, B.; Sun, L.; Wang, X.-G. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials 2013, 34, 9519–9534.
- Elvati, P.; Baumeister, E.; Violi, A. Graphene quantum dots: Effect of size, composition and curvature on their assembly. RSC Adv. 2017, 7, 17704–17710.
- Jorns, M.; Pappas, D. A review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications. Nanomaterials 2021, 11, 1448.
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316.
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195.
- Dekaliuk, M.O.; Viagin, O.; Malyukin, Y.V.; Demchenko, A.P. Fluorescent carbon nanomaterials: ‘‘quantum dots’’ or nanoclusters? Phys. Chem. Chem. Phys. 2014, 16, 16075–16084.
- Fu, M.; Erhart, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolyarczyk, J.K.; Urban, A.S.; Feldman, J. Carbon dots: A unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett. 2015, 15, 6030–6035.
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. Engl. 2013, 52, 7800–7804.
- Yang, Z.; Xu, M.; Liu, Y.; He, F.; Gao, F.; Su, Y.; Wei, H.; Zhang, Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895.
- Ganguly, S.; Das, P.; Banerjee, S.; Das, N.C. Advancement in science and technology of carbon dot-polymer hybrid composites: A review. Funct. Compos. Struct. 2019, 1, 022001.
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757.
- Chandra, A.; Singh, N. Cell microenvironment pH sensing in 3D microgels using fluorescent carbon dots. ACS Biomater. Sci. Eng. 2017, 3, 3620–3627.
- Xu, J.; Wang, C.; Li, H.; Zhao, W. Synthesis of green-emitting carbon quantum dots with double carbon sources and their application as a fluorescent probe for selective detection of Cu2+ ions. RSC Adv. 2020, 10, 2536–2544.
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768.
- Vasin, A.V.; Ishikawa, Y.; Shibata, N.; Salonen, J.; Lehto, V.-P. Strong white photoluminescence from carbon-Incorporated silicon oxide fabricated by preferential oxidation of silicon in nano-structured Si:C layer. Jpn. J. Appl. Phys. 2007, 46, L465–L467.
- Ishikawa, Y.; Vasin, A.V.; Salonen, J.; Muto, S.; Lysenko, V.S.; Nazarov, A.N.; Shibata, N.; Lehto, V.-P. Color control of white photoluminescence from carbon incorporated silicon oxide. J. Appl. Phys. 2008, 104, 083522-1–083522-6.
- Vasin, A.V.; Muto, S.; Ishikawa, Y.; Salonen, J.; Nazarov, A.N.; Lysenko, V.S.; Okholin, P. Attribution of white-light emitting centers with carbonized surface in nano-structured SiO2:C layers. Thin Solid Films 2011, 519, 4008–4011.
- Savchenko, D.; Vasin, A.; Muto, S.; Kalabukhova, E.; Nazarov, A. EPR study of porous Si:C and SiO2:C layers. Phys. Status Solidi B 2018, 255, 1700559.
- Vasin, A.; Rusavsky, A.; Nazarov, A.; Lysenko, V.; Rudko, G.; Piryatinski, Y.; Blonsky, I.; Salonen, J.; Makila, E.; Starik, S. Excitation effects and luminescence stability in porous SiO2:C layers. Phys. Status Solidi A 2012, 209, 1015–1021.
- Sundaram, K.B.; Alizadeh, Z.; Chow, L. The effects of oxidation on the optical properties of amorphous SiC films. Mater. Sci. Eng. B 2002, 90, 47–49.
- Vasin, A.V.; Ishikawa, Y.; Kolesnik, S.P.; Konchits, A.A.; Lysenko, V.S.; Nazarov, A.N.; Rudko, G.Y. Light-emitting properties of amorphous Si:C:O:H layers fabricated by oxidation of carbon-rich a-Si:C:H films. Solid State Sci. 2009, 11, 1833–1837.
- Vasin, A.V.; Muto, S.; Ishikawa, Y.; Rusavsky, A.V.; Kimura, T.; Lysenko, V.S.; Nazarov, A.N. Comparative study of annealing and oxidation effects inSiC:H and a-SiC thin films deposited by radio-frequency magnetron sputtering techniques. Thin Solid Films 2011, 519, 2218–2222.
- Wang, Y.; Townsend, P.D. Common mistakes in luminescence analysis. J. Phys. Conf. Ser. 2012, 398, 012003.
- Vivaldo, I.; Ambrosio, R.C.; López, R.; Flores-Méndez, J.; Sánchez-Gaspariano, L.A.; Moreno, M.; Candia, F. Enhanced photoluminescence of hydrogenated amorphous silicon carbide thin films by means of a fast thermal annealing process. Materials 2020, 13, 2643.
- Vasin, A.V.; Rusavsky, A.V.; Kysil, D.V.; Prucnal, S.; Piryatinsky, Y.P.; Starik, S.P.; Nasieka, I.; Strelchuk, V.V.; Lysenko, V.S.; Nazarov, A.N. The effect of deposition processing on structural and luminescent properties of a-SiOC:H thin films fabricated by RF-magnetron sputtering. J. Lumin. 2017, 191, 102–106.
More
Information
Subjects:
Acoustics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
953
Revisions:
4 times
(View History)
Update Date:
20 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




