Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael Bouvet | + 2595 word(s) | 2595 | 2021-12-07 10:32:12 | | | |
| 2 | Amina Yu | Meta information modification | 2595 | 2021-12-20 04:33:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bouvet, M. Fluorescent Anti-CEA Antibodies and Cancers. Encyclopedia. Available online: https://encyclopedia.pub/entry/17292 (accessed on 07 February 2026).
Bouvet M. Fluorescent Anti-CEA Antibodies and Cancers. Encyclopedia. Available at: https://encyclopedia.pub/entry/17292. Accessed February 07, 2026.
Bouvet, Michael. "Fluorescent Anti-CEA Antibodies and Cancers" Encyclopedia, https://encyclopedia.pub/entry/17292 (accessed February 07, 2026).
Bouvet, M. (2021, December 18). Fluorescent Anti-CEA Antibodies and Cancers. In Encyclopedia. https://encyclopedia.pub/entry/17292
Bouvet, Michael. "Fluorescent Anti-CEA Antibodies and Cancers." Encyclopedia. Web. 18 December, 2021.
Copy Citation
Carcinoembryonic antigen (CEA) is a membrane-bound glycoprotein expressed in over 80% of colorectal cancers as well as lung, breast, pancreatic, gallbladder, bladder, ovarian and gastric cancer. Antibodies to CEA conjugated to a near-infrared dye have been studied to help detect, resect and treat a variety of GI cancers. This review covers all the near-infrared anti-CEA antibodies used in mouse models and clinical trials since 1990.
carcinoembryonic antigen
CEA
fluorescence-guided surgery
fluorescence
1. Colorectal Cancer
1.1. Subcutaneous Mouse Models
The first in vivo imaging study to evaluate an anti-CEA antibody conjugated to a fluorophore was performed by Pèlegrin et al. [1]. Human CRC cell line (T380) was used to establish subcutaneous tumors in mice which were then injected with MoAB 35 (CEA specific antibody) conjugated to fluorescein (non-specific IgG antibody was used as a control). Mice were imaged between 6 and 96 h, and at all time points, the fluorescence signal was greater in the tumor than liver or muscle. Pèlegrin et al. also used dye alone as a control. The authors found the tumor-to-liver ratio with MoAB 35-fluorescein was 120:1 compared to 1:1 with dye alone.
The most common human CRC cell line used to establish murine models was the CEA-positive, commercially available cell line, LS174T. In an LS174T subcutaneous murine model, Berk et al. used a fluorescently tagged anti-CEA antibody (ZCEO25) to quantify ligand-receptor density and to calculate an association constant confirming high affinity binding of the fluorescently labeled antibody to the tumor [2]. Lisy et al. [3] and Kaushal et al. [4] also investigated different anti-CEA fluorescent antibodies to LS174T subcutaneous murine models, showing higher TBR with anti-CEA fluorescent antibodies than either the control arm, nonspecific IgG conjugated dye, or a low CEA-expressing arm. Different CRC cell lines (e.g., SW1222, C15A3), with different anti-CEA fluorescent antibodies, showed similar successful results in subcutaneous murine models [5][6].
The subcutaneous models of CRC convincingly demonstrated that anti-CEA fluorescent antibodies can selectively label subcutaneous tumors and provided vital information regarding timing and dosing of different antibody-dye conjugates.
1.2. Orthotopic and Intraperitoneal Mouse Models
Kaushal et al. reported the first use of anti-CEA florescent antibodies in a patient-derived orthotopic xenograft (PDOX) model of CRC (Colo4104) [4]. The PDOX tumor was brightly and specifically labeled by an anti-CEA fluorescent antibody compared to the nonspecific IgG control. Fluorescence-guided surgery (FGS) with anti-CEA fluorescent antibodies led to improved rates of R0 resection in a CRC PDOX model [7] and increased disease-free survival (DFS) and overall survival (OS) [8]. Anti-CEA fluorescent antibodies also labeled HT-29 cell line CRC tumors in orthotopic models with improved operative outcomes [9][10][11].
Other preclinical models can be created by tumor cell injection. Intraperitoneal (IP) murine CRC metastasis models established via IP injection and liver metastasis models established via CRC cell injection into the spleen (allowing them to “seed” the liver) have been used in anti-CEA fluorescent antibody studies [11]. Gutowski et al. [12] intraperitoneally injected mice with LS174T cells and demonstrated successful labeling of “very small” nodules (<1 mg in weight or <1 mm in diameter) with fluorescent anti-CEA antibody. Tumors as small as <3 mm were successfully resected. The authors reported a sensitivity of 90.7%, specificity of 97.2%, positive predictive value (PPV) of 94.7% and negative predictive value (NPV) of 94.9%. These results were confirmed in an LS174T intraperitoneal mouse model using a dual radio- and fluorescently labeled anti-CEA antibody [13]. Hekman et al. established a metastasis model created by injecting a human CRC cell line (GW-39) into the mouse lung [14]. The antibody-dye conjugate was able to detect early micrometastasis undetectable by bright light alone. FGS allowed for all fluorescent nodules to be resected with no fluorescence signal visualized in the post-resection tumor bed. Hiroshima et al. established CRC liver metastasis murine models via splenic HT-29 cell injection [11]. The anti-CEA fluorescent antibody allowed for detection of deep hepatic tumors. However liver background signal is an important limitation in liver fluorescence imaging [10]. Conjugating long polyethylene glycol chains to the dye (“PEGylation”), the antibody-dye had increased serum half-life and decreased liver signal [15]. Maawy et al. [15] demonstrated higher TBRs with lower hepatic signals in a PEGylated anti-CEA fluorescent antibody versus the non-PEGylated fluorescent antibody. There was also decreased signal in liver, lung, and lymph node using a PEGylated fluorescent dye conjugated to an anti-CEA antibody [15].
SGM-101 was created from an anti-CEA antibody, SGM—ch511 conjugated to BM104, a fluorophore with an absorbance band centered at 700 nm [16]. Gutowski et al. [16] evaluated SGM-101 in 4 different murine models, 3 of which were CRC (LS174T intra-peritoneal, LS174T liver metastasis via spleen injection, HT29 cecal orthotopic). After resection of all tumors identified with single-photon emission computed tomography (SPECT) and visual inspection, the mice underwent FGS with NIR imaging, identifying and removing submillimeter tumor deposits. Laboratory has established liver metastasis orthotopic models and shown that SGM-101 selectively labeled the tumor in the liver bed (Figure 1) [17]. SGM-101 is notable as the only anti-CEA fluorescent antibody in phase III clinical trials (NCT03659448 and NCT04642924).
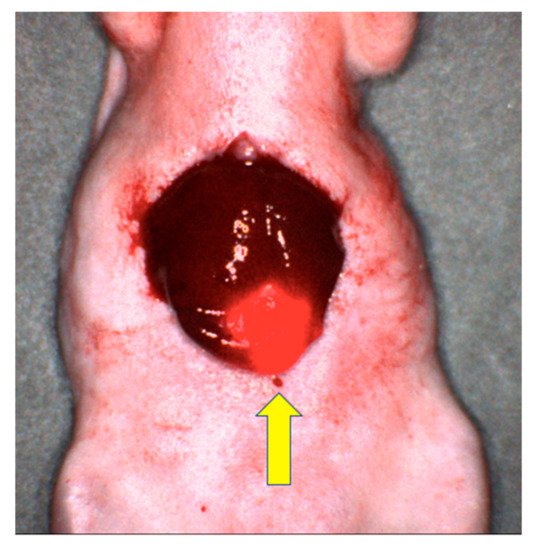
Figure 1. Image of LS174T liver metastasis in a mouse model after labeling with SGM-101. The yellow arrow points to the brightly labeled tumor with surrounding normal liver tissue [17].
Photoimmunotherapy (PIT) is a type of theranostics as it can both diagnose and treat disease. PIT utilizes a tumor-specific antibody conjugated to a photoactivatable dye to selectively bind cancer cells of interest and cause cell death when exposed to activating energy. Conventional photodynamic therapy (PDT) has limited use in cancer treatment due to lack of selective photosensitizers, limited tissue penetration and its reliance on reactive oxygen species (ROS) (problematic in solid tumors with a hypoxic milieu) [18][19]. However, recent studies using the NIR dye IR-700 conjugated to an anti-CEA antibody showed improved tissue penetration and cytotoxicity only when bound to a cell membrane, suggesting a different mechanism of action than ROS generation [18][19]. Elekonawo et al. used a CEA-expressing human CRC cell line, LoVo, subcutaneously implanted in 18 mice demonstrating labetuzumab-IR700 plus PIT significantly slowed tumor growth compared to PIT alone or labetuzumab-IR700 alone [20]. Hollandsworth et al. demonstrated similar findings in an LS174T orthotopic murine model [21].
1.3. Clinical Trials
Clinical trials with fluorescent anti-CEA antibodies in CRC have been performed using SGM-101, as noted above. Initially, Boogerd et al. enrolled 26 patients with CRC into a safety and effectiveness study with SGM-101 [22]. Of the 9 patients in the dose escalation portion of the study, 4 of the 9 patients had an intraoperative signal (TBR = 1.83). An additional 3 patients had a fluorescence signal when the tumor was imaged after resection (the remaining 2 patients had complete pathological response). The other 17 patients had recurrent or peritoneal metastasis. After receiving SGM-101, a total of 44 malignant lesions were resected. Thirty-four of these lesions had an intraoperative fluorescence signal, and after excision, 43/44 lesions had a fluorescence signal. Importantly, 19 of these lesions were detected by fluorescence imaging only and were not clinically visible prior to NIR im-aging. Two false positives were recorded, one classified as dysplasia of the bladder urothelial lining and one as a peritoneal lesion containing blue ink particles due to endoscopic tattooing of the tumor. Neither of the false positives were CEA-positive by immunohistochemistry (IHC) staining. A total of 6 patients had their original treatment plan altered due to these findings in the clinical trial.
In 2021, de Valk et al. repeated the experiment with 37 patients and found similar optimal dose/timing and TBR with SGM-101 [23]. Seven true negative (no fluorescence, confirmed complete pathologic response after neoadjuvant therapy) and 2 false positives (one with CEA expressing mucin but no malignancy and one showing weak CEA ex-pression in the epithelial tissue) were recorded. Including primary and recurrent tumors and metastasis, a total of 97 lesions were resected, 49 of which were malignant. Of the 49 malignant lesions, 47 were fluorescent, although in the majority (27), the fluorescence signal was obscured due to anatomical positioning and only apparent after excision. Of the 48 benign lesions, 22 were false positives. Twelve patients had their original surgical plan altered due to fluorescence imaging, 9 of which were deemed appropriate (7 had additional tissue removed and 2 were downstaged due to lack of fluorescence signal and confirmed benign by frozen-section analysis). Schaap et al. conducted a non-randomized, multi-center, single-arm open-label study for patients with CRC peritoneal metastasis using SGM-101 [24]. Fourteen patients were scheduled to have a hyperthermic intraperitoneal chemotherapy (HIPEC) procedure and received SGM-101 prior to the procedure. Twelve patients had the HIPEC procedure (2 cases were aborted due to extensive, unresectable disease). The patients had their clinical peritoneal cancer index (PCI) calculated under bright light, then it was recalculated under fluorescence imaging (fPCI). Seven patients had their PCI increased due to fluorescence imaging, 4 of which were determined to be accurate based on histopathological analysis. In two patients, the PCI was incorrectly increased after fluorescence imaging (false positive fluorescent nodules confirmed to be benign by histopathological analysis). Histology of these false positives showed benign, hypervascularized, collagen-rich connective tissue with inflammatory changes. In one patient, the PCI decreased from 5 to 4 based on fluorescence imaging; however, histopathology showed that the PCI should have been 3 (1 false positive).
Folli et al. [25] used a different anti-CEA fluorescent antibody (CGP44290) in 6 patients with known CRC. After infusion with the anti-CEA fluorescent antibody, all 6 patients’ tumors became fluorescent. Keller et al. evaluated 27 patients with documented colonic polypoid lesions on a previous examination for a total of 33 colonic polypoid lesions (25 carcinomas, 8 adenomas) [26]. After an anti-CEA fluorescent antibody was applied directly to the lesions and allowed to incubate for 10 min, 19/25 carcinoma lesions and 3/8 adenomas were fluorescent. None of the surrounding normal tissue had a fluorescence signal. In reviewing the false negatives, bleeding or ulceration of the mucosa was a common finding and appeared to limit the sensitivity of fluorescent labeling. No false positives were recorded. Elekonawo et al. evaluated 10 patients, scheduled to undergo HIPEC for CRC peritoneal carcinomatosis and stratified them into dual radio- and fluorescence-labeled labetuzumab at 2 mg (n = 5) or 10 mg (n = 5) treatment arms [27]. Imaging of the resected lesions showed that 17/28 (61%) malignant lesions in the 2 mg group could be detected with fluorescence compared to 16/17 (95%) malignant lesions in the 10 mg group. However, the 10 mg group also had 4 false positives (3 of the lesions were classified as granulocytic inflammatory process with necrosis, fibrotic inflammation, and local colitis, while the 4th lesion was too damaged to undergo further histological analysis). No false positives were reported in the 2 mg group.
These clinical trials show the potential of fluorescent anti-CEA antibodies to augment intraoperative decision making. Ongoing phase III clinical trials may shed more light on which patients will most benefit from this emerging technology.
2. Pancreatic Cancer
2.1. Subcutaneous Mouse Models
Kaushal et al. used an anti-CEA fluorescent monoclonal antibody to label 5 different human pancreatic cancer cell lines growing subcutaneously in nude mice, including BxPC3, a common pancreatic cancer cell line used in several FGS experiments [4]. All 5 subcutaneous tumor models had a specific fluorescence signal after receiving the antibody-dye conjugate. Animals with large tumors were selected to undergo tumor resection. After careful resection under a dissecting microscope with bright light, the tumor beds were imaged showing residual fluorescent disease in all mice (confirmed by histology). Knutson et al. also successfully labeled BxPC3 tumors in nude mice with an anti-CEA fluorescent antibody [28].
Maawy et al. compared PEGylated vs. non PEGylated anti-CEA fluorescent antibodies using a subcutaneous BxPC3 murine model [29]. The PEGylated dyes had a higher TBR than non-PEGylated dyes in both subcutaneous tumor models.
2.2. Orthotopic and Intraperitoneal Mouse Models
Kaushal et al. used subcutaneous BxPC3 tumors to establish a pancreatic orthotopic murine model [4]. Small pancreatic tumors difficult to visualize under bright light, became obvious with fluorescence imaging after receiving fluorescent anti-CEA antibodies. Next, using IP pancreatic cancer cell injections to establish an intraperitoneal metastasis model, Kaushal et al. demonstrated peritoneal deposits, invisible by bright light, imaged brightly with fluorescence imaging after receiving fluorescent anti-CEA antibodies. In these experiments, a nonspecific IgG antibody-dye conjugate was used as a control and did not target the tumors. Similar studies confirmed the ability of anti-CEA fluorescent antibodies to selectively label BxPC3 orthotopic tumors [5][9][16][30][31]. Anti-CEA fluorescent antibodies in BxPC3 orthotopic models also have faster intraoperative tumor identification and improved sensitivity of intraperitoneal metastasis nodule identification with fluorescence imaging [32]. BxPC3 orthotopic models also demonstrated decreased local recurrence with improved DFS with FGS using anti-CEA fluorescent antibodies compared to bright-light surgery (BLS) alone [33]. FGS using anti-CEA fluorescent antibodies and neoadjuvant chemotherapy (NAC) also demonstrated improved R0 rates (92% vs. 45.5%), cure rates (40% vs. 4.5%), survival at 1 year (28% vs. 0%), median DFS (11 weeks vs. 5 weeks), and median OS (22 weeks vs. 13.5 weeks) in BxPC3 orthotopic models compared to BLS with NAC [34].
Lwin et al. developed a humanized anti-CEA hT84.66-M5A-IR800m (M5A-IR800) fluorescence antibody to image green fluorescence protein (GFP) labeled BxPC3 pancreatic cancer orthotopic murine models [35]. M5A-IR800 had a stronger fluorescence signal than GFP labeling. Serial imaging between 6 and 72 h showed peak signal strength with M5A-IR800 in the orthotopic model at 48 h. As in the CRC experiments [10], there was high liver signal with M5A-IR800. To address this problem, Yazaki et al. conjugated the M5A antibody with long linear PEG molecules allowing 6–7 IR-800 dyes per antibody [36]. The new PEGylated M5A-sidewinder-IR800 (M5A-SW-IR800) had decreased hepatic accumulation and a longer serum half-life, resulting in decreased liver signal and an increased TBR in a BxPC3 pancreatic cancer orthotopic murine model (Figure 2).
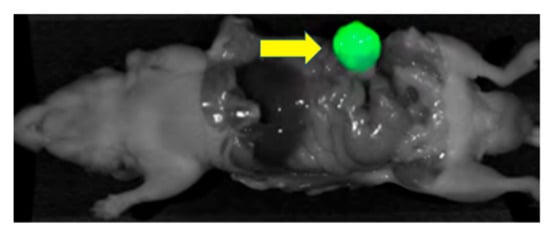
Figure 2. BxPC3 pancreatic orthotopic mouse model after receiving M5A-SW-IR800 (75 μg). The arrow points to the brightly labeled tumor [36].
Patient pancreatic tumors have also been used to establish orthotopic models. Using a patient tumor, Hiroshima et al. [37] investigated the efficacy of FGS with NAC in a CEA-negative, CA 19-9-positive pancreatic PDOX murine model. While the anti-CA 19-9 antibody-dye conjugate brightly labeled the PDOX tumor, the signal from the fluorescent anti-CEA antibody was “very weak”. In contrast, when Hiroshima et al. [38] used FGS with NAC in a CEA-positive pancreatic PDOX model, the tumors were labeled brightly. One in 8 mice in the FGS arm and 0/8 mice in the FGS + NAC had tumor recurrence 12 weeks after FGS compared to 6/8 in both the BLS and BLS + NAC arm. Lwin et al. used M5A-IR800 to image a pancreatic PDOX murine model using a patient’s liver metastasis from a pancreatic primary tumor and establishing it in the pancreas of a mouse [39]. M5A-IR800 brightly labeled the primary pancreatic tumor as well as the splenic and abdominal wall metastasis. Florescence signal was noted in the liver and bladder, with the average liver fluorescence signal 52% as strong as the average tumor signal.
In another example of theranostics, Maawy et al. used a chimeric anti-CEA antibody conjugated with IRDye 700DX NHS Ester to perform PIT in mice with BxPC3 orthotopic pancreatic cancer [40]. Mice received PIT and then were imaged weekly to assess tumor size. At the end of 5 weeks, the mice were euthanized, and tumor weight was recorded. Compared to control (PIT only), PIT with anti-CEA antibodies conjugated to IR700CW had significantly lower tumor weight at all time points. There was no difference in the weight of the mice minus the tumor between the two arms suggesting PIT was well tolerated.
2.3. Clinical Trials
There is only one clinical trial with anti-CEA fluorescent antibodies for pancreatic cancer. Hoogstins et al. enrolled 12 patients with pancreatic ductal adenocarcinoma into the SGM-101 trial [41]. SGM-101 brightly labeled primary and metastatic tumors in 11 patients (one patient’s procedure was abandoned before visualizing the primary tumor due to the extent of metastatic disease). Seven of the primary tumors were resected (TBR 1.67 ± 0.37), 6 of which were confirmed adenocarcinoma (1 tumor sample was diagnosed as IPMN, a premalignant lesion, and considered a false positive). Three of the patients had peritoneal or liver metastasis. A total of 5 fluorescent, clinically suspicious nodules were removed, all demonstrated to be malignant with moderate-to-strong CEA expression (TBR 1.7 ± 0.42). An additional 8 non-fluorescent, clinically suspicious nodules were removed, 2 of which were malignant and thus classified as false negatives.
References
- Pèlegrin, A.; Folli, S.; Buchegger, F.; Mach, J.-P.; Wagnières, G.; Bergh, H.V.D. Antibody–fluorescein conjugates for photoimmunodiagnosis of human colon carcinoma in nude mice. Cancer 1991, 67, 2529–2537.
- Berk, D.A.; Yuan, F.; Leunig, M.; Jain, R.K. Direct in vivo measurement of targeted binding in a human tumor xenograft. Proc. Natl. Acad. Sci. USA 1997, 94, 1785–1790.
- Lisy, M.-R.; Goermar, A.; Thomas, C.; Pauli, J.; Resch-Genger, U.; Kaiser, W.A.; Hilger, I. In Vivo Near-infrared Fluorescence Imaging of Carcinoembryonic Antigen–expressing Tumor Cells in Mice. Radiology 2008, 247, 779–787.
- Kaushal, S.; McElroy, M.K.; Talamini, M.A.; Moossa, A.R.; Bouvet, M. Fluorophore-conjugated anti-CEA Antibody for the Intraoperative Imaging of Pancreatic and Colorectal Cancer. J. Gastrointest. Surg. 2008, 12, 1938–1950.
- Zhou, X. Near-Infrared Fluorescent Imaging of Pancreatic Cancer in Mice Using a Novel Antibody to CEACAM5. Ph.D. Thesis, Kiel University, Kiel, Germany, 2021. Available online: https://macau.uni-kiel.de/receive/macau_mods_00001232 (accessed on 29 August 2021).
- Lu, Z.; Pham, T.T.; Rajkumar, V.; Yu, Z.; Pedley, R.B.; Årstad, E.; Maher, J.; Yan, R. A Dual Reporter Iodinated Labeling Reagent for Cancer Positron Emission Tomography Imaging and Fluorescence-Guided Surgery. J. Med. Chem. 2018, 61, 1636–1645.
- Metildi, C.A.; Kaushal, S.; Luiken, G.A.; Talamini, M.A.; Hoffman, R.M.; Bouvet, M. Fluorescently labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Surg. Oncol. 2014, 109, 451–458.
- Hiroshima, Y.; Maawy, A.; Metildi, C.A.; Zhang, Y.; Uehara, F.; Miwa, S.; Yano, S.; Sato, S.; Murakami, T.; Momiyama, M.; et al. Successful Fluorescence-Guided Surgery on Human Colon Cancer Patient-Derived Orthotopic Xenograft Mouse Models Using a Fluorophore-Conjugated Anti-CEA Antibody and a Portable Imaging System. J. Laparoendosc. Adv. Surg. Tech. 2014, 24, 241–247.
- Boonstra, M.C.; Tolner, B.; Schaafsma, B.E.; Boogerd, L.S.F.; Prevoo, H.A.J.M.; Bhavsar, G.; Kuppen, P.J.K.; Sier, C.F.M.; Bonsing, B.A.; Frangioni, J.V.; et al. Preclinical evaluation of a novel CEA-targeting near-infrared fluorescent tracer delineating colorectal and pancreatic tumors. Int. J. Cancer 2015, 137, 1910–1920.
- DeLong, J.C.; Murakami, T.; Yazaki, P.J.; Hoffman, R.M.; Bouvet, M. Near-infrared-conjugated humanized anti-carcinoembryonic antigen antibody targets colon cancer in an orthotopic nude-mouse model. J. Surg. Res. 2017, 218, 139–143.
- Hiroshima, Y.; Lwin, T.M.; Murakami, T.; Maawy, A.A.; Kuniya, T.; Chishima, T.; Endo, I.; Clary, B.M.; Hoffman, R.; Bouvet, M. Effective fluorescence-guided surgery of liver metastasis using a fluorescent anti-CEA antibody. J. Surg. Oncol. 2016, 114, 951–958.
- Gutowski, M.; Carcenac, M.; Pourquier, D.; Larroque, C.; Saint-Aubert, B.; Rouanet, P.; Pèlegrin, A. Intraoperative immunophotodetection for radical resection of cancers: Evaluation in an experimental model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 1142–1148.
- Rijpkema, M.; Oyen, W.J.; Bos, D.; Franssen, G.M.; Goldenberg, D.M.; Boerman, O.C. SPECT- and Fluorescence Image–Guided Surgery Using a Dual-Labeled Carcinoembryonic Antigen–Targeting Antibody. J. Nucl. Med. 2014, 55, 1519–1524.
- Hekman, M.C.H.; Rijpkema, M.; Bos, D.L.; Oosterwijk, E.; Goldenberg, D.M.; Mulders, P.F.A.; Boerman, O.C. Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model. J. Nucl. Med. 2017, 58, 706–710.
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Polyethylene glycol (PEG) linked to near infrared (NIR) dyes conjugated to chimeric anti-carcinoembryonic antigen (CEA) antibody enhances imaging of liver metastases in a nude-mouse model of human colon cancer. PLoS ONE 2014, 9, e97965.
- Gutowski, M.; Framery, B.; Boonstra, M.C.; Garambois, V.; Quenet, F.; Dumas, K.; Scherninski, F.; Cailler, F.; Vahrmeijer, A.L.; Pèlegrin, A. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017, 26, 153–162.
- Nishino, H.; Turner, M.A.; Amirfakhri, S.; Hollandsworth, H.M.; Lwin, T.M.; Yamamoto, J.; Framery, B.; Cailler, F.; Hoffman, R.M.; Bouvet, M. Spectrally Distinct Double Labeling of Colon-Cancer Liver Metastases and Adjacent Liver Segment with a Near-Infrared-labeled Anti-Carcinoembryonic Antigen (CEA) Antibody and Indocyanine Green in an Orthotopic Mouse Model | Elsevier Enhanced Reader. J. Am. Coll. Surg. 2021, 233, S154.
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell–selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691.
- Shirasu, N.; Yamada, H.; Shibaguchi, H.; Kuroki, M.; Kuroki, M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int. J. Cancer 2014, 135, 2697–2710.
- Elekonawo, F.M.K.; Bos, D.L.; Goldenberg, D.M.; Boerman, O.C.; Rijpkema, M. Carcinoembryonic antigen-targeted photodynamic therapy in colorectal cancer models. EJNMMI Res. 2019, 9, 108.
- Hollandsworth, H.M.; Amirfakhri, S.; Filemoni, F.; Molnar, J.; Hoffman, R.M.; Yazaki, P.J.; Bouvet, M. Near-infrared photoimmunotherapy is effective treatment for colorectal cancer in orthotopic nude-mouse models. PLoS ONE 2020, 15, e0234643.
- Boogerd, L.S.F.; Hoogstins, C.E.S.; Schaap, D.P.; Kusters, M.; Handgraaf, H.J.M.; van der Valk, M.J.M.; Hilling, D.E.; Holman, F.A.; Peeters, K.C.M.J.; Mieog, J.S.D.; et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: A dose-escalation pilot study. Lancet Gastroenterol. Hepatol. 2018, 3, 181–191.
- De Valk, K.S.; Deken, M.M.; Schaap, D.P.; Meijer, R.P.; Boogerd, L.S.; Hoogstins, C.E.; van der Valk, M.J.; Kamerling, I.M.; Bhairosingh, S.S.; Framery, B.; et al. Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 1832–1844.
- Schaap, D.P.; de Valk, K.S.; Deken, M.M.; Meijer, R.P.J.; Burggraaf, J.; Vahrmeijer, A.L.; Kusters, M. Carcinoembryonic antigen-specific, fluorescent image-guided cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer. Br. J. Surg. 2020, 107, 334–337.
- Folli, S.; Wagnières, G.; Pèlegrin, A.; Calmes, J.M.; Braichotte, D.; Buchegger, F.; Chalandon, Y.; Hardman, N.; Heusser, C.; Givel, J.C.; et al. Immunophotodiagnosis of colon carcinomas in patients injected with fluoresceinated chimeric antibodies against carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA 1992, 89, 7973–7977.
- Keller, R.; Winde, G.; Terpe, H.J.; Foerster, E.C.; Domschke, W. Fluorescence Endoscopy Using a Fluorescein-Labeled Monoclonal Antibody Against Carcinoembryonic Antigen in Patients with Colorectal Carcinoma and Adenoma. Endoscopy 2002, 34, 801–807.
- Elekonawo, F.M.K.; de Gooyer, J.M.; Boerman, O.C.; Bremers, A.J.A.; Aarnntzen, E.; Nagtegaal, I.D.; Rijpkema, M.; de Wilt, J.H.W. Multimodal image-guided surgery of colorectal peritoneal carcinomatosis: A phase 1 clinical trial. In Improving the Surgical Treatment of Peritoneal Metastases of Colorectal Origin; Radbound University: Nijmegen, The Netherlands, 2020; pp. 184–201.
- Knutson, S.; Raja, E.; Bomgarden, R.; Nlend, M.; Chen, A.; Kalyanasundaram, R.; Desai, S. Development and Evaluation of a Fluorescent Antibody-Drug Conjugate for Molecular Imaging and Targeted Therapy of Pancreatic Cancer. PLoS ONE 2016, 11, e0157762.
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Specific tumor labeling enhanced by polyethylene glycol linkage of near infrared dyes conjugated to a chimeric anti-carcinoembryonic antigen antibody in a nude mouse model of human pancreatic cancer. J. Biomed. Opt. 2014, 19, 101504.
- Maawy, A.A.; Hiroshima, Y.; Kaushal, S.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Comparison of a chimeric anti-carcinoembryonic antigen antibody conjugated with visible or near-infrared fluorescent dyes for imaging pancreatic cancer in orthotopic nude mouse models. J. Biomed. Opt. 2013, 18, 126016.
- Metildi, C.A.; Kaushal, S.; Lee, C.; Hardamon, C.R.; Synder, C.S.; Luiken, G.A.; Talamini, M.A.; Hoffman, R.M.; Bouvet, M. An LED light source and novel fluorophore combinations improve fluorescence laparoscopic detection of metastatic pancreatic cancer in orthotopic mouse models. J. Am. Coll. Surg. 2012, 214, 997–1007.
- Tran Cao, H.S.; Kaushal, S.; Metildi, C.A.; Menen, R.S.; Lee, C.; Synder, C.S.; Messer, K.; Pu, M.; Luiken, G.A.; Talamini, M.A.; et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology 2012, 59, 1994–1999.
- Metildi, C.A.; Kaushal, S.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Advantages of fluorescence-guided laparoscopic surgery of pancreatic cancer labeled with fluorescent anti-carcinoembryonic antigen antibodies in an orthotopic mouse model. J. Am. Coll. Surg. 2014, 219, 132–141.
- Metildi, C.A.; Kaushal, S.; Pu, M.; Messer, K.A.; Luiken, G.A.; Moossa, A.R.; Hoffman, R.M.; Bouvet, M. Fluorescence-guided Surgery with a Fluorophore-conjugated Antibody to Carcinoembryonic Antigen (CEA), that Highlights the Tumor, Improves Surgical Resection and Increases Survival in Orthotopic Mouse Models of Human Pancreatic Cancer. Ann. Surg. Oncol. 2014, 21, 1405–1411.
- Lwin, T.M.; Murakami, T.; Miyake, K.; Yazaki, P.J.; Shively, J.E.; Hoffman, R.M.; Bouvet, M. Tumor-Specific Labeling of Pancreatic Cancer Using a Humanized Anti-CEA Antibody Conjugated to a Near-Infrared Fluorophore. Ann. Surg. Oncol. 2018, 25, 1079–1085.
- Yazaki, P.; Lwin, T.M.; Minnix, M.; Li, L.; Sherman, A.; Molnar, J.; Miller, A.; Frankel, P.; Chea, J.; Poku, E.; et al. Improved antibody-guided surgery with a near-infrared dye on a pegylated linker for CEA-positive tumors. J. Biomed. Opt. 2019, 24, 1–9.
- Hiroshima, Y.; Maawy, A.; Zhang, Y.; Murakami, T.; Momiyama, M.; Mori, R.; Matsuyama, R.; Katz, M.H.G.; Fleming, J.B.; Chishima, T.; et al. Metastatic Recurrence in a Pancreatic Cancer Patient Derived Orthotopic Xenograft (PDOX) Nude Mouse Model Is Inhibited by Neoadjuvant Chemotherapy in Combination with Fluorescence-Guided Surgery with an Anti-CA 19-9-Conjugated Fluorophore. PLoS ONE 2014, 9, e114310.
- Hiroshima, Y.; Maawy, A.; Zhang, Y.; Murakami, T.; Momiyama, M.; Mori, R.; Matsuyama, R.; Chishima, T.; Tanaka, K.; Ichikawa, Y.; et al. Fluorescence-guided surgery, but not bright-light surgery, prevents local recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) model resistant to neoadjuvant chemotherapy (NAC). Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al. 2015, 15, 295–301.
- Lwin, T.M.; Miyake, K.; Murakami, T.; DeLong, J.C.; Amirfakhri, S.; Filemoni, F.; Yoon, S.N.; Yazaki, P.J.; Shivley, J.E.; Datnow, B.; et al. Fluorescent humanized anti-CEA antibody specifically labels metastatic pancreatic cancer in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 2018, 9, 37333–37342.
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Heim, R.; Makings, L.; Garcia-Guzman, M.; Luiken, G.A.; Kobayshi, H.; Hoffman, R.M.; Bouvet, M. Near Infra-Red Photoimmunotherapy with Anti-CEA-IR700 Results in Extensive Tumor Lysis and a Significant Decrease in Tumor Burden in Orthotopic Mouse Models of Pancreatic Cancer. PLoS ONE 2015, 10, e0121989.
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Mulder, B.G.S.; Mieog, J.S.D.; Swijnenburg, R.J.; van de Velde, C.J.H.; Sarasueta, A.F.; Bonsing, B.A.; Framer, B.; Pèlegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
716
Revisions:
2 times
(View History)
Update Date:
20 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




