
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Pablo López-Cervantes | + 8196 word(s) | 8196 | 2021-12-06 04:38:17 |
Video Upload Options
Emerging research suggests environmental exposures before conception may adversely affect allergies and lung diseases in future generations. Most studies are limited as they have focused on single exposures, not considering that these diseases have a multifactorial origin in which environmental and lifestyle factors are likely to interact. Traditional exposure assessment methods fail to capture the interactions among environmental exposures and their impact on fundamental biological processes, as well as individual and temporal factors. A valid estimation of exposure preconception is difficult since the human reproductive cycle spans decades and the access to germ cells is limited. The exposome is defined as the cumulative measure of external exposures on an organism (external exposome), and the associated biological responses (endogenous exposome) throughout the lifespan, from conception and onwards. An exposome approach implies a targeted or agnostic analysis of the concurrent and temporal multiple exposures, and may, together with recent technological advances, improve the assessment of the environmental contributors to health and disease. This review describes the current knowledge on preconception environmental exposures as related to respiratory health outcomes in offspring. We discuss the usefulness and feasibility of using an exposome approach in this research, advocating for the preconception exposure window to become included in the exposome concept.
1. Introduction
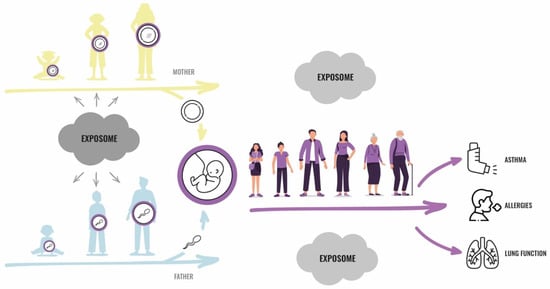
2. Preconception Exposures and Respiratory Health: Evidence from Multigeneration Studies on Humans
2.1. Smoking
2.2. Occupational Exposures
2.3. Environmental Exposures
2.4. Metabolic and Hormonal Exposures
2.5. Infections and Immunity across Generations
2.6. Miscellaneous Exposures
3. Discussion
The reviewed literature strongly suggests that preconception exposures may be important for the development of asthma and lung health outcomes in future offspring. Associations with lung health outcomes of offspring are suggested for a range of single chemical exposures (smoking, occupation, and air pollution), hormonal metabolic exposures (being overweight and using oral contraceptives), and disease processes (infections, and asthmatic and allergic disease activity). In the context of preconception exposures, there is more evidence on the paternal line, and, in particular, for the childhood and early puberty exposure windows in males. The importance of these exposure windows may possibly be high; for instance, the associations between the father’s smoking starting before the age of 15 years and the offspring’s asthma risk appear to be at least as strong as for the associations with the mother’s smoking in pregnancy. Within the maternal line, associations with the asthma of a grandmother who smoked tobacco in pregnancy are reported in several studies. There is also some evidence of a maternal preconception exposure window in young adulthood.
The literature on the importance of preconception exposure for allergies and lung diseases in future offspring is supported by the evidence on preconception exposures for other health outcomes, such as obesity and growth [19][105]. Furthermore, the concept of preconception origins of health and disease is supported by mechanistic and experimental studies showing biological plausibility for the transfer of environmentally induced adverse impacts across generations. A role of the influence of germ cells, both for the female and male lines, is the most plausible pathway for the transfer of environmental effects across generations, and the mechanism most suitable for a discussion of the exposome effects, i.e., the effect of the totality of multiple concurrent or temporal exposures from all sources. One may speculate that the transfer of microbiome, or immune features influenced by the microbiome or infections, may become better documented and more relevant for future research on the preconception exposome.
3.1. The Exposome Concept
The exposome concept is divided into three different domains: the general external exposome, the specific external exposome, and the internal exposome [14][105][106]. The general external exposome includes the wider social, economic, and psychological influences (e.g., socioeconomic status, education, psychological and mental stress, urban and rural environments, etc.). This domain can partly be captured by questionnaires, registry data, and geocoding, while the use of personal apps and the global positioning system (GPS) to capture these domains is likely to increase in the years to come. The specific external exposome includes exposures such as chemical agents and environmental/occupational pollutants, infectious agents, radiation, noise, diet, lifestyle factors, and medication [107]. Traditionally, this type of information has been collected by questionnaires, expert-based exposure matrices, and through measurements of the agent in question in the ambient air, drinking water, or food. Loh and coworkers (2017) reviewed the possibilities and limitations in the use of wearable sensor technology and smart technologies as a means to measure the specific external exposure domain, including the use of the GPS for the measurement of personal location, which together with activity affect all three exposome domains [108]. Finally, the internal exposome includes chemicals or their metabolites in biological fluids and tissues, as well as nongenotoxic processes (metabolome, epigenome, transcriptome, proteome, adductome, and microbiome processes). In general, the high-resolution and high-throughput technologies needed for investigating the multiple omics above are already in place. A major challenge in the field of exposome research is that the biostatistical and bioinformatical methods needed for analyzing the vast number of combined omics and exposure data are lacking. This technology and knowledge are imperative in order to facilitate the translation and interpretation of the exposome to derive new biological knowledge that can be used in prevention and that has implications for human health. Even though the complexity will be even higher with data from two generations, methodological and technological advances should aspire to be able to also include the preconception window in the exposome concept.
3.2. What and When to Measure?
To have a beneficial or adverse effect on human health, the environmental exposure in question must interact with the biological system at some level, i.e., it must be bioavailable for the target cell at the right time and be able to interact with biomolecules. An optimal assessment of the exposome during the preconception period would require access to the germ cells of the parent and, perhaps, of previous generations, as well as (surrogate) target tissues in the next generation [6]. The human germ cells and the microenvironments of these in the ovaries/testes are unavailable for research in the prepuberty window, in both males and females. For the susceptibility window, from puberty to conception, it is possible to collect mature sperm and parts of its microenvironment from the paternal line. The sampling of egg cells (ova) from the maternal line would be excessively invasive to be feasible in large cohorts, and in relation to ovum retrieval, the microenvironment will most likely be affected by the treatment cycle prior to the procedure (e.g., hormonal and inflammatory effects).
The first obstacle for applying the exposome approach in the preconception period is the need to document the bioavailability and exposure intensities of the compounds of interest at the target cell. While the external exposome domain does not give us any information on the bioavailability of the compound, the endogenous exposome accounts for uptake from all sources (e.g., air, food, cosmetics, etc.) and routes (e.g., inhalation, oral, or dermal) of exposure. The use of the biological monitoring of exposure (chemical or its metabolites) in the assessment of health risk or prognosis requires that the biomarker is quantifiable, sufficiently correlated to the exposure of interest, and biologically relevant for the health effect or biological effect. Hence, although the internal concentrations of the environmental compounds of interest can be measured as proxies in accessible tissues (i.e., cord blood, blood, and urine) in future parents from birth and until conception, the true concentration available for the target cells or tissues (germ cells) during specific time points is not available.
For the same reasons described for bioavailability above, when judging whether it is biologically plausible that the human germ cells may be affected by preconception environmental exposures that also affect the immune and respiratory systems of future generations, we must rely on proxies of both exposures and effects, from existing and future human cohorts, combined with data from mechanistic and animal studies. The latter provides, for example, the possibility to gain detailed knowledge on how, and at what stages during testicular development and epididymal maturation, environmental information is transferred to the spermatozoa, and how the transfer of spermatozoan epigenetic information to the oocyte regulates transcriptional programs in the early embryo and the resulting later phenotypes. The experimental manipulation of epigenetic information, e.g., by injecting noncoding RNAs of interest into the early zygote, may potentially establish a cause–effect relationship.
3.3. Ethical, Legal, and Social Issues Related to the Use of Exposome Technologies
In parallel with the increased use of exposome technologies and the collection of high-resolution and high-dimensional data in health sciences, there is also a continuing need to address the ethical, legal, and social issues related to biobanking, archiving, and analysis of the large amount of biological material and data generated. These issues apply to all types of health research, but future offspring is a particularly vulnerable population. A main concern for discussion in large-scale epigenetic and genetic studies is the procedures for reporting pertinent and incidental findings back to the participants. This issue also applies to exposome research. At present, there seems to be a consensus that at least three requirements should be fulfilled prior to reporting research results: the results must be analytically valid, clinically significant, and actionable [109][110]. However, the criteria for what is valid, clinically significant, and actionable are not established for many of the exposures and outcomes that are being investigated nowadays, and must, hence, often be decided after a discretionary assessment.
For studies that investigate how the exposome at the preconception time window may affect future offspring, informed consent is provided by the parents or previous generations, but not by the subject at risk. This raises several ethical considerations for reporting back research results to the study participants and their offspring. How can we ensure that the offspring want to know whether he or she has an increased susceptibility to respiratory diseases in adult life, and how will this information affect future choices with respect to education and occupation, family life, or leisure time? Moreover, will his or her future employer or insurance company have the right to obtain this information before employment or before accepting him or her as a customer?
Finally, although there are analytical methods that allow examinations of thousands of chemicals in biological samples among the general population, the health risk for most of these chemicals cannot yet be determined. This hinders a liable risk communication back to the participants and, consequently, the biomonitoring of these chemicals is not performed. However, if we do not start collecting this type of biological material and information in ongoing and planned cohort studies for use in later agnostic and untargeted analysis, there will be important limitations in understanding exposure effects and interactions in the future.
3.4. Other Challenges
There are considerable challenges related to addressing the preconception environmental impact on the health of offspring in studies of humans, for whom the reproductive cycle spans over decades. Few cohorts have exposure data from parental prepubertal and pubertal years. Statistical methods for the optimal analyses of complex multigeneration data, with multiple mediators, confounders, exposures, and outcomes, in several generations, are becoming available, but there is a need for statistical expertise and methodology that also combine omics and exposure data representing the exposome across generations. Even with advanced methodology, the interpretation of negative results may pose a particular challenge, as they may reflect truly negative findings or a sum of limitations in complex data. Two partly negative papers have been published, showing an attention to publishing negative papers, but the publication bias of the statistically significant and stronger results is likely, as in other areas with less complexity.
4. Conclusions
Overall, the literature is sufficient to justify major research efforts on the preconception origins of asthma, allergies, and lung health. The research may have wide public health implications, indicating the potential benefit of interventions—or harm from negative exposures—for not only the exposed person, but also for future generations. Thus, it is urgent to bring this research forward. We argue that the benefits of an exposome approach in addressing complex, multiple, and concurrent lifestyles, behaviors, and exposures that interplay with each other may be equally important when considering the preconception environment as when addressing exposures during the life span. An exposome approach may be particularly useful to elucidating the germ cell environment at different developmental stages, from intrauterine life until the reproductive age of the parents. Exposure information from the preconception time window will, however, often be far more limited than for other life-stages, and the biomaterial from the preconception stages is generally not available, except for sperm, rarely available in humans and, if so, sampled after the offspring were born. This underscores the need for the close integration of epidemiological and mechanistic studies. Still, obtaining valid information about the preconception period should be kept in mind when planning questionnaires, the collection of biomaterials, the use of novel technologies, and data for validation analyses, in future inter- and transgenerational studies. We advocate for explicitly including the preconception period in the exposome concept in the quest to identify the environmental contributors to health and disease (Table 1).
Table 1. Summary of multigeneration human studies associating preconception environmental exposures to asthma, allergies, and lung function.
|
Exposure |
Outcome |
Exposure Window |
Main Findings |
Study Cohorts 1 |
Reference |
|
Smoking |
|||||
|
Smoking |
Asthma |
Grandmaternal pregnancy |
Grandmother’s smoking during mother’s fetal period increased the risk of asthma in her grandchildren. |
CHS |
Li et al. Chest, 2005 [23] |
|
Smoking |
Asthma and wheezing |
Grandmaternal pregnancy |
Grandmother’s smoking during father’s fetal period increased the risk of asthma in the paternal daughter, in the absence of mother’s smoking during her daughter’s pregnancy. |
ALSPAC |
Miller et al. Chest, 2014 [27] |
|
Smoking |
Asthma |
Grandmaternal pregnancy |
Grandmother’s smoking during mother’s fetal period increased the risk of asthma in her grandchild, in the absence of the mother’s smoking during her offspring's pregnancy. |
MoBa |
Magnus et al. Thorax, 2015 [26] |
|
Smoking |
Nonallergic early-onset asthma |
Paternal prepuberty: paternal grandmother’s pregnancy |
Father’s smoking in prepuberty increased the risk of asthma in his offspring, in the absence of grandmother’s smoking during the father's fetal period. |
RHINE |
Svanes et al. Int J Epidemiol, 2017 [28] |
|
Smoking |
Allergic and nonallergic asthma |
Paternal prepuberty; pregnancy |
Father’s smoking in prepuberty increased the risk of nonallergic asthma in his offspring; grandmother’s smoking during mother’s fetal period increased the risk of allergic asthma in her grandchild. |
ECRHS |
Accordini et al. Int J Epidemiol, 2018 [29] |
|
Smoking |
Asthma |
Grandmaternal pregnancy |
Grandmother’s smoking during mother’s fetal period increased the risk of asthma in her grandchild, independent of the mother’s smoking during her offspring's pregnancy. |
NSC |
Lodge et al. Clin Exp Allergy, 2018 [25] |
|
Smoking |
Persistent childhood asthma |
Grandmaternal pregnancy |
Grandmother’s smoking during pregnancy was related to an increased risk of early persistent childhood asthma in grandchildren. No risk for other asthma phenotypes was found. |
Swedish national health registry-based cohort |
Bråbäck et al. Pediatr Allergy Immunol, 2018 [24] |
|
Smoking |
Lung function |
Paternal prepuberty; grandmaternal pregnancy |
Father’s smoking in prepuberty reduced offspring’s FEV1 and FVC; grandmother’s smoking during father’s fetal period reduced the grandchild’s FEV1/FVC ratio. |
Parents: ECRHS Offspring: RHINESSA |
Accordini et al. Eur Respir J, 2021 [30] |
|
Occupational exposures |
|||||
|
Welding |
Nonallergic asthma |
Paternal adolescence |
Fathers’ preconception welding was associated with nonallergic asthma in offspring. |
RHINE |
Svanes et al. Int J Epidemiol, 2017 [28] |
|
Allergens, reactive chemicals, microorganisms, and pesticides |
Asthma |
Before conception of child; pre- and postconception combined |
Preconception maternal and paternal exposure to occupational agents was, in general, not associated with asthma in offspring. One exception was a higher risk of early-onset asthma if the mother had been occupationally exposed to allergens and/or reactive chemicals both before and after conception. |
Parents: ECRHS Offspring: RHINESSA |
Pape et al. Int Epidemiol, 2020 [50] |
|
Cleaning products and disinfectants |
Asthma and/or wheeze |
Before conception of child; around conception and pregnancy |
Mother’s exposure to indoor cleaning, starting before conception, was associated with offspring’s childhood allergic and nonallergic asthma, and/or wheeze. |
Parents: RHINE Offspring: RHINESSA |
Tjalvin et al. J Allergy Clin Immunol, 2021 [51] |
|
Environmental exposures |
|||||
|
Outdoor pollutants and indoor new furniture/redecoration |
Asthma and allergies |
Before conception of the child |
Preconception exposure to outdoor pollutants increased the risk of asthma and allergic rhinitis in childhood, while redecoration was associated with rhinitis-like symptoms. |
CCHH |
Deng et al. Chemosphere, 2016 [54] |
|
Air pollution |
Asthma and allergies |
Parental childhood |
Parental exposure to air pollution during childhood increased the risk of asthma and allergies in offspring. |
RHINESSA |
Kuiper et al. Int. J. Environ. Res. Public Health 2020 [55] |
|
Farm exposure |
Asthma |
Parental childhood |
Farm upbringing in previous generations was not associated with offspring asthma —either for parental or grandparental upbringing. |
Parents: ECRHS/RHINE Offspring: RHINESSA |
Timm et al. Int J Epidemiol, 2021 [56] |
|
Metabolic and hormonal exposures |
|||||
|
Oral contraceptive pills |
Childhood wheeze, asthma, and allergies |
Before conception of child |
Use of oral contraceptive pills increased the risk for wheeze, asthma, and rhinitis. Extended use of contraceptives increased risk for wheeze and rhinitis. |
T-CHILD |
Yamamoto-Hanada et al. Allergol Int, 2016 [74] |
|
High BMI |
Asthma |
Parental childhood and adolescence |
Father’s high BMI in childhood and adolescence associated with higher risk of asthma in offspring. |
TAHS |
Bowatte et al. J Allergy Clin Immunol, 2021 [72] |
|
Overweight |
Nonallergic asthma |
Parental childhood, adolescence, and adulthood |
Father's onset of being overweight in puberty associated with offspring's asthma without nasal allergies. The effect was independent of offspring’s overweight. |
Parents: ECRHS Offspring: RHINESSA |
Johannessen et al. J Allergy Clin Immunol, 2020 [69] |
|
Overweight |
Lung function |
Paternal childhood/puberty |
Father being overweight during childhood and/or puberty may cause lower lung function in offspring. |
Parents: ECRHS Offspring: RHINESSA |
Lønnebotn et al. Eur Respir J, 2021 [73] |
|
Infections and disease processes |
|||||
|
Helminth infection |
Allergies |
Not known |
Toxocara spp seropositivity in parents was associated with allergic outcomes in their offspring. |
Parents: ECRHS Offspring: RHINESSA |
Jogi et al. Clin Exp Allergy, 2018 [83] |
|
Tuberculosis |
Asthma |
Parental childhood |
Parental tuberculosis in childhood is associated to asthma in offspring. |
Norwegian national health registries |
López-Cervantes et al. Trop Med Int Health, 2021 [85] |
|
Miscellaneous exposures |
|||||
|
Asthmatic and allergic disease activity (bronchial hyperresponsiveness and IgE levels) |
Asthma and allergies |
Before conception of child |
Parental asthmatic and allergic disease activity measured before conception was associated to offspring asthma and hay fever. |
ECRHS |
Bertelsen et al. Clin Exp Allergy, 2017 [102] |
|
Depression/anxiety |
Asthma |
Before conception of the child, pregnancy, postnatal and current |
Cumulative exposure to maternal depression or anxiety was associated to asthma in offspring, but no specific period was found to be associated. |
Swedish national health registries |
Brew et al. Int J Epidemiol, 2018 [104] |
|
Asthma medication |
Asthma |
Before conception of child |
Parental use of asthma medication (inhaled steroids) before conception was associated with asthma in offspring. |
ECRHS |
Banjara et al. Trop Med Int Health, 2021 [103] |
1 Study cohort: CHS: Children’s Health study in southern California; ALSPAC: Avon Longitudinal Study of Parents and Children; MoBa: Norwegian Mother and Child Cohort Study; RHINE: Respiratory Health In Northern Europe; ECRHS: European Community Respiratory Health Survey; NSC: Nationwide Swedish Cohort; RHINESSA: Respiratory Health In Northern Europe Spain and Australia; CCHH: China-Children-Homes-Health epidemiology study; T-CHILD: Tokyo-Children’s Health, Illness and Development Study; TAHS: Tasmanian Longitudinal Health Study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jawaid, A.; Jehle, K.L.; Mansuy, I.M. Impact of Parental Exposure on Offspring Health in Humans. Trends Genet. 2021, 37, 373–388.
- Mørkve Knudsen, T.; Rezwan, F.I.; Jiang, Y.; Karmaus, W.; Svanes, C.; Holloway, J.W. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018, 142, 765–772.
- Soubry, A. Epigenetics as a Driver of Developmental Origins of Health and Disease: Did We Forget the Fathers? Bioessays 2018, 40, 1700113.
- Pembrey, M.; Saffery, R.; Bygren, L.O. Human transgenerational responses to early-life experience: Potential impact on development, health and biomedical research. J. Med. Genet. 2014, 51, 563–572.
- Schwartz, D.; Collins, F. Medicine. Environmental biology and human disease. Science 2007, 316, 695–696.
- Svanes, C.; Bertelsen, R.J.; Accordini, S.; Holloway, J.W.; Júlíusson, P.; Boateng, E.; Krauss-Etchmann, S.; Schlünssen, V.; Gómez-Real, F.; Skulstad, S.M. Exposures during the prepuberty period and future offspring’s health: Evidence from human cohort studies. Biol. Reprod. 2021, 105, 667–680.
- Ly, L.; Chan, D.; Trasler, J.M. Developmental windows of susceptibility for epigenetic inheritance through the male germline. Semin. Cell Dev. Biol. 2015, 43, 96–105.
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The preconception environment and sperm epigenetics. Andrology 2020, 8, 924–942.
- Mitamura, R.; Yano, K.; Suzuki, N.; Ito, Y.; Makita, Y.; Okuno, A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, and testosterone secretion before the onset of male puberty. J. Clin. Endocrinol. Metab. 1999, 84, 29–37.
- Wu, H.; Hauser, R.; Krawetz, S.A.; Pilsner, J.R. Environmental Susceptibility of the Sperm Epigenome During Windows of Male Germ Cell Development. Curr. Environ. Health Rep. 2015, 2, 356–366.
- Miller, G.W.; Jones, D.P. The nature of nurture: Refining the definition of the exposome. Toxicol. Sci. 2014, 137, 1–2.
- Schulte, P.A.; Hauser, J.E. The use of biomarkers in occupational health research, practice, and policy. Toxicol. Lett. 2012, 213, 91–99.
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396.
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850.
- Agier, L.; Basagaña, X.; Maitre, L.; Granum, B.; Bird, P.K.; Casas, M.; Oftedal, B.; Wright, J.; Andrusaityte, S.; de Castro, M.; et al. Early-life exposome and lung function in children in Europe: An analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet. Health 2019, 3, e81–e92.
- Guillien, A.; Cadiou, S.; Slama, R.; Siroux, V. The Exposome Approach to Decipher the Role of Multiple Environmental and Lifestyle Determinants in Asthma. Int. J. Environ. Res. Public Health 2021, 18, 1138.
- Guillien, A.; Lepeule, J.; Seyve, E.; Le Moual, N.; Pin, I.; Degano, B.; Garcia-Aymerich, J.; Pépin, J.L.; Pison, C.; Dumas, O.; et al. Profile of exposures and lung function in adults with asthma: An exposome approach in the EGEA study. Environ. Res. 2021, 196, 110422.
- Soubry, A. POHaD: Why we should study future fathers. Environ. Epigenet. 2018, 4, dvy007.
- Golding, J.; Gregory, S.; Northstone, K.; Iles-Caven, Y.; Ellis, G.; Pembrey, M. Investigating Possible Trans/Intergenerational Associations With Obesity in Young Adults Using an Exposome Approach. Front. Genet. 2019, 10, 314.
- James M Antonini; Vamsi Kodali; Mohammad Shoeb; Michael Kashon; Katherine A Roach; Gregory Boyce; Terence Meighan; Samuel Stone; Walter McKinney; Theresa Boots; et al.Jenny R RobertsPatti C Zeidler-ErdelyAaron Erdely Effect of a High-Fat Diet and Occupational Exposure in Different Rat Strains on Lung and Systemic Responses: Examination of the Exposome in an Animal Model. Toxicological Sciences 2019, 174, 100-111, 10.1093/toxsci/kfz247.
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Reports of the Surgeon General. In The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays 2014, 36, 359–371.
- Li, Y.F.; Langholz, B.; Salam, M.T.; Gilliland, F.D. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 2005, 127, 1232–1241.
- Bråbäck, L.; Lodge, C.J.; Lowe, A.J.; Dharmage, S.C.; Olsson, D.; Forsberg, B. Childhood asthma and smoking exposures before conception-A three-generational cohort study. Pediatr. Allergy Immunol. 2018, 29, 361–368.
- Lodge, C.J.; Bråbäck, L.; Lowe, A.J.; Dharmage, S.C.; Olsson, D.; Forsberg, B. Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin. Exp. Allergy 2018, 48, 167–174.
- Magnus, M.C.; Håberg, S.E.; Karlstad, Ø.; Nafstad, P.; London, S.J.; Nystad, W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: The Norwegian Mother and Child Cohort Study. Thorax 2015, 70, 237–243.
- Miller, L.L.; Henderson, J.; Northstone, K.; Pembrey, M.; Golding, J. Do Grandmaternal Smoking Patterns Influence the Etiology of Childhood Asthma? Chest 2014, 145, 1213–1218.
- Svanes, C.; Koplin, J.; Skulstad, S.M.; Johannessen, A.; Bertelsen, R.J.; Benediktsdottir, B.; Bråbäck, L.; Elie Carsin, A.; Dharmage, S.; Dratva, J.; et al. Father’s environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int. J. Epidemiol. 2017, 46, 235–245.
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117.
- Accordini, S.; Calciano, L.; Johannessen, A.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Dharmage, S.C.; Forsberg, B.; Gómez Real, F.; Holloway, J.W.; et al. Prenatal and prepubertal exposures to tobacco smoke in men may cause lower lung function in future offspring: A three-generation study using a causal modelling approach. Eur. Respir. J. 2021.
- Muthén, B.O.; Muthén, L.K.; Asparouhov, T. Regression and Mediation Analysis Using Mplus; Muthén & Muthén: Los Angeles, CA, USA, 2016.
- Lutz, S.M.; Thwing, A.; Schmiege, S.; Kroehl, M.; Baker, C.D.; Starling, A.P.; Hokanson, J.E.; Ghosh, D. Examining the role of unmeasured confounding in mediation analysis with genetic and genomic applications. BMC Bioinform. 2017, 18, 344.
- Kuiper, I.N.; Svanes, C.; Benediktsdottir, B.; Bertelsen, R.J.; Bråbäck, L.; Dharmage, S.C.; Holm, M.; Janson, C.; Jögi, R.; Malinovschi, A.; et al. Agreement in reporting of asthma by parents or offspring—the RHINESSA generation study. BMC Pulm. Med. 2018, 18, 122.
- Pape, K.; Svanes, C.; Malinovschi, A.; Benediktsdottir, B.; Lodge, C.; Janson, C.; Moratalla, J.; Sánchez-Ramos, J.L.; Bråbäck, L.; Holm, M.; et al. Agreement of offspring-reported parental smoking status: The RHINESSA generation study. BMC Public Health 2019, 19, 94.
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32.
- Marcon, A.; Pesce, G.; Calciano, L.; Bellisario, V.; Dharmage, S.C.; Garcia-Aymerich, J.; Gislasson, T.; Heinrich, J.; Holm, M.; Janson, C.; et al. Trends in smoking initiation in Europe over 40 years: A retrospective cohort study. PLoS ONE 2018, 13, e0201881.
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373.
- Mørkve Knudsen, G.T.; Rezwan, F.I.; Johannessen, A.; Skulstad, S.M.; Bertelsen, R.J.; Real, F.G.; Krauss-Etschmann, S.; Patil, V.; Jarvis, D.; Arshad, S.H.; et al. Epigenome-wide association of father’s smoking with offspring DNA methylation: A hypothesis-generating study. Environ. Epigenet. 2019, 5, dvz023.
- Hammer, B.; Kadalayil, L.; Boateng, E.; Buschmann, D.; Rezwan, F.I.; Wolff, M.; Reuter, S.; Bartel, S.; Knudsen, T.M.; Svanes, C.; et al. Preconceptional smoking alters spermatozoal miRNAs of murine fathers and affects offspring’s body weight. Int. J. Obes. 2021, 45, 1623–1627.
- Rehan, V.K.; Liu, J.; Naeem, E.; Tian, J.; Sakurai, R.; Kwong, K.; Akbari, O.; Torday, J.S. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012, 10, 129.
- Wu, C.C.; Hsu, T.Y.; Chang, J.C.; Ou, C.Y.; Kuo, H.C.; Liu, C.A.; Wang, C.L.; Chuang, H.; Chen, C.P.; Yang, K.D. Paternal Tobacco Smoke Correlated to Offspring Asthma and Prenatal Epigenetic Programming. Front. Genet. 2019, 10, 471.
- Patil, V.K.; Holloway, J.W.; Zhang, H.; Soto-Ramirez, N.; Ewart, S.; Arshad, S.H.; Karmaus, W. Interaction of prenatal maternal smoking, interleukin 13 genetic variants and DNA methylation influencing airflow and airway reactivity. Clin. Epigenetics 2013, 5, 22.
- Baur, X.; Bakehe, P. Allergens causing occupational asthma: An evidence-based evaluation of the literature. Int. Arch. Occup. Environ. Health 2014, 87, 339–363.
- Dumas, O.; Le Moual, N. Do chronic workplace irritant exposures cause asthma? Curr. Opin. Allergy Clin. Immunol. 2016, 16, 75–85.
- Christensen, B.H.; Thulstrup, A.M.; Hougaard, K.S.; Skadhauge, L.R.; Hansen, K.S.; Frydenberg, M.; Schlünssen, V. Maternal occupational exposure to asthmogens during pregnancy and risk of asthma in 7-year-old children: A cohort study. BMJ Open 2013, 3, e002401.
- Magnusson, L.L.; Wennborg, H.; Bonde, J.P.; Olsen, J. Wheezing, asthma, hay fever, and atopic eczema in relation to maternal occupations in pregnancy. Occup. Environ. Med. 2006, 63, 640–646.
- Tagiyeva, N.; Devereux, G.; Semple, S.; Sherriff, A.; Henderson, J.; Elias, P.; Ayres, J.G. Parental occupation is a risk factor for childhood wheeze and asthma. Eur. Respir. J. 2010, 35, 987–993.
- Li, X.; Sundquist, K.; Sundquist, J. Parental occupation and risk of hospitalization for asthma in children and adolescents. J. Asthma 2009, 46, 815–821.
- Yu, W.; Sipowicz, M.A.; Haines, D.C.; Birely, L.; Diwan, B.A.; Riggs, C.W.; Kasprzak, K.S.; Anderson, L.M. Preconception urethane or chromium(III) treatment of male mice: Multiple neoplastic and non-neoplastic changes in offspring. Toxicol. Appl. Pharmacol. 1999, 158, 161–176.
- Pape, K.; Svanes, C.; Sejbæk, C.S.; Malinovschi, A.; Benediktsdottir, B.; Forsberg, B.; Janson, C.; Benke, G.; Tjalvin, G.; Sánchez-Ramos, J.L.; et al. Parental occupational exposure pre- and post-conception and development of asthma in offspring. Int. J. Epidemiol. 2020, 49, 1856–1869.
- Tjalvin, G.; Svanes, Ø.; Igland, J.; Bertelsen, R.J.; Benediktsdóttir, B.; Dharmage, S.; Forsberg, B.; Holm, M.; Janson, C.; Jõgi, N.O.; et al. Maternal preconception occupational exposure to cleaning products and disinfectants and offspring asthma. J. Allergy Clin. Immunol. 2021.
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021.
- Nordeide Kuiper, I.; Svanes, C.; Markevych, I.; Accordini, S.; Bertelsen, R.J.; Bråbäck, L.; Heile Christensen, J.; Forsberg, B.; Halvorsen, T.; Heinrich, J.; et al. Lifelong exposure to air pollution and greenness in relation to asthma, rhinitis and lung function in adulthood. Environ. Int. 2021, 146, 106219.
- Deng, Q.; Lu, C.; Ou, C.; Chen, L.; Yuan, H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere 2016, 152, 459–467.
- Kuiper, I.N.; Markevych, I.; Accordini, S.; Bertelsen, R.J.; Bråbäck, L.; Christensen, J.H.; Forsberg, B.; Halvorsen, T.; Heinrich, J.; Hertel, O.; et al. Associations of Preconception Exposure to Air Pollution and Greenness with Offspring Asthma and Hay Fever. Int. J. Environ. Res. Public Health 2020, 17, 5828.
- Timm, S.; Svanes, C.; Frydenberg, M.; Sigsgaard, T.; Holm, M.; Janson, C.; Bråbäck, L.; Campbell, B.; Kjaer Madsen, M.; Jõgi, N.O.; et al. Does parental farm upbringing influence the risk of asthma in offspring? A three-generation study. Int. J. Epidemiol. 2021, 49, 1874–1882.
- Timm, S.; Schlünssen, V.; Benediktsdottir, B.; Bertelsen, R.J.; Bråbäck, L.; Holm, M.; Jogi, R.; Malinovschi, A.; Svanes, C.; Frydenberg, M. Offspring Reports on Parental Place of Upbringing: Is It Valid? Epidemiology 2019, 30, e16–e18.
- Meng, X.; Zhang, L.; Hou, J.; Ma, T.; Pan, C.; Zhou, Y.; Han, R.; Ding, Y.; Peng, H.; Xiang, Z.; et al. The mechanisms in the altered ontogenetic development and lung-related pathology in microcystin-leucine arginine (MC-LR)-paternal-exposed offspring mice. Sci. Total Environ. 2020, 736, 139678.
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179.
- Akinbami, L.J.; Fryar, C.D. Current Asthma Prevalence by Weight Status Among Adults: United States, 2001–2014. NCHS Data Brief. 2016, 239, 1–8.
- Mamun, A.A.; Lawlor, D.A.; Alati, R.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M. Increasing body mass index from age 5 to 14 years predicts asthma among adolescents: Evidence from a birth cohort study. Int. J. Obes. 2007, 31, 578–583.
- Zhang, Z.; Lai, H.J.; Roberg, K.A.; Gangnon, R.E.; Evans, M.D.; Anderson, E.L.; Pappas, T.E.; Dasilva, D.F.; Tisler, C.J.; Salazar, L.P.; et al. Early childhood weight status in relation to asthma development in high-risk children. J. Allergy Clin. Immunol. 2010, 126, 1157–1162.
- Weinmayr, G.; Forastiere, F.; Büchele, G.; Jaensch, A.; Strachan, D.P.; Nagel, G. Overweight/obesity and respiratory and allergic disease in children: International study of asthma and allergies in childhood (ISAAC) phase two. PLoS ONE 2014, 9, e113996.
- Dumas, O.; Varraso, R.; Gillman, M.W.; Field, A.E.; Camargo, C.A., Jr. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 2016, 71, 1295–1304.
- Harpsøe, M.C.; Basit, S.; Bager, P.; Wohlfahrt, J.; Benn, C.S.; Nøhr, E.A.; Linneberg, A.; Jess, T. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: A study within the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2013, 131, 1033–1040.
- Forno, E.; Han, Y.Y.; Mullen, J.; Celedón, J.C. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 570–581.e510.
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571.
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 2015, 39, 650–657.
- Johannessen, A.; Lønnebotn, M.; Calciano, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Dharmage, S.; Franklin, K.A.; Gislason, T.; Holm, M.; et al. Being overweight in childhood, puberty, or early adulthood: Changing asthma risk in the next generation? J. Allergy Clin. Immunol. 2020, 145, 791–799.e794.
- Dratva, J.; Bertelsen, R.; Janson, C.; Johannessen, A.; Benediktsdóttir, B.; Bråbäck, L.; Dharmage, S.C.; Forsberg, B.; Gislason, T.; Jarvis, D.; et al. Validation of self-reported figural drawing scales against anthropometric measurements in adults. Public Health Nutr. 2016, 19, 1944–1951.
- Lønnebotn, M.; Svanes, C.; Igland, J.; Franklin, K.A.; Accordini, S.; Benediktsdóttir, B.; Bentouhami, H.; Blanco, J.A.G.; Bono, R.; Corsico, A.; et al. Body silhouettes as a tool to reflect obesity in the past. PLoS ONE 2018, 13, e0195697.
- Bowatte, G.; Dinh, B.; Priyankara, S.; Lowe, A.J.; Perret, J.L.; Lodge, C.J.; Hamilton, G.S.; Erbas, B.; Thomas, P.; Thompson, B.; et al. Parental preconception BMI trajectories from childhood to adolescence and asthma in the future offspring. J. Allergy Clin. Immunol. 2021. In Press.
- Lønnebotn, M.; Calciano, L.; Accordini, S.; Benediktsdóttir, B.; Bråbäck, L.; Holm, M.; Jogi, N.O.; Johannessen, A.; Malinovschi, A.; Pereira-Vega, A.; et al. Lung function in adult offspring as associated with their father’s overweight in childhood/puberty. Eur. Resp. J. 2020, 56, 2066, Abstract.
- Yamamoto-Hanada, K.; Futamura, M.; Yang, L.; Shoda, T.; Narita, M.; Kobayashi, F.; Saito, H.; Ohya, Y. Preconceptional exposure to oral contraceptive pills and the risk of wheeze, asthma and rhinitis in children. Allergol Int. 2016, 65, 327–331.
- Hancock, D.B.; Håberg, S.E.; Furu, K.; Whitworth, K.W.; Nafstad, P.; Nystad, W.; London, S.J. Oral contraceptive pill use before pregnancy and respiratory outcomes in early childhood. Pediatr. Allergy Immunol. 2011, 22, 528–536.
- Jacobsen, H.; Walendy-Gnirß, K.; Tekin-Bubenheim, N.; Kouassi, N.M.; Ben-Batalla, I.; Berenbrok, N.; Wolff, M.; Dos Reis, V.P.; Zickler, M.; Scholl, L.; et al. Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat. Commun. 2021, 12, 4957.
- Straubinger, K.; Paul, S.; Prazeres da Costa, O.; Ritter, M.; Buch, T.; Busch, D.H.; Layland, L.E.; Prazeres da Costa, C.U. Maternal immune response to helminth infection during pregnancy determines offspring susceptibility to allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 1271–1279.e1210.
- Mpairwe, H.; Ndibazza, J.; Webb, E.L.; Nampijja, M.; Muhangi, L.; Apule, B.; Lule, S.; Akurut, H.; Kizito, D.; Kakande, M.; et al. Maternal hookworm modifies risk factors for childhood eczema: Results from a birth cohort in Uganda. Pediatr. Allergy Immunol. 2014, 25, 481–488.
- Ateba-Ngoa, U.; Mombo-Ngoma, G.; Zettlmeissl, E.; van der Vlugt, L.E.; de Jong, S.E.; Matsiegui, P.B.; Ramharter, M.; Kremsner, P.G.; Yazdanbakhsh, M.; Adegnika, A.A. CD4 + CD25hiFOXP3+ cells in cord blood of neonates born from filaria infected mother are negatively associated with CD4+Tbet+ and CD4+RORgammat+ T cells. PLoS ONE 2014, 9, e114630.
- Lima, C.; Souza, V.M.; Faquim-Mauro, E.L.; Hoshida, M.S.; Bevilacqua, E.; Macedo, M.S.; Tavares-de-Lima, W.; Vargaftig, B.B. Modulation of the induction of lung and airway allergy in the offspring of IFN-gamma-treated mother mice. J. Immunol. 2005, 175, 3554–3559.
- Polte, T.; Hennig, C.; Hansen, G. Allergy prevention starts before conception: Maternofetal transfer of tolerance protects against the development of asthma. J. Allergy Clin. Immunol. 2008, 122, 1022–1030.e1025.
- Happle, C.; Jirmo, A.C.; Meyer-Bahlburg, A.; Habener, A.; Hoymann, H.G.; Hennig, C.; Skuljec, J.; Hansen, G. B cells control maternofetal priming of allergy and tolerance in a murine model of allergic airway inflammation. J. Allergy Clin. Immunol. 2018, 141, 685–696.e686.
- Jõgi, N.O.; Svanes, C.; Siiak, S.P.; Logan, E.; Holloway, J.W.; Igland, J.; Johannessen, A.; Levin, M.; Real, F.G.; Schlunssen, V.; et al. Zoonotic helminth exposure and risk of allergic diseases: A study of two generations in Norway. Clin. Exp. Allergy 2018, 48, 66–77.
- Stokholm, J.; Sevelsted, A.; Bønnelykke, K.; Bisgaard, H. Maternal propensity for infections and risk of childhood asthma: A registry-based cohort study. Lancet Respir. Med. 2014, 2, 631–637.
- López-Cervantes, J.P.; Shigdel, R.; Mustafa, T.; Accordini, S.; Svanes, C. Does parental tuberculosis infection increase the risk of asthma in their offspring? A Norwegian registry-based study. Trop. Med. Int. Health 2021, 26, 3–251, Abstract 117.
- Nyangahu, D.D.; Darby, M.; Havyarimana, E.; Brown, B.P.; Horsnell, W.; Jaspan, H.B. Preconception helminth infection alters offspring microbiota and immune subsets in a mouse model. Parasite. Immunol. 2020, 42, e12721.
- Darby, M.G.; Chetty, A.; Mrjden, D.; Rolot, M.; Smith, K.; Mackowiak, C.; Sedda, D.; Nyangahu, D.; Jaspan, H.; Toellner, K.M.; et al. Pre-conception maternal helminth infection transfers via nursing long-lasting cellular immunity against helminths to offspring. Sci. Adv. 2019, 5, eaav3058.
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191.
- Matson, A.P.; Thrall, R.S.; Rafti, E.; Puddington, L. Breastmilk from allergic mothers can protect offspring from allergic airway inflammation. Breastfeed. Med. 2009, 4, 167–174.
- Matson, A.P.; Thrall, R.S.; Rafti, E.; Lingenheld, E.G.; Puddington, L. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin. Mol. Allergy 2010, 8, 9.
- Verhasselt, V.; Milcent, V.; Cazareth, J.; Kanda, A.; Fleury, S.; Dombrowicz, D.; Glaichenhaus, N.; Julia, V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 2008, 14, 170–175.
- Hamada, K.; Suzaki, Y.; Goldman, A.; Ning, Y.Y.; Goldsmith, C.; Palecanda, A.; Coull, B.; Hubeau, C.; Kobzik, L. Allergen-independent maternal transmission of asthma susceptibility. J. Immunol. 2003, 170, 1683–1689.
- Leme, A.S.; Hubeau, C.; Xiang, Y.; Goldman, A.; Hamada, K.; Suzaki, Y.; Kobzik, L. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J. Immunol. 2006, 176, 762–769.
- Romano-Keeler, J.; Weitkamp, J.H. Maternal influences on fetal microbial colonization and immune development. Pediatr. Res. 2015, 77, 189–195.
- Neu, J.; Rushing, J. Cesarean versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol 2011, 38, 321–331.
- Ghosh, M.K.; Nguyen, V.; Muller, H.K.; Walker, A.M. Maternal Milk T Cells Drive Development of Transgenerational Th1 Immunity in Offspring Thymus. J. Immunol. 2016, 197, 2290–2296.
- Tingö, L.; Ahlberg, E.; Johansson, L.; Pedersen, S.A.; Chawla, K.; Sætrom, P.; Cione, E.; Simpson, M.R. Non-Coding RNAs in Human Breast Milk: A Systematic Review. Front. Immunol. 2021, 12, 725323.
- Caminati, M.; Pham, D.L.; Bagnasco, D.; Canonica, G.W. Type 2 immunity in asthma. World Allergy Organ. J. 2018, 11, 13.
- Restori, K.H.; Srinivasa, B.T.; Ward, B.J.; Fixman, E.D. Neonatal Immunity, Respiratory Virus Infections, and the Development of Asthma. Front. Immunol. 2018, 9, 1249.
- Dawod, B.; Marshall, J.S. Cytokines and Soluble Receptors in Breast Milk as Enhancers of Oral Tolerance Development. Front. Immunol. 2019, 10, 16.
- Tyebji, S.; Hannan, A.J.; Tonkin, C.J. Pathogenic Infection in Male Mice Changes Sperm Small RNA Profiles and Transgenerationally Alters Offspring Behavior. Cell Rep. 2020, 31, 107573.
- Bertelsen, R.J.; Rava, M.; Carsin, A.E.; Accordini, S.; Benediktsdóttir, B.; Dratva, J.; Franklin, K.A.; Heinrich, J.; Holm, M.; Janson, C.; et al. Clinical markers of asthma and IgE assessed in parents before conception predict asthma and hayfever in the offspring. Clin. Exp. Allergy 2017, 47, 627–638.
- Banjara, S.; Shigdel, R.; Svanes, C. Association between parental asthma medication and asthma in offspring. Trop. Med. Int. Health 2021, 26, 3–251, Abstract 147.
- Brew, B.K.; Lundholm, C.; Viktorin, A.; Lichtenstein, P.; Larsson, H.; Almqvist, C. Longitudinal depression or anxiety in mothers and offspring asthma: A Swedish population-based study. Int. J. Epidemiol. 2018, 47, 166–174.
- Jere R Behrman; Maria C Calderon; Samuel H Preston; John Hoddinott; Reynaldo Martorell; Aryeh D Stein; Nutritional supplementation in girls influences the growth of their children: prospective study in Guatemala. The American Journal of Clinical Nutrition 2009, 90, 1372-1379, 10.3945/ajcn.2009.27524.
- Paolo Vineis; Oliver Robinson; Marc Chadeau-Hyam; Abbas Dehghan; Ian Mudway; Sonia Dagnino; What is new in the exposome?. Environment International 2020, 143, 105887, 10.1016/j.envint.2020.105887.
- Christopher Paul Wild; The exposome: from concept to utility. International Journal of Epidemiology 2012, 41, 24-32, 10.1093/ije/dyr236.
- Miranda Loh; Dimosthenis Sarigiannis; Alberto Gotti; Spyros Karakitsios; Anjoeka Pronk; Eelco Kuijpers; Isabella Annesi-Maesano; Nour Baiz; Joana Madureira; Eduardo Oliveira Fernandes; et al.Michael JerrettJohn W. Cherrie How Sensors Might Help Define the External Exposome. International Journal of Environmental Research and Public Health 2017, 14, 434, 10.3390/ijerph14040434.
- Stephanie O. M. Dyke; Katie M. Saulnier; Charles Dupras; Amy P. Webster; Karen Maschke; Mark Rothstein; Reiner Siebert; Jörn Walter; Stephan Beck; Tomi Pastinen; et al.Yann Joly Points-to-consider on the return of results in epigenetic research. Genome Medicine 2019, 11, 31, 10.1186/s13073-019-0646-6.
- Susan M. Wolf; Brittney N. Crock; Brian Van Ness; Frances Lawrenz; Jeffrey P. Kahn; Laura M. Beskow; Mildred K. Cho; Michael F. Christman; Robert C. Green; Ralph Hall; et al.Judy IllesMoira KeaneBartha M. KnoppersBarbara A. KoenigIsaac S. KohaneBonnie LeRoyKaren J. MaschkeWilliam McGeveranPilar OssorioLisa S. ParkerGloria M. PetersenHenry S. RichardsonJoan A. ScottSharon F. TerryBenjamin S. WilfondWendy A. Wolf Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genetics in Medicine 2012, 14, 361-384, 10.1038/gim.2012.23.




