
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wojciech Grodzicki | + 2464 word(s) | 2464 | 2021-11-29 02:02:50 | | | |
| 2 | Conner Chen | Meta information modification | 2464 | 2021-12-20 03:36:35 | | | | |
| 3 | Wojciech Grodzicki | + 2 word(s) | 2466 | 2022-01-10 13:39:40 | | | | |
| 4 | Wojciech Grodzicki | + 19 word(s) | 2485 | 2022-03-07 17:47:10 | | | | |
| 5 | Conner Chen | -17 word(s) | 2449 | 2022-03-08 01:33:27 | | | | |
| 6 | Conner Chen | -17 word(s) | 2449 | 2022-03-08 01:36:53 | | | | |
| 7 | Lindsay Dong | Meta information modification | 2449 | 2022-03-28 04:36:50 | | |
Video Upload Options
The widespread usage of plastic places a significant burden on the environment and impacts numerous aquatic and terrestrial species. Humans in particular can be affected by plastic pollution, predominantly via inhalation and ingestion, as well as trophic transfer along the food chain. Under natural conditions synthetic materials undergo degradation into micro- and nanoparticles, especially prone to interact with biological systems. Organisms exposed to nanoplastic accumulate it in multiple tissues, including the gut and the brain. The scarce but consistent evidence shows that exposure to plastic nanoparticles can indeed affect both the digestive and the nervous system, therefore, potentially pose a threat to the complex network of mutual interactions between them, known as the gut-brain axis.
1. Plastics in Human Environment
The current era of the Earth’s history is frequently referred to as the Plasticene, the “Plastic Age”. Although coined informally, the term seems to appropriately reflect the state of the global environment, ubiquitously polluted with synthetic litter [1]. During the last decades plastic production worldwide has been steadily increasing, reaching 368 million tons in 2019 [2][3]. The total amount of plastic ever produced by humans has been estimated to be more than 8 billion tons. Approximately 60% of that amount was discarded as waste and accumulated in the environment [4]. This rejected, unrecycled material not only contaminates the land, but also ends up in the aquatic biome, forming plastic debris both on and beneath the water surface [2]. Due to physicochemical and biological processes, such as UV-induced decomposition and digestion by marine species, under environmental conditions plastic can undergo degradation into micro- (defined as smaller than 5 mm in diameter) and nanoparticles (defined as smaller than 1000 or 100 nm in diameter). Compared with larger fragments, these micro- and nanoplastics (MNPs) pose a less tangible, but not less dangerous threat to organisms. As it turns out, they can be harmful especially in regards to digestive and nervous systems of aquatic organisms and other elements of the food chain, probably also including humans [2][5][6][7].
2. The Gut-Brain Axis
The gastrointestinal tract (GI tract) and the central nervous system (CNS) are connected by a complex network of mutual interactions, known as the gut-brain axis (GB axis) [8]. The core of the GB axis consists of the vagus nerve, the X cranial nerve and simultaneously a branch of the autonomic nervous system. It sends information about the state of the inner organs, via afferent fibers, to the brain and connects the CNS to the enteric nervous system (ENS) [8][9][10]. Simultaneously, the CNS interacts with the GI tract via the hypothalamic-pituitary-adrenal axis (HPA axis), also integrated into the gut-brain communication pathways [9]. Direct effects of diverse neuronal and hormonal stimulation on the GI tract are possible due to the ENS, an intricate complex of nerves situated beneath the intestinal mucosa [9][10]. The ENS is located in close vicinity to the epithelium, which creates a tight barrier between the gut lumen and underlying tissues, preventing the unwanted passage of food and microbial antigens deep into the mucosa. A disruption of the barrier has been observed in several psychiatric disorders, including anxiety and depression, which suggests a link between gut permeability and CNS function [11][10].
Besides the physical defense provided by the intestinal barrier, the digestive tract is protected against multiple environmental factors by immune cells in the gut-associated lymphoid tissue (GALT) [9][11][12]. GALT contains the highest concentration of immune cells in the entire human organism and provides the primary space of exposure to microbial agents and their metabolites [11][12]. The totality of these microorganisms is known as the gut microbiota and, as a growing body of evidence demonstrates, heavily affects CNS functioning, blood-brain barrier (BBB) permeability, brain cells development and neuron maturation [11][12][13][14]. Additionally, microbial metabolites act as neuromodulators, while short-chain fatty acids (SCFA) produced by gut bacteria might stimulate the vagus nerve, affect neurotransmitter metabolism and have an impact on behavior [11]. Changes in microbiota have been linked to the development of different CNS-related disorders, including pathogeneses of Alzheimer’s disease, Parkinson’s disease, autism and depression [10]. Taken together, there are multifarious ways of communication between the gut and the brain. Given the crucial physiological function of the GB axis and its involvement in numerous neurological disorders, any potential disrupting agents are a cause of particular concern.
3. Impact of Nanoplastic Exposure on the Gut-Brain Axis
Data regarding nanoplastics (NPs) and their impact on living organisms indicate that the main risk associated with plastic exposure is a non-acute toxicity, particularly with respect to the digestive and the nervous system [6][7]. Moreover, there is a parallelism between the effects provoked by NPs and other nanoparticles, since many nanomaterials, especially metallic nanoparticles, have well-documented neurotoxic properties [15][16]. Therefore, NPs of similar size can be expected to produce analogous outcomes. The following is the description of experimental studies investigating NPs-related toxicity in regards to several elements of the GB axis.
3.1. In Vitro Studies on Cellular Cultures
Currently, in vitro research exploring the toxicity of NPs with regards to the GI tract and CNS cells is still scarce. In addition, in the limited number of existing studies different types, sizes and concentrations of nanoplastics have been used, which complicates direct comparisons. Nevertheless, those preliminary findings share certain commonalities, allowing for some generalization. NPs seem to be able to penetrate gut cells, creating an opportunity for further distribution. In brain cells models nanoplastic is internalized, elicits oxidative stress and reduces cell viability, particularly in the more realistic, “aged” form. The summary of these research is presented in Table 1.
Table 1. In vitro NPs toxicity related to the GB axis.
|
Cell Models |
NPs Type and Size |
Exposure |
Effects Related to the GB axis |
Reference |
|
Human intestinal Caco-2, HT29-MTX-E12 and THP-1 monocultures; triple culture human intestinal Caco-2/HT29-MTX-E12/THP-1 model (healthy or inflamed) |
Pristine/amino-modified polystyrene NPs (59 nm); polyvinyl chloride NPs (279 nm) |
24 h (1–50 µg/mL) |
Monocultures/amino-modified polystyrene: metabolic disruption, inflammation, DNA damage; healthy triple culture model/amino-modified polystyrene: increased cytotoxicity, decrease of tight junction protein 1; inflamed triple culture model/polyvinyl chloride: loss of nuclei |
[17] |
|
Human intestinal Caco-2/HT29 and Caco-2/HT29 + Raji-B cells |
Polystyrene NPs (5–100 nm) |
24 h (1–100 µg/mL) |
No significant toxic effects |
[18] |
|
Human intestinal Caco-2/HT29-MTX-E12 co-culture model |
Carboxylated polystyrene NPs (50 and 500 nm) |
24 h (0.1–100 µg/mL) |
Uptake of NPs |
[19] |
|
Human intestinal Caco-2/HT29-MTX co-culture model |
Pristine/positively/ negatively charged polystyrene NPs (50 nm), non-digested or digested in vitro |
24 h (250 µg/mL) |
Digested NPs: enhanced translocation across cells; positively charged NPs: increased intestinal barrier permeability |
[20] |
|
Murine mixed neuronal cells; primary astrocytes |
Polystyrene NPs (100 nm) |
48 h (50–200 µg/mL) |
Uptake of NPs; mixed neuronal cells: reduced cell viability, altered expression of Tubb3 and Gfap genes; primary astrocytes: increased expression of Tnfa and Il1b genes |
[21] |
|
Human neuronal T98G cells |
Polyethylene NPs (100–600 nm); polystyrene NPs (40–250 nm) |
24 h (0.05–10 µg/mL) |
Increased reactive oxygen species generation |
[22] |
|
Murine NE-4C Neuroectodermal stem cells; neuron-enriched primary brain cell cultures; primary astrocytes; microglial cultures; brain vascular endothelial cell cultures |
Carboxylated/PEGylated polystyrene NPs (45–70 nm), “fresh” or “aged” (6 months< of storage) |
1 h (50 µg/mL) 24 h (7.8–250 µg/mL) |
“Fresh” carboxylated NPs: uptake by microglia; “aged” NPs: uptake and cytotoxicity in NE-4C neuronal stem cells and microglia; enhanced cellular uptake of NPs caused by lipopolysaccharide adsorption |
[23] |
|
Embryonic stem cell (hESC)-derived 3-dimensional model of human neural development |
Polyethylene NPs (33 nm) |
48 h (5.6–1440 µg/mL); 18 days (5.6–360 µg/mL) |
Uptake of NPs; reduced cell viability; oxidative stress; down-regulation of HES5, NOTCH1, FOXG1, NEUROD1 and ASCL1 genes |
[24] |
3.2. In Vivo Studies on Fish
Data regarding NPs impact on GB axis derived from in vivo studies on aquatic vertebrates are still insufficient to draw definitive conclusions. Furthermore, the size and concentration of particles applied are often too dissimilar to confidently compare results derived from different experiments. However, studies conducted up to date are consistent in at least several aspects. They clearly show size-dependent differences in toxicity, NPs being more harmful than microplastics (MPs), possibly due to their smaller diameter and, consequently, higher bioactivity. There is also convincing preliminary evidence for translocation of NPs from the gut to the brain and their ability to cross the BBB. In conjunction with behavioral alterations, possible neurodevelopmental disturbances, impact on enzymatic activity, induction of oxidative stress and immune system activation, the influence of nanoplastic on the GB axis becomes a plausible phenomenon. The summary of these research is presented in Table 2.
Table 2. Summarized data derived from in vivo experiments on fish regarding toxic effects of NPs related to the GB axis.
|
Fish |
NPs Type and Size |
Exposure |
Effects Related to the GB Axis |
Reference |
|
Zebrafish (D. rerio) |
Polystyrene NPs (700 nm) |
Single-dose injection |
Altered expression of 26 genes 1 day and 51 genes 3 days post-injection; activation of the complement system; activation of oxidative stress-related pathways |
[25] |
|
Marine medaka (O. melastigma) |
Polystyrene NPs (50 nm) |
In water for 24 h (10 µg/mL) |
NPs accumulation in the digestive system; induction of apoptosis in the gut; increased activity of superoxide dismutase and catalase in the gut |
[26] |
|
Japanese medaka (O. latipes) |
Polystyrene NPs (39.4 nm) |
In water for 7 days (10 µg/mL) |
NPs accumulation in the gut and brain |
[27] |
|
Zebrafish (D. rerio) |
Polystyrene NPs (51 nm) |
In water for 114 h (0.1–10 µg/mL) |
NPs accumulation in the gut and head; behavioral alterations |
[28] |
|
Chinese medaka (O. sinensis); Dark chub (Z. temminckii) |
Polystyrene NPs (51 nm) |
In water for 7 days (5 µg/mL, individual toxicity); for 48 h (O. sinensis) or 24 h (Z. temminckii) via trophic transfer (C. reinhardtii→ |
Individual toxicity: behavioral alterations; O. sinensis/trophic transfer: NPs accumulation in the gut; Z. temminckii/trophic transfer: NPs accumulation in the gut and stomach |
[29] |
|
Crucian carp (C. carassius) |
Sulfonated polystyrene NPs (24 and 27 nm) |
For 61 days via trophic transfer (Scenedesmus sp.→D. magna→C. carassius) |
Histological changes in the brain; behavioral alterations |
[30] |
|
Crucian carp (C. carassius) |
Amino-modified polystyrene NPs (53 and 180 nm) |
For 67 days via trophic transfer (Scenedesmus sp.→D. magna→C. carassius) |
NPs accumulation in the brain; behavioral alterations |
[31] |
|
Zebrafish (D. rerio) |
Polystyrene NPs (50 nm) |
In water for 117 h (1 µg/mL) |
Up-regulation of Gfap and α1-tubulin genes; decreased acetylcholinesterase activity; decreased levels of reduced glutathione; decreased body length; behavioral alterations |
[32] |
|
Zebrafish (D. rerio) |
Polystyrene NPs (70 nm) |
7 days (0.5 and 1.5 µg/mL); 30 days (1.5 µg/mL); 7 weeks (5 µg/mL) |
NPs accumulation in the gut and brain; lowered levels of acetylcholinesterase, dopamine, melatonin, vasopressin, 5-hydroxytryptophan, kisspeptin, γ-aminobutyric acid and oxytocin; behavioral alterations |
[33] |
3.3. In Vivo Studies on Rodents
Research into plastic toxicity in mammalian species is currently very scarce and in most part focused on the effects caused by MPs. Consequently, studies investigating nanoplastic effects in regards to the GB axis are even more lacking. In fact, a recent review by Yong et al. mentioned only 10 articles describing MNPs effects in mice, whereas another review by Prüst et al. identified only one such publication directly related to NPs neurotoxicity [6][7]. Nevertheless, the existing evidence allows to formulate some initial remarks and is definitely worth exploring. Summarized data of in vivo studies on rodents are presented in Table 3.
Table 3. Summarized data derived from in vivo experiments on rodents regarding toxic effects of NPs related to the GB axis.
|
Rodent |
NPs Type and Size |
Exposure |
Effects Related to the GB Axis |
Reference |
|
Fischer rat |
Pristine/positively/ negatively charged polystyrene NPs (50 nm) |
Single-dose orally (125 mg/kg bw) |
NPs accumulation in the gut |
[34] |
|
Sprague-Dawley rat |
Polystyrene NPs (500 nm) |
Orally for 5 h (100–125 mg/kg bw) |
Accumulation in the GI tract and brain |
[35] |
|
Sprague-Dawley rat (pregnant) |
Polystyrene NPs (20 nm) |
Single-dose inhalation |
NPs accumulation in fetal brain |
[36] |
|
ICR mouse |
Polystyrene NPs (500 nm) |
Orally in drinking water for 5 weeks (0.1 or 1 µg/mL) |
Higher load: decreased body weight; decrease in gut mucin secretion; lowered expression |
[37] |
|
Wistar rat |
Polystyrene NPs (38.9 nm) |
Orally for 35 days (1–10 mg/kg bw) |
No changes in behavior |
[38] |
|
C57BL/6J mice |
Polystyrene NPs (around 50 nm) |
Orally for 30 days (0.2–10 mg/kg bw) |
No changes in behavior; no inflammation/oxidative stress in the gut and brain; highest dose: damage to the intestinal wall; changes in microbiota composition |
[39] |
|
BALB/c mice |
Pristine/carboxyl-/amino- |
Orally for 28 days (1 mg/day) |
NPs accumulation in the gut and brain; histological damage to the gut and brain; inflammation in the brain; intestinal cells penetration confirmed in vitro |
[40] |
Research regarding the influence of NPs on different components of the GB axis is scarce and only begins to scratch the surface of possible toxicity. Available data come exclusively from experiments performed on cellular cultures and animal models, therefore, any indications of potential risks for human health have to be extrapolated from these results. In vitro studies demonstrate that nanoscale plastic particles undergo internalization, both in intestinal and cerebral cells, provoking reduced viability and oxidative damage. Moreover, under environmentally realistic conditions, they are able to adsorb other toxins, which contribute to their harmfulness. In vivo experiments on aquatic vertebrates confirm these observations, proving NPs capable of effectively distribute over the body, affecting the digestive tract and the brain. They trigger the immune response, disturb the intestinal microbiota homeostasis, induce oxidative stress and cause behavioral alterations. Finally, the few studies conducted on rodents are in line with the aforementioned research and show several alarming effects taking place upon exposure to NPs. In mammals nanoplastic accumulates in the GI tract, induces dysbiosis and undermines the intestinal barrier integrity. Furthermore, it translocates to multiple organs and passes across biological barriers, including the placental-blood barrier and the BBB, to ultimately enter the brain. Summarized effects of NPs exposure on the GB axis are depicted in Figure 1.
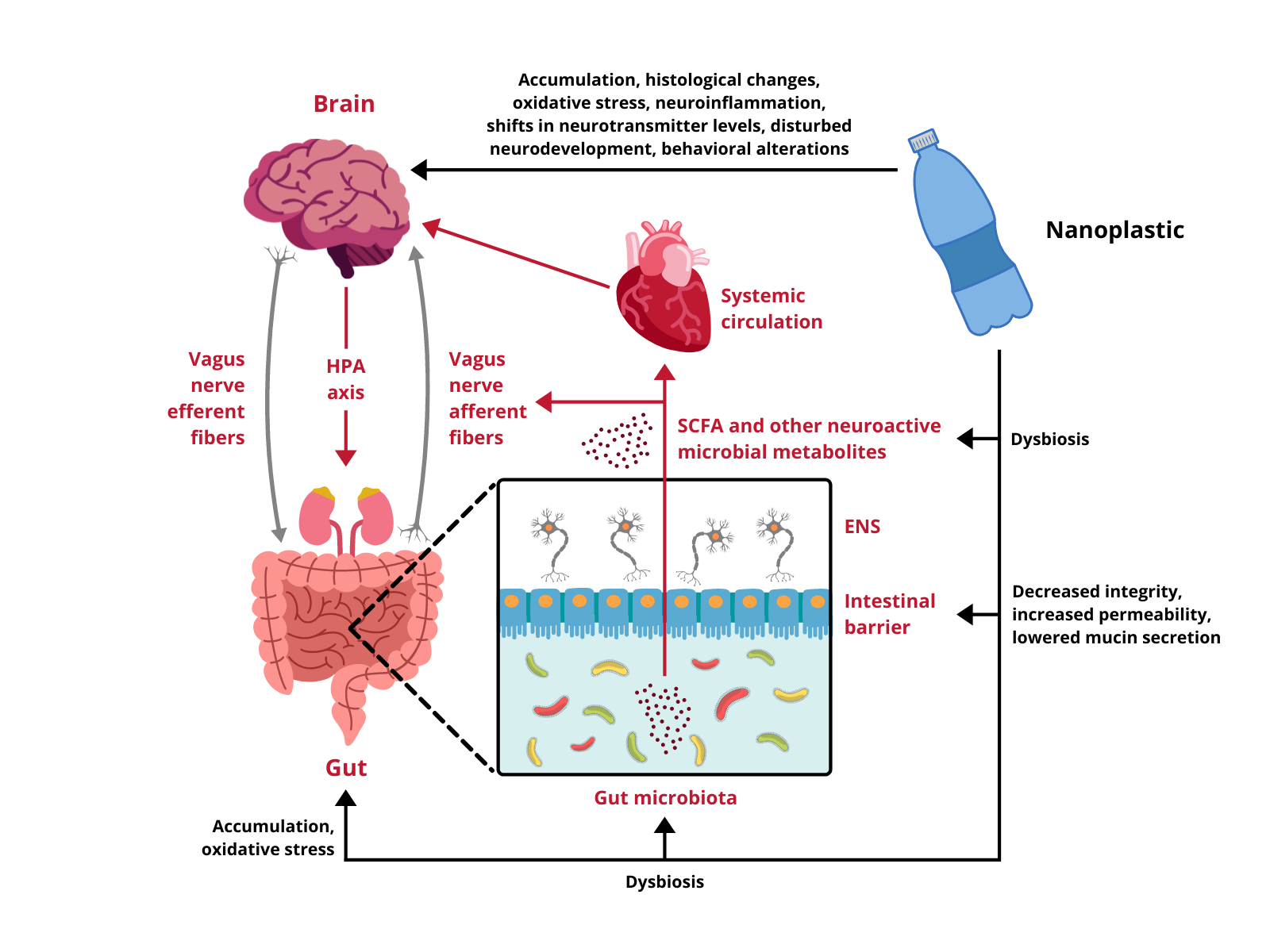
Figure 1. Impact of nanoplastic exposure on the gut-brain axis. HPA axis, hypothalamic-pituitary-adrenal axis; ENS, enteric nervous system; SCFA, short-chain fatty acids.
4. Future Perspectives
Although the experimental data regarding nanoplastic impact on mammalian systems are just beginning to build up, the evidence gathered up to date sheds some light on the consequences NPs exposure could have for both the GI tract and the CNS. The accumulation in the digestive system seems to be a factual phenomenon that might lead to dysbiosis and jeopardize the integrity of the intestinal barrier. Further biodistribution of NPs also takes place, as their presence in multiple tissues is shown consistently. One of the target organs is the brain, which suggests that nanoparticulate plastic possesses the ability to cross the BBB. Even though specific behavioral or biochemical alterations in the CNS are yet to be proven, the fact that NPs can reach cerebral compartments and affect the gut environment opens up the alarming possibility of compromised functioning of the GB axis. Toxicity determinants, such as plastic type, particle size and load, surface modification or adsorption of chemicals, as well as impact on gene expression and specific biochemical pathways involved in the gut-brain communication are examples of topics that future investigation should aim to address. Regardless of the outcomes, the widespread plastic contamination in the human environment makes preventive measures and caution highly advisable.
References
- Linsey E. Haram; James T. Carlton; Gregory M. Ruiz; Nikolai A. Maximenko; A Plasticene Lexicon. Marine Pollution Bulletin 2019, 150, 110714, 10.1016/j.marpolbul.2019.110714.
- Chibuisi Gideon Alimba; Caterina Faggio; Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environmental Toxicology and Pharmacology 2019, 68, 61-74, 10.1016/j.etap.2019.03.001.
- Plastics—The Facts 2020 . Plastics Europe. Retrieved 2021-12-13
- Roland Geyer; Jenna R. Jambeck; Kara Lavender Law; Production, use, and fate of all plastics ever made. Science Advances 2017, 3, e1700782-1700782, 10.1126/sciadv.1700782.
- Julien Gigault; Alexandra ter Halle; Magalie Baudrimont; Pierre-Yves Pascal; Fabienne Gauffre; Thuy-Linh Phi; Hind El Hadri; Bruno Grassl; Stéphanie Reynaud; Current opinion: What is a nanoplastic?. Environmental Pollution 2018, 235, 1030-1034, 10.1016/j.envpol.2018.01.024.
- Minne Prüst; Jonelle Meijer; Remco H. S. Westerink; The plastic brain: neurotoxicity of micro- and nanoplastics. Particle and Fibre Toxicology 2020, 17, 1-16, 10.1186/s12989-020-00358-y.
- Cheryl Yong; Suresh Valiyaveettil; Bor Tang; Toxicity of Microplastics and Nanoplastics in Mammalian Systems. International Journal of Environmental Research and Public Health 2020, 17, 1509, 10.3390/ijerph17051509.
- Marilia Carabotti; Annunziata Scirocco; Maria Antonietta Maselli; Carola Severi; The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology 2015, 28, 203-209.
- John F. Cryan; Kenneth J. O'riordan; Caitlin S. M. Cowan; Kiran V. Sandhu; Thomaz F. S. Bastiaanssen; Marcus Boehme; Martin G. Codagnone; Sofia Cussotto; Christine Fulling; Anna V. Golubeva; et al.Katherine E. GuzzettaMinal JaggarCaitriona M. Long-SmithJoshua M. LyteJason MartinAlicia Molinero-PerezGerard MoloneyEmanuela MorelliEnrique MorillasRory O'connorJoana S. Cruz-PereiraVeronica PetersonKieran ReaNathaniel L. RitzEoin SherwinSimon SpichakEmily TeichmanMarcel van de WouwAna Paula Ventura-SilvaShauna E. Wallace-FitzsimonsNiall HylandGerard ClarkeTimothy G. Dinan The Microbiota-Gut-Brain Axis. Physiological Reviews 2019, 99, 1877-2013, 10.1152/physrev.00018.2018.
- Livia H. Morais; Henry L. Schreiber Iv; Sarkis K. Mazmanian; The gut microbiota–brain axis in behaviour and brain disorders. Nature Reviews Genetics 2020, 19, 241-255, 10.1038/s41579-020-00460-0.
- Le Shen; Functional Morphology of the Gastrointestinal Tract. Current Topics in Microbiology and Immunology 2009, 337, 1-35, 10.1007/978-3-642-01846-6_1.
- Shan Liang; Xiaoli Wu; Feng Jin; Gut-Brain Psychology: Rethinking Psychology From the Microbiota–Gut–Brain Axis. Frontiers in Integrative Neuroscience 2018, 12, 33, 10.3389/fnint.2018.00033.
- Qianquan Ma; Changsheng Xing; Wenyong Long; Helen Y. Wang; Qing Liu; Rong-Fu Wang; Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. Journal of Neuroinflammation 2019, 16, 1-14, 10.1186/s12974-019-1434-3.
- Daniel Erny; Anna Lena Hrabě De Angelis; Diego Adhemar Jaitin; Peter Wieghofer; Ori Staszewski; Eyal David; Hadas Keren-Shaul; Tanel Mahlakoiv; Kristin Jakobshagen; Thorsten Buch; et al.Vera SchwierzeckOlaf UtermöhlenEunyoung ChunWendy GarrettKathy D McCoyAndreas DiefenbachPeter StaeheliBärbel StecherIdo AmitMarco Prinz Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience 2015, 18, 965-977, 10.1038/nn.4030.
- Lidia Strużyńska; Joanna Skalska; Mechanisms Underlying Neurotoxicity of Silver Nanoparticles. Advances in Experimental Medicine and Biology 2018, 1048, 227-250, 10.1007/978-3-319-72041-8_14.
- Daniel Mihai Teleanu; Cristina Chircov; Alexandru Mihai Grumezescu; Raluca Ioana Teleanu; Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96, 10.3390/nano9010096.
- Mathias Busch; Gerrit Bredeck; Angela A.M. Kämpfer; Roel P.F. Schins; Investigations of acute effects of polystyrene and polyvinyl chloride micro- and nanoplastics in an advanced in vitro triple culture model of the healthy and inflamed intestine. Environmental Research 2020, 193, 110536, 10.1016/j.envres.2020.110536.
- Josefa Domenech; Alba Hernández; Laura Rubio; Ricard Marcos; Constanza Cortés; Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Archives of Toxicology 2020, 94, 2997-3012, 10.1007/s00204-020-02805-3.
- Michelle Hesler; Leonie Aengenheister; Bernhard Ellinger; Roland Drexel; Susanne Straskraba; Carsten Jost; Sylvia Wagner; Florian Meier; Hagen von Briesen; Claudia Büchel; et al.Peter WickTina Buerki-ThurnherrYvonne Kohl Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicology in Vitro 2019, 61, 104610, 10.1016/j.tiv.2019.104610.
- Agata P. Walczak; Evelien Kramer; Peter Hendriksen; Richard Helsdingen; Meike van der Zande; Ivonne M. C. M. Rietjens; Hans Bouwmeester; In vitrogastrointestinal digestion increases the translocation of polystyrene nanoparticles in anin vitrointestinal co-culture model. Nanotoxicology 2015, 9, 886-894, 10.3109/17435390.2014.988664.
- Byung-Kwon Jung; Seung-Woo Han; So-Hyun Park; Jin-Sil Bae; Jinhee Choi; Kwon-Yul Ryu; Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. NeuroToxicology 2020, 81, 189-196, 10.1016/j.neuro.2020.10.008.
- Gabriella Francesca Schirinzi; Ignacio Pérez-Pomeda; Josep Sanchís; Cesare Rossini; Marinella Farré; Damià Barceló; Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environmental Research 2017, 159, 579-587, 10.1016/j.envres.2017.08.043.
- Kumarasamy Murali; Kata Kenesei; Yang Li; Kornél Demeter; Zsuzsanna Környei; Emilia Madarász; Uptake and bio-reactivity of polystyrene nanoparticles is affected by surface modifications, ageing and LPS adsorption: in vitro studies on neural tissue cells. Nanoscale 2015, 7, 4199-4210, 10.1039/c4nr06849a.
- Lisa Hoelting; Benjamin Scheinhardt; Olesja Bondarenko; Stefan Schildknecht; Marion Kapitza; Vivek Tanavde; Betty Tan; Qian Yi Lee; Stefan Mecking; Marcel Leist; et al.Suzanne Kadereit A 3-dimensional human embryonic stem cell (hESC)-derived model to detect developmental neurotoxicity of nanoparticles. Archives of Toxicology 2012, 87, 721-733, 10.1007/s00204-012-0984-2.
- Wouter J. Veneman; Herman P. Spaink; Nadja R. Brun; Thijs Bosker; Martina G. Vijver; Pathway analysis of systemic transcriptome responses to injected polystyrene particles in zebrafish larvae. Aquatic Toxicology 2017, 190, 112-120, 10.1016/j.aquatox.2017.06.014.
- Hye-Min Kang; Eunjin Byeon; Haksoo Jeong; Min-Sub Kim; Qiqing Chen; Jae-Seong Lee; Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. Journal of Hazardous Materials 2021, 405, 124207.
- Shosaku Kashiwada; Distribution of Nanoparticles in the See-through Medaka (Oryzias latipes). Environmental Health Perspectives 2006, 114, 1697-1702, 10.1289/ehp.9209.
- Jordan A. Pitt; Jordan S. Kozal; Nishad Jayasundara; Andrey Massarsky; Rafael Trevisan; Nick Geitner; Mark Wiesner; Edward D. Levin; Richard T. Di Giulio; Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquatic Toxicology 2017, 194, 185-194, 10.1016/j.aquatox.2017.11.017.
- Yooeun Chae; Dokyung Kim; Shin Woong Kim; Youn-Joo An; Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Scientific Reports 2018, 8, 1-11, 10.1038/s41598-017-18849-y.
- Karin Mattsson; Mikael T. Ekvall; Lars-Anders Hansson; Sara Linse; Anders Malmendal; Tommy Cedervall; Altered Behavior, Physiology, and Metabolism in Fish Exposed to Polystyrene Nanoparticles. Environmental Science & Technology 2014, 49, 553-561, 10.1021/es5053655.
- Karin Mattsson; Elyse V. Johnson; Anders Malmendal; Sara Linse; Lars-Anders Hansson; Tommy Cedervall; Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Scientific Reports 2017, 7, 1-7, 10.1038/s41598-017-10813-0.
- Qiqing Chen; Michael Gundlach; Shouye Yang; Jing Jiang; Mirna Velki; Daqiang Yin; Henner Hollert; Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Science of The Total Environment 2017, 584-585, 1022-1031, 10.1016/j.scitotenv.2017.01.156.
- Sreeja Sarasamma; Gilbert Audira; Petrus Siregar; Nemi Malhotra; Yu-Heng Lai; Sung-Tzu Liang; Jung-Ren Chen; Kelvin H.-C. Chen; Chung-Der Hsiao; Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. International Journal of Molecular Sciences 2020, 21, 1410, 10.3390/ijms21041410.
- Agata P. Walczak; Peter Hendriksen; Ruud A. Woutersen; Meike van der Zande; Anna Undas; Richard J R Helsdingen; Hans H. J. Van Den Berg; Ivonne M. C. M. Rietjens; Hans Bouwmeester; Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. Journal of Nanoparticle Research 2015, 17, 1-13, 10.1007/s11051-015-3029-y.
- Joshua Reineke; Daniel Cho; Yu-Ting Dingle; A. Peter Morello; Jules Jacob; Christopher G. Thanos; Edith Mathiowitz; Unique insights into the intestinal absorption, transit, and subsequent biodistribution of polymer-derived microspheres. Proceedings of the National Academy of Sciences 2013, 110, 13803-13808, 10.1073/pnas.1305882110.
- Sara B. Fournier; Jeanine N. D’Errico; Derek S. Adler; Stamatina Kollontzi; Michael J. Goedken; Laura Fabris; Edward J. Yurkow; Phoebe A. Stapleton; Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Particle and Fibre Toxicology 2020, 17, 1-11, 10.1186/s12989-020-00385-9.
- Liang Lu; Zhiqin Wan; Ting Luo; Zhengwei Fu; Yuanxiang Jin; Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Science of The Total Environment 2018, 631-632, 449-458, 10.1016/j.scitotenv.2018.03.051.
- Mohammad Rafiee; Leila Dargahi; Akbar Eslami; Elmira Beirami; Mahsa Jahangiri-Rad; Siamak Sabour; Fatemeh Amereh; Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere 2018, 193, 745-753, 10.1016/j.chemosphere.2017.11.076.
- Junting Xiao; Xuejun Jiang; Yujian Zhou; Golamaully Sumayyah; Lixiao Zhou; Baijie Tu; Qizhong Qin; Jingfu Qiu; Xia Qin; Zhen Zou; et al.Chengzhi Chen Results of a 30-day safety assessment in young mice orally exposed to polystyrene nanoparticles. Environmental Pollution 2021, 292, 118184, 10.1016/j.envpol.2021.118184.
- Dihui Xu; Yuhan Ma; Xiaodong Han; Yabing Chen; Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. Journal of Hazardous Materials 2021, 417, 126092, 10.1016/j.jhazmat.2021.126092.




