
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simone Brogi | + 2588 word(s) | 2588 | 2020-11-12 10:10:04 | | | |
| 2 | Catherine Yang | + 1 word(s) | 2589 | 2021-12-20 02:08:24 | | |
Video Upload Options
The human eye is a specialized organ with a complex anatomy and physiology, because it is characterized by different cell types with specific physiological functions. Given the complexity of the eye, ocular tissues are finely organized and orchestrated. In the last few years, many in vitro models have been developed in order to meet the 3Rs principle (Replacement, Reduction and Refinement) for eye toxicity testing.
1. Introduction
The human eye is a deeply specialized organ with a singular anatomy and physiology, comprehending several structures with specific physiological functions. Due to the complexity of the eye, ocular tissues are finely organized and orchestrated. As a result, optimal visual function is maintained while the passage of solutes, fluids, and also drugs is highly controlled [1]. Briefly, the human eye is characterized by three main layers, which enclose many anatomical structures. The outermost layer is the fibrous tunic, composed of the cornea and sclera. The cornea and opaque sclera, its non-transparent extension, are inelastic structures that provide mechanical support to the eye globe, also protecting the eye from the external environment [2][3][4]. Moreover, the cornea is covered by the tear film, whose composition ensures hydration, provides nutrients, and further limits the entering of toxins or particles into the eye [3][5][6][7]. The middle layer (uvea or vascular tunic) includes the iris, pigmented epithelium, choroid, and ciliary body [8]. Finally, the innermost layer of the eye is represented by the retina, which is a neurosensory structure fundamental for the vision process [9][10][11]. According to its crucial role in regulating the vision process, many pathological conditions affecting the retina may progressively lead to an altered vision or blindness [12][13].
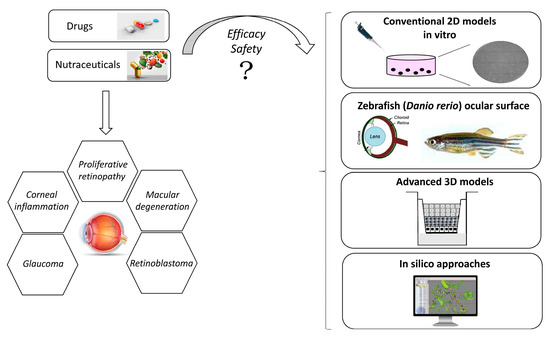
Based on these observations, along with the necessity to reduce tests on animals for evaluating the pharmacological profile of possible ocular drug candidates for given ophthalmic disorders (drug delivery/drug efficacy), including possible toxicity issues, the development of suitable and robust in vitro ocular models is a challenging task. These models allow to investigate the different aspects of the ocular pathophysiology of different diseases as well as the potential efficacy of possible therapeutic agents [14]. Furthermore, the use of these in vitro tools can be relevant for studying cell surface biomarkers for drug delivery. In the last years, along the ocular in vitro models, isolated primary cultures are expected to reproduce in vivo cellular functions and morphology in a more accurate way; however, these kinds of cells are difficult to cultivate since they arrest their growth quickly. Moreover, considering the human primary cells, it is very problematic to obtain numerous isolates for the restricted availability of human donor eyes. In order to overcome this issue, several attempts aimed at exploiting immortalized cell lines have been described to be used for pharmacological and biological investigations [15]. Unfortunately, the immortalized cell lines are characterized by altered gene expression patterns that often do not reflect the comportment of ocular cells in vivo, partially lacking the ability to mimic the complexity of the physiology of the human eye. However, the development of improved ocular cell-based models established also by reconstructing ocular tissues is fundamental for speeding up the discovery of safe ocular drugs with a relevant pharmacological profile. In this review, we report the most advance in vitro ocular models along with the computational approaches in the field of ophthalmic research. In fact, in the next sections, actual in vitro ocular models are discussed in detail, considering conventional two-dimensional (2D) models and advanced corneal three-dimensional (3D) models, with a particular focus on the application of the human cornea-like epithelium system and the potential models resembling human corneal diseases such as the zebrafish ocular surface. In addition, possible pharmacological application of 3D reconstructed human corneal tissues are reported as well as the most advanced in silico approaches in the field of ocular pharmacology and toxicology.
2. In Vitro Ocular Models
2.1. Opportunity and Application
2.2. Conventional 2D Models
2.3. Advanced Corneal 3D Models
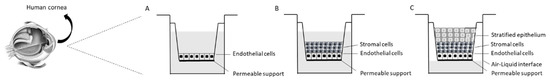
2.4. Pharmacological Application of 3D Reconstructed Human Corneal Tissues: The Dry Eye Model
Dry eye syndrome is caused by chronic dehydration of the conjunctiva and cornea, which induces irritation [7]. It is mainly due to a quantitative reduction or qualitative alteration of the tear film, which physiologically covers, lubricates, and protects corneal tissue [27]. Poor production or excessive evaporation of tears can be a complication of blepharitis, conjunctivitis (including allergic forms), and other inflammatory eye diseases [28]. Dry eye syndrome can also result from systemic diseases such as systemic lupus erythematosus and rheumatoid arthritis. Moreover, the disorder is typical in elderly patients (for the atrophy of the tear glands), in menopausal women (for the new hormonal balance), and in those who wear contact lenses [29]. Dry eye can be also related to iatrogenic causes for the use of several systemic drugs (antihypertensives, anxiolytics, sleeping pills, antihistamines). The most common symptoms due to dry eye syndrome are itching, burning, irritation, and annoyance to light (photophobia). In addition, a sensation of a foreign body pulling and scratching inside the eye, blurring of vision, difficulty opening the eyelid when waking up, eye pain, and hyperemia (red eyes) may also occur [30]. Tiredness or fatigue of the eyes may also appear and, in some patients, the appearance of mucus inside or around the eye is observed. All these disorders increase as a result of prolonged visual strain or under particular environmental conditions, such as exposure to wind or heat or staying in dusty, smoky, air-conditioned, or heated environments. In the most serious cases, the eye is exposed to increased friction due to eyelid movement and an increased risk of infections. In addition, it can degenerate to the appearance of lesions to the external structures of the eye: scarring, neovascularization, infections, and ulceration. Treatment for dry eye syndrome includes therapies that may vary depending on the cause and type of the disorder. Generally, medication is prescribed with eye drops or lubricating gels to help the eye to stay moist and clean [31]. When the patient’s eye allows it, it is also possible to prescribe protective contact lenses to protect the organ from rubbing with the eyelid. Developing novel medical devices for the treatment of dry eye syndrome is nowadays a challenging issue and 3D human corneal tissues have been recently used for the setup of in vitro dry eye model [32]. In particular, several research papers describe the realization of in vitro dry eye condition by exposing the tissues to specific conditions. Reconstructed corneal tissues are first treated with 0.6 M sorbitol in order to create a hyperosmolar environment mimicking the qualitative alteration of tear film. Furthermore, the tissues are exposed to 40 °C and 40% humidity for simulating the dryness [33]. After 24 h, tissues can be treated with the tested medical devices, and several biomarkers can be investigated. In particular, besides the tissue viability, also pro-inflammatory biomarkers (e.g., TNF, interleukins) and specific metalloproteinases which are responsible for corneal remodeling processes can be measured in order to characterize the efficacy of medical devices or drugs [34].
References
- Barar, J.; Asadi, M.; Mortazavi-Tabatabaei, S.A.; Omidi, Y. Ocular Drug Delivery; Impact of in vitro Cell Culture Models. J. Ophthalmic Vis. Res. 2009, 4, 238–252.
- Kasthurirangan, S.; Markwell, E.L.; Atchison, D.A.; Pope, J.M. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2531–2540.
- Estlack, Z.; Bennet, D.; Reid, T.; Kim, J. Microengineered biomimetic ocular models for ophthalmological drug development. Lab Chip 2017, 17, 1539–1551.
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Trans. Res. 2016, 6, 735–754.
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812.
- Dartt, D.A.; Willcox, M.D. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3.
- Zhang, X.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; Li, W. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398.
- Pradeep, T.; Mehra, D.; Le, P.H. Histology, Eye. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544343/ (accessed on 3 July 2020).
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1250–1264.
- Correia Barao, R.; Pinto Ferreira, N.; Abegao Pinto, L. A Camera without a Diaphragm. Ophthalmol. Glaucoma 2020, 3, 138.
- Kels, B.D.; Grzybowski, A.; Grant-Kels, J.M. Human ocular anatomy. Clin. Dermatol. 2015, 33, 140–146.
- Haderspeck, J.C.; Chuchuy, J.; Kustermann, S.; Liebau, S.; Loskill, P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert Opin. Drug Discov. 2019, 14, 47–57.
- Chemi, G.; Brindisi, M.; Brogi, S.; Relitti, N.; Butini, S.; Gemma, S.; Campiani, G. A light in the dark: State of the art and perspectives in optogenetics and optopharmacology for restoring vision. Future Med. Chem. 2019, 11, 463–487.
- Shafaie, S.; Hutter, V.; Cook, M.T.; Brown, M.B.; Chau, D.Y. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores. Open Access 2016, 5, 94–108.
- Vasconcelos, T.; da Silva, S.B.; Ferreira, D.; Pintado, M.; Marques, S. Cell-based in vitro models for ocular permeability studies. In Concepts and Models for Drug Permeability Studies; Woodhead Publishing: Cambridge, UK, 2016; pp. 129–154.
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672.
- Swaminathan, S.; Kumar, V.; Kaul, R. Need for alternatives to animals in experimentation: An Indian perspective. Indian J. Med. Res. 2019, 149, 584–592.
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419.
- Burden, N.; Benstead, R.; Benyon, K.; Clook, M.; Green, C.; Handley, J.; Harper, N.; Maynard, S.K.; Mead, C.; Pearson, A.; et al. Key Opportunities to Replace, Reduce, and Refine Regulatory Fish Acute Toxicity Tests. Environ. Toxicol. Chem. 2020, 39, 2076–2089.
- Piccinno, M.S.; Petrachi, T.; Resca, E.; Strusi, V.; Bergamini, V.; Mulas, G.A.; Mari, G.; Dominici, M.; Veronesi, E. Label-free toxicology screening of primary human mesenchymal cells and iPS-derived neurons. PLoS ONE 2018, 13, e0201671.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083.
- Crespo-Moral, M.; Garcia-Posadas, L.; Lopez-Garcia, A.; Diebold, Y. Histological and immunohistochemical characterization of the porcine ocular surface. PLoS ONE 2020, 15, e0227732.
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919.
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218.
- Tong, L.; Diebold, Y.; Calonge, M.; Gao, J.; Stern, M.E.; Beuerman, R.W. Comparison of gene expression profiles of conjunctival cell lines with primary cultured conjunctival epithelial cells and human conjunctival tissue. Gene Exp. 2009, 14, 265–278.
- Blazejewska, E.A.; Schlotzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009, 27, 642–652.
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77.
- Rolando, M.; Cantera, E.; Mencucci, R.; Rubino, P.; Aragona, P. The correct diagnosis and therapeutic management of tear dysfunction: Recommendations of the P.I.C.A.S.S.O. board. Int. Ophthalmol. 2018, 38, 875–895.
- Ziaragkali, S.; Kotsalidou, A.; Trakos, N. Dry Eye Disease in Routine Rheumatology Practice. Mediterr. J. Rheumatol. 2018, 29, 127–139.
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57.
- Javadi, M.A.; Feizi, S. Dry eye syndrome. J. Ophthalmic Vis. Res. 2011, 6, 192–198.
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13.
- Abusharha, A.A.; Pearce, E.I. The effect of low humidity on the human tear film. Cornea 2013, 32, 429–434.
- Acera, A.; Vecino, E.; Duran, J.A. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8285–8291.




