Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simone Brogi | + 6495 word(s) | 6495 | 2021-11-16 04:35:21 | | | |
| 2 | Vicky Zhou | Meta information modification | 6495 | 2021-12-17 11:05:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Brogi, S. Artificial Intelligence in Translational Medicine. Encyclopedia. Available online: https://encyclopedia.pub/entry/17258 (accessed on 07 February 2026).
Brogi S. Artificial Intelligence in Translational Medicine. Encyclopedia. Available at: https://encyclopedia.pub/entry/17258. Accessed February 07, 2026.
Brogi, Simone. "Artificial Intelligence in Translational Medicine" Encyclopedia, https://encyclopedia.pub/entry/17258 (accessed February 07, 2026).
Brogi, S. (2021, December 17). Artificial Intelligence in Translational Medicine. In Encyclopedia. https://encyclopedia.pub/entry/17258
Brogi, Simone. "Artificial Intelligence in Translational Medicine." Encyclopedia. Web. 17 December, 2021.
Copy Citation
Between preclinical and clinical research, translational research is benefitting from computer-based approaches like Artificial Intelligence, resulting in breakthroughs for advancing human health.
translational medicine
machine learning
artificial intelligence
1. Introduction
Nowadays, artificial intelligence (AI), as well as the related specialties of machine learning (ML) and deep learning (DL), are rapidly gaining traction in many sectors, including the scientific (e.g., healthcare), with the potential to transform lives and improve patient outcomes in various fields of medicine. Accordingly, AI companies attracted approximately USD 40 billion worldwide in unveiled investment in 2019 alone [1], reaching USD 232 billion by 2025 [2]. Regarding the scientific areas, these revolutionary computer-based approaches have the potential to revolutionize how clinicians assist patients in clinical practice (precision medicine, virtual diagnosis, and patient monitoring) as well as how scientists discover and deliver new drugs and diagnostic tools [3][4][5].
According to one description, ML is “the fundamental technology required to meaningfully process data that exceed the capacity of the human brain to comprehend” [6]. A large number of data points is used to train ML computer-based models. Existing information about specific data items and relationships between data elements is learned via repeated cycles of mapping between inputs and outputs, rather than being explicitly coded into the model. Therefore, cooperation between ML and clinical specialists is critical, and there are many computational approaches that include various degrees of clinical experience into model parameters [7]. Currently, the generation of ML models is mainly grouped into four categories: supervised learning, unsupervised learning, semi-supervised learning, and reinforcement learning. Briefly, the output labels, such as a disorder, are known in advance in supervised ML models. In fact, the objective is to generate a computational tool for predicting an output from a set of input data (i.e., the output is usually termed a target value, response variable, or label, while inputs are termed predictors or features). The method “learns” the best model by analyzing the data contained in the training set, which include many observations, each of which holds values for its characteristics as well as its label. Additionally, there are two kinds of supervised ML: classification and regression. In the classification, the output variable is divided into categories, such as “present” or “absent”, “disorder” or “no-disorder”, or “grading” (Grade1, Grade2, etc.), while in regression the output variable is an actual value, such as “weight”, “dose”, or “concentration” (IC50, EC50, TC50, etc.) for performing predictions on novel samples. Such ML may be employed in medical imaging in a variety of areas, including radiology, pathology, and other imaging fields, as well as in epidemiology. Recently, with the outbreak of the COVID-19 pandemic, some ML approaches have been applied for predicting the infection rate, starting from an epidemiology dataset [8], as well as from environmental conditions [9]. Furthermore, in recent years, supervised ML has also been used in drug discovery and development [10][11][12]. These approaches employing supervised ML are valuable, but they must be approached with prudence because they need huge and reliable data sets containing high-quality data to become accurate, and the data must be correctly categorized [13].
On the other hand, unsupervised ML models aim to identify relationships in data that we would not see otherwise. In particular, there are no labels on the data sets, but they do contain features. As a result, the unsupervised ML algorithm must produce groups and classes based on data set similarities. Unsupervised ML, in contrast to supervised ML, predicts unknown outcomes, uncovering previously undiscovered patterns.
Unsupervised ML is exemplified through clustering. This latter is the process of dividing data into various groups or clusters. Accordingly, when the exact information about the clusters is unknown, we can utilize unsupervised ML to cluster them [14]. Various scientific fields benefit from the application of unsupervised ML. For instance, in a recent report, the unsupervised ML technique was applied for identifying subjects showing a high likelihood of dementia in a population-based survey, with no need of a medical diagnosis of dementia in the subsample [15]. Another study investigated healthcare professionals’ feelings toward a digital simulator, technology, and mentality for elucidating their effects on neonatal resuscitation performance in simulation-based assessments [16]. In general pathology, unsupervised ML is becoming a crucial tool for accelerating the transition to autonomous pathological tissue analysis [17]. In another study, an unsupervised ML approach was used to discover patient clusters established by genetic signatures [18]. Additionally, in this case, in drug discovery and development, unsupervised ML has been successfully applied in atomistic simulations or to understand the comportment of chemicals (e.g., drugs) as well as materials [19]. Recently, in a randomized clinical study, unsupervised ML was applied to cluster septic patients to determine optimal treatment (NCT03752489). To further clarify the difference between supervised and unsupervised ML models, a supervised ML model can be used to identify which subjects will develop a given disorder, a known entity, while an unsupervised ML model will be able to identify unknown subgroups of patients suffering from a given pathology since unsupervised models assume that the output labels are unknown. Most of the computer-based models incorporated into clinical workflows, as clinical decision support, are supervised ML models. For improving the performances of ML models, unsupervised and supervised ML can be combined into semi-supervised ML (Figure 1). Ma and colleagues successfully reported a combination of the two strategies for phenotyping complex diseases. They applied this technique to obstructive sleep apnea, highlighting that the phenotyping framework constructed by combining unsupervised and supervised ML techniques can be employed for other heterogeneous, complex diseases to phenotype patients, distinguishing significant features for high-risk phenotypes [20]. Omta and coworkers, by combining unsupervised and supervised ML-based tools, showed that they have a great capacity to increase the capability of detecting new knowledge in functional genomics screening. Firstly, they applied unsupervised exploratory ML models to the dataset to gain better insight into the quality of the data. This latter approach enhances the selection and labeling of data for establishing reliable training sets prior to applying ML. For demonstrating the validity of the approach, they used a high-content genome-wide small interfering RNA (siRNA) screen. By applying unsupervised ML models, they easily identified four robust phenotypes that were consequently used as a training set for developing a high-quality random forest (RF) ML tool for differentiating four phenotypes (accuracy = 91.1%; kappa = 0.85). The reported approach significantly improved the ability to obtain novel information from a screening compared with the usage of unsupervised ML techniques alone [21].
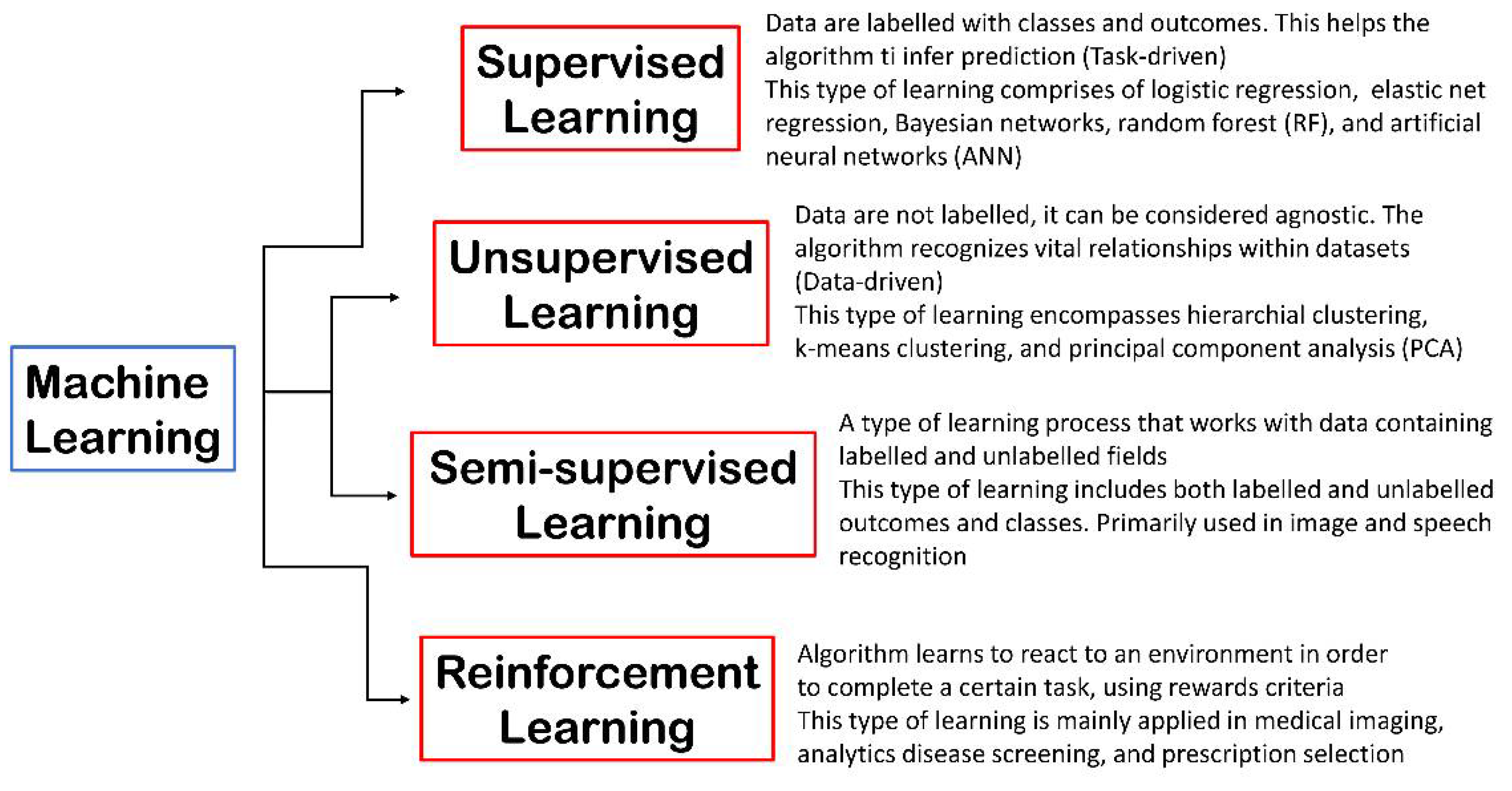 Figure 1. ML is mainly classified into four classes: supervised learning, semi-supervised learning, unsupervised learning, and reinforcement learning.
Figure 1. ML is mainly classified into four classes: supervised learning, semi-supervised learning, unsupervised learning, and reinforcement learning.However, it is important to highlight that the accuracy of these analyses is strongly dependent on the quality of the training sets employed to generate ML models.
Finally, the reinforcement ML method allows the computational tool to learn from its failures, generating an algorithm based on what it has learned. Thus, this learning is constructed upon the trial-and-error process [22]. In the scientific field, for example, different tasks can include training an algorithm to understand the treatment regimens of medical registry data and to find the optimal strategy for treating patients with chemotherapy. A recent study reported the successful use of a reinforcement ML model for establishing an effective formulation of clinical trial dosing. The algorithm was trained with proper dosing regimens for reducing tumor diameters in patients treated by means of chemotherapy and radiation [23]. In Figure 1 is reported a schematic illustration of the mentioned ML approaches.
This brief excursion about the different ML techniques and how they can be applied to scientific fields, for improving and enhancing the understanding of complex systems, highlights the potential of ML methods. To this end, there is growing attention being paid to the application of these methodologies in the framework of translational medicine, enhancing the ability of translational scientists to provide novel effective treatments and diagnostics for healthcare.
2. Artificial Intelligence (AI) and Machine Learning (ML) in Translational Medicine
2.1. Drug Discovery and Development, and Drug Target Prediction
2.1.1. Drug Discovery and Development
Beyond the classical computational approaches in drug discovery, such as ligand- (mainly QSAR methods and pharmacophore modeling) [24][25][26][27][28][29][30] and structure-based strategies (mainly based on molecular docking and molecular dynamics) [31][32][33][34][35][36][37][38][39] or a combination of them [40][41][42][43][44][45], currently these computational methods are integrated with ML technologies for improving the reliability of the calculation, avoiding false positive outcomes and enhancing the success ratio in identifying safer hit compounds. Some examples are represented by QSAR-ML models [46][47][48][49], and multi- and combi-QSAR approaches [50][51][52][53][54][55][56]. Furthermore, in the drug discovery field, advanced computational models, based on ML technology, have demonstrated strong potential in selecting effective hit compounds [57][58][59][60][61][62][63][64]. Moreover, ML-based approaches represent a valuable resource also in the drug repurposing field [65][66]. Interestingly, these approaches have provided potential drugs for treating COVID-19 in a short time [67]68]. Currently, protein structural modeling and design, as well as protein structure prediction, which can increase the proficiency in the drug discovery pipeline, are emerging areas of application of ML models [68][69][70][71]. In fact, ML methods offer a theoretical framework for identifying and prioritizing bioactive molecules possessing suitable pharmacological profile, as well as optimizing them as drug-like lead compounds before clinical investigation [64]. Generally, three steps allow the development of a computational protocol enabling ML-based models: (a) the selection of appropriate descriptors for capturing crucial features of compounds involved in the study; (b) the selection of a suitable metric or scoring system for comparing the set of molecules; (c) a proper ML-based technique for determining the characteristic traits of the features that help to qualitatively or quantitatively discriminate active molecules from inactive ones [72][73]. ML/DL approaches suitable in the drug discovery field include RF, Artificial Neural Networks (ANN), Deep Neural Networks (DNN), Graph Convolutional Neural Networks (GCNN), Convolutional Neural Networks (CNN), Naïve Bayesian techniques, Multiple Linear Regression (MLR), natural language processing (NLP), decision trees (DT), Logistic Regression (LR), Linear Discriminant Analysis (LDA), Multi-Layer Perceptron (MLP), Probabilistic Neural Networks (PNN), k-nearest neighbors (k-NN), and Support Vector Machine (SVM), only considering some of them in the context of ML [74][75][76].
Table 1. Main examples of AI/ML in the drug discovery and development field.
| AI Technique | Target | Dataset | Statistical Parameters |
Outcomes | |
|---|---|---|---|---|---|
| Bayesian ML models | GSK-3β AD |
2368 compounds | Cross-validation, ROC curve = 0.905 | Virtual screening found ruboxistaurin (CHEMBL91829) as GSK-3β (IC50 = 97.3 nM) and GSK-3α (IC50 = 695.9 nM) inhibitor | |
| Bayesian ML and RP algorithms for developing a multi-QSAR approach | 25 crucial cellular targets in AD | 18,741 active compounds against the selected targets | Internal and external validation (area under the ROC curve for the test set 0.741–1.0, average 0.965) | Identification of various MTDLs against AD (seven AChE inhibitors (IC50 = 0.442–72.26 μM); four H3R antagonists (IC50 = 0.308–58.6 μM). The best performing MTDL (DL0410) showed a dual cholinesterase inhibitor behavior (IC50 AChE = 0.442 μM; IC50 BuChE = 3.57 μM), and behaved as a H3R antagonist (IC50 = 0.308 μM) | |
| ML-based approach | DRIAD for drug repurposing in AD | DRIAD was applied to find relationships between the pathology of AD severity (the Braak stage) and molecular mechanisms as determined in records of gene names by using 80 FDA-approved and investigational drugs | Model performance was evaluated through leave-pair-out cross-validation, area under the ROC curve ranging from 0.6 to 0.8 | 33 FDA-approved drugs can be used for repurposing immediately | |
| SVM models coupled with Tanimoto similarity-based clustering analysis | A2A and D2 receptor subtypes as targets for PD | 135 compounds (96 from A2A and 39 from D2) | Experimental validation | Virtual screening of over 13.5 million compounds from PubChem and MDDR databases. Two compounds behaved as multifunctional ligands against human A2A (Ki = 8.7 and 11.2 μM) and D2 receptors (EC50 = 22.5 and 40.2 μM) | |
| SVM and SVR | PD drug discovery A2A vs. A3 receptor subtype selectivity profiles and related binding affinities | For SVM, 104 selective N7- and N8-substituted pyrazolo–triazolo–pyrimidine analogs. For SVR, 104 N8-substituted pyrazolo–triazolo–pyrimidine derivatives. A test set of 51 N8-substituted pyrazolo–triazolo–pyrimidine analogs to validate both SVM and SVR models |
LOO-cv Correct prediction 93.3, sensitivity 92.0, specificity 94.4 |
51 novel pyrazolo–triazolo–pyrimidine containing compounds that confirmed the predicted receptor subtype selectivity and the related binding affinity profiles | |
| SVM and RF | Anticancer drug discovery—target FEN1 | The training set contained 1163 FEN1 inhibitors and 281,583 non-inhibitors; the test set 388 inhibitors and 93,861 non-inhibitors | For the test set: sensitivity 0.54, specificity 0.99, MCC 0.67 |
The computational tool was used in a virtual screening employing the Maybridge database (53,000 molecules). Five top-ranked compounds were experimentally validated. The molecule JFD00950 behaved as a FEN1 inhibitor in the micromolar range, inhibiting Flap cleavage activity, showing cytotoxic activity against colon cancer cells (DLD-1, IC50 = 16.7 µM) | |
| ML models using naïve Bayesian and RP techniques | Indoleamine 2,3-dioxygenase (IDO), a promising target for cancer immunotherapy | The model was trained using a library of established IDO inhibitors (504 compounds, 242 active and 262 inactive) | The Q values for the test set of the top 10 models are greater than 0.76, the MCC values >0.53, the area under ROC curve >0.89 | Virtual screening campaign using a proprietary chemical library. This step provided 50 potential IDO inhibitors that were experimentally validated. In vitro tests confirmed the prediction of the ML model, since three new IDO inhibitors, belonging to the tanshinone family, were identified (IC50s = 1.30, 4.10, and 4.68 μM) | |
| ML model using naïve Bayesian technique coupled with a molecular docking calculation | VEGFR-2, a drug target for developing anticancer compounds with anti-angiogenic activity | The model was trained using 3464 VEGFR-2 inhibitors | MCC of 0.966 and 0.951 considering the test set and external validation set | Virtual screening protocol for identifying VEGFR-2 inhibitors using a chemical library containing 1841 FDA-approved drugs. Papaverine, rilpivirine, and flubendazole were able to inhibit VEGFR-2 (IC50 = 0.47–6.29 μM) | |
| Four distinct ML algorithms to train the model (LR, naïve Bayesian, SVM, and RF) | Anticancer drug discovery—target BCRP | The dataset contained 433 inhibitors and 545 noninhibitors, collected from 47 publications | Cross-validation (area under ROC curve = 0.9) and predictivity in prospective validation (area under ROC curve = 0.7) | Virtual screening approach using a drug library (1702 compounds). 10 drugs as potential BCRP inhibitors were identified (inhibition of mitoxantrone efflux in BCRP-expressing PLB985 cells). Among the drugs tested two of them behaved as BCRP inhibitors (cisapride and roflumilast, IC50 = 0.4 µM and 0.9 µM, respectively) | |
| ML model, based on Laplacien-modified naïve Bayesian classifiers. The ML model for EGFR was coupled with a structure-based technique regarding the bromodomain | Anticancer drug discovery—target EGFR/BRD4 | Two ML models for EGFR were developed considering ECFP4 based on a total of 591,744 unique kinase compounds (one with 3058 active molecules, pIC50/pKi ≥ 7, and another with 4785 active compounds, pIC50/pKi ≥ 6). | Area under ROC curve values of 0.98 to 0.99 based on 50/50 training/test set and assessed employing LOO-cv | Virtual screening campaign employing a large database (eMolecules > 6 million compounds). Among them, a first-in-class dual EGFR–BRD4 inhibitor (compound 2870) was found (EGFR IC50 = 44 nM; ERBB2, ERBB4, and BRD4 IC50 = 8.73, 24.2, and 9.02 μM, respectively) | |
| ML model based on a GCNN algorithm | DeepMalaria antimalarial drug discovery | 13,446 potential antimalarials contained in GSK database | Accuracy from 44.13% in the whole library to 87.75%. Accuracy of 100% for all nanomolar active compounds | The developed model was validated by predicting hit molecules from an additional chemical collection and a FDA-approved drug database. DeepMalaria identified all molecules showing nanomolar activity and 87.5% of chemicals with greater percentage of inhibition | |
| DL method DNN model |
Discovery of novel antibiotic agents, possessing a broad-spectrum antibacterial profile | Dataset of 2335 molecules | Area under ROC curve of 0.896 considering the test data | Virtual screening of various chemical libraries. From this screening step, they identify an existing drug, namely, halicin (SU-3327), showing interesting bactericidal activity in vitro as well as in vivo. It was found to be effective against M. tuberculosis. Virtual screening of ZINC15 (>100 million compounds) provided eight further antibacterial agents, chemically unrelated to known antibiotics. ZINC000100032716 and ZINC000225434673 showed strong broad-spectrum activity, overcoming a range of frequent resistance factors | |
| ML models, employing naïve Bayesian and RP techniques | DNA gyrase to find broad-spectrum antibacterial agents | 137 DNA gyrase inhibitors spanning several orders of magnitude | The overall predictive accuracy, considering the training and test sets, was greater than 80% | ML models used for virtual screening of a chemical library. The potential hits were experimentally validated against DNA gyrase, E. coli, methicillin-resistant S. aureus and other bacteria. For compounds able to inhibit DNA gyrase, MIC values range between 1 and 32 μg/mL, and the relative inhibition rates of inhibitors, range from 42% to 75% at 1 μM | |
| Bayesian ML model | Antiviral research—Ebola virus | 868 molecules viral pseudotype entry assay and the Ebola virus replication assay data | Cross-validation showed ROC values greater than 0.8 | Virtual screening campaign using the MicroSource library of drugs, for selecting possible antiviral compounds. Among the retrieved potential hit compounds, three promising antiviral candidates were found (quinacrine, pyronaridine, and tilorone EC50 = 350, 420, and 230 nM, respectively, against Ebola virus replication). | |
| GENTRL | For de novo small molecule design acting as inhibitors of DDR1 kinase | The model was generated using six data sets: (i) molecules from the ZINC database; (ii) inhibitors of DDR1 kinase; (iii) common kinase inhibitors (positive set); (iv) actives against non-kinase targets (negative set); (v) patent data of biological actives; (vi) 3D structures for DDR1 inhibitors | Experimental validation—GENTRL allowed indication of several compounds for the synthesis, and the authors synthesized six lead compounds | Two molecules strongly inhibited DDR1 activity (IC50 = 10–21 nM), the other two compounds showed moderate potency (IC50 = 0.278–1 μM) | |
| ML models RF and GCNN |
Three drug targets (sEH, a hydrolase, ERα, a nuclear receptor, c-KIT, a kinase) | Models were trained on the DEL selection data for classifying molecules (over 2000) | Experimental validation | Virtual screening of large chemical databases (∼88 million compounds). The outcomes revealed that the technique is efficient, with a global hit rate of ∼30% at 30 μM, discovering powerful compounds (IC50 < 10 nM) for each drug target | |
| DL and reinforcement learning DNNs |
De novo design of small molecules with desired profile, and JAK2 as the target protein | The generative network was trained with ~1.5 million structures from the ChEMBL21 database | Experimental validation | ReLeaSE was successfully applied for generating a series of libraries containing chemical entities with a precise profile: (a) satisfactory drug-likeness, regarding physchem properties, for which the authors chose Tm and n-octanol/water partition coefficient (logP); (b) desired biological activity, for which the authors selected Janus protein kinase 2 (JAK2) as the target protein |
Abbreviation: A2A—adenosine receptor 2A subtype; A3—adenosine receptor 3 subtype; AChE—acetylcholinesterase; AD—Alzheimer’s disease; BCRP—breast cancer resistance protein; BuChE—butyrylcholinesterase; D2—dopamine receptor type 2; DDR1—discoidin domain receptor 1; DEL—DNA-encoded small molecule library; DNN—deep neural network; DRIAD—Drug Repurposing In AD; ECFP4—extended connectivity fingerprints; EGFR—epidermal growth factor receptor; FDA—United States Food and Drug Administration; FEN1—flap endonuclease1; GENTRL—generative tensorial reinforcement learning; GCNN—graph convolutional neural networks; GSK—GlaxoSmithKline; GSK-3β—glycogen synthase kinase 3 beta; H3R—histamine receptor 3; IDO—indoleamine 2,3-dioxygenase; JAK2—Janus protein kinase 2; LOO-cv—leave-one-out cross-validation; LR—logistic regression; MCC—Matthews’s correlation coefficient; MIC—minimum inhibitory concentration; ML—machine learning; MTDLs—multitarget-directed ligands; PD—Parkinson’s disease; QSAR—quantitative structure-activity relationship; ReLeaSE—reinforcement learning for structural evolution; RF—random forest; RP—recursive partitioning; ROC—receiver operating characteristic; SVM—support vector machine; SVR—support vector regression; VEGFR-2—vascular endothelial growth factor receptor 2.
2.1.2. Drug Target Prediction and Biomarker Identification
Noteworthy is that in addition to the previously discussed ML approaches to identify promising drug candidates, AI techniques are also emerging in drug target prediction, with remarkable success. For instance, in the field of neurodegenerative disorders, we report here significant progress in ML approaches applied to drug target identification in the drug discovery/drug repurposing field (Table 2).
Table 2. Main examples of AI/ML in drug target prediction and biomarker identification.
| AI Technique | Target | Dataset | Statistical Parameters |
Outcomes | |
|---|---|---|---|---|---|
| DL methodology deepDTnet | Multiple sclerosis | DeepDTnet was generated using 732 FDA-approved for training | Area under the ROC curve = 0.963 | Topotecan was predicted as an inhibitor of ROR-γt, (IC50 = 0.43 μM), showing potential therapeutic effects in multiple sclerosis, being effective in reverting the pathological phenotype in vivo in an EAE mouse model at 10 mg/kg | |
| Bayesian ML algorithm BANDIT |
Prediction of drug targets combining various kinds of data | A total of 20 million data points derived from six diverse types of data such as drug efficacy, post-treatment transcriptional response, drug structure, described undesirable effects, bioassay results, and well-established targets | Using over 2000 compounds, BANDIT showed an accuracy of ~90% in identifying correct targets | BANDIT was validated using 14,000 molecules with no target, producing ~4000 molecule target predictions. Fourteen molecules were predicted as microtubule binders and validated in vitro, supporting the predictions. BANDIT was applied to ONC201 (anticancer in clinical with no target). ONC201 was predicted and validated as a D2 receptor antagonist and will be evaluated in pheochromocytomas, a rare cancer overexpressing D2 receptor NCT03034200 | |
| ML-based approach RF algorithm |
Druggability score of novel unidentified drug targets | The ML model included 70 features obtained from drug targets, generating 10,000 ML models using a training set enclosing 102 complexes drug targets/drugs, and a “negative” set enclosing 102 non-drug targets | The ML models discriminated drug targets. The approach was validated using an external test set of 277 clinically relevant drug targets (area under the ROC curve of 0.89) | The output reported in this work provided new potential drug targets for developing innovative anticancer drugs |
Abbreviation: BANDIT—Bayesian ANalysis to determine Drug Interaction Targets; D2—dopamine receptor type 2; DL—deep learning; FDA—United States Food and Drug Administration; ML—machine learning; RF—random forest; ROC—receiver operating characteristic; ROR-γt—human retinoic-acid-receptor-related orphan receptor-gamma t.
2.1.3. AI/ML in Quantitative Systems Pharmacology (QSP)
Following the identification of prospective therapeutic drug targets, analysis must be performed to validate them. Computational approaches offer affordable, time-saving strategies to evaluate the likelihood that potential targets could provide an efficient method for treating a given disorder. Accordingly, a pivotal step in target validation is represented by the construction of a confidence interval for a given potential therapeutic hypothesis, employing quantitative systems pharmacology (QSP) models [77]. QSP is a stimulating and effective conjunction of biological pathways, pharmacology, and mathematical models for drug development. QSP possesses potential for providing a considerable impact on modern medicine as a result of the discovery and deployment of new molecular pathways and drug targets in the quest for innovative therapeutic agents and personalized medicine. The combination of these specialties is triggering substantial attention in pharma companies to expand predictions from a pharmacodynamic (PD) and pharmacokinetics (PK) perspective, and through improvements in computing capacity, QSP is currently capable of improving outcomes in the drug discovery trajectory. In fact, QSP models can combine information on PK/PD properties, biological processes of interest, and mechanisms of action, resulting from prior knowledge and available preclinical and clinical data, to quantitatively predict efficacy and safety responses over time and translate molecular data to clinical outcomes [78][79][80][81]. QSP provides a perfect quantitative framework for integrating different big data sources, including omics (i.e., proteomics, transcriptomics, metabolomics, and genomics) and imaging, the dimensionality of which can be reduced using ML methods. By allowing the identification of relevant association and data representations, the development of QSP platforms with higher granularity and enhanced predictive power can be further enhanced [82]. Moreover, the opportunity to implement a QSP platform with ML techniques to enhance the capacity to handle big data can offer great opportunities for systems pharmacology modeling. In fact, with the high availability of processed and organized data for building interpretable and actionable computational models, supporting decision making in the whole process of drug discovery and development, QSP can improve the reliability of predictions, providing more complex analysis, a better understanding of biomedical systems, and ultimately the design of optimized treatments. We report some examples regarding this approach.
2.2. Imaging, Biomarkers, Diagnosis, and Disease Progression
With the growing accessibility to high-quality amounts of cell imaging data, there are currently relevant possibilities to use ML-based methods to aid researchers in cell image processing. In fact, the image features that are supposed to be crucial in producing predictions or diagnoses can be generally processed using ML algorithms. The latter offers the possibility of predictive, descriptive, and prescriptive assessment to acquire relevant information that would otherwise be impossible to obtain by human analysis, providing accurate medical diagnoses [83][84]. Accordingly, in recent years, numerous clinical investigations have enabled the use of AI in several fields, providing general pathological classification, risk evaluation, diagnosis, prognosis, and the prediction of appropriate therapy and possible responses to a given pharmacological treatment [85][86]. In particular, DL, a class of ML that employs ANN (CNN and recurrent neural networks (RNN)) resembling human cognitive capabilities, has proven undeniable superiority over conventional ML approaches owing to algorithm improvement, better processing hardware, and access to massive amounts of imaging data [87]. The successful incorporation of DL technology into normal clinical practice has determined that the diagnosis accuracy is comparable to that of healthcare experts. Furthermore, DL model integration provides additional advantages, including speed, efficiency, affordability, increased accessibility, and ethical behavior [88]. For these reasons, the FDA has approved the use of specific DL-driven diagnostic computational tools for clinical usage (Table 3) [89][90][91]. The application of AI encompasses several medical and biomedical fields, including radiology [92], gastroenterology [93][94], neurology [95][96], ophthalmology [97][98], cardiology [99][100], dermatology [101], general pathology [102], oncology [103], healthcare [104][105], and clinical medicine [105][106].
| Device/Algorithm (Company) |
Type of Algorithm | Description | FDA Approval Number | Medical Field(s) | Date |
|---|---|---|---|---|---|
| Accipio Ix (MaxQ-Al Ltd.), Tel Aviv, Israel |
AI | The tool is used for an automatic, rapid, highly accurate identification and prioritization of suspected intracranial hemorrhage | K182177 | Radiology Neurology |
October 2018 |
| Advanced Intelligent Clear-IQ Engine (AiCE) (Canon Medical Systems Corporation, Ōtawara, Japan) |
Deep CNN | AiCE system is used for reducing noise-boosting signals to quickly deliver sharp, clear, and distinct images | K183046 | Radiology | June 2019 |
| AI-Rad Companion (Cardiovascular) (Siemens Medical Solutions USA, Inc., Malvern, PA, USA) | DL | The software is used for detecting cardiovascular risks from CT images | K183268 | Radiology | October 2019 |
| AI-Rad Companion (Pulmonary) (Siemens Medical Solutions USA, Inc., Malvern, PA, USA) |
DL | The software is used for detecting lung nodules from CT images | K183271 | Radiology | July 2019 |
| AI Segmentation (Varian Medical Systems, Inc., Crawley, UK) |
AI | The software is used for providing fast, accurate, and intelligent contouring for improving the reproducibility of structure delineation in radiation oncology | K203469 | Radiology Oncology |
April 2021 |
| AmCAD-UO (AmCad BioMed Corporation, Taipei City, Taiwan) |
AI | The tool is used for detecting OSA in awake patients; it can precisely scan upper airway and analyze the gap between normal breathing and Müller Maneuver models | K180867 | Radiology | December 2018 |
| AmCAD-US (AmCad BioMed Corporation, Taipei City, Taiwan) |
AI | The tool is used to view and quantify ultrasound image data of backscattered signals acquired from ultrasound data | K162574 | Radiology | May 2017 |
| AmCAD-UT Detection 2.2 (AmCad BioMed Corporation, Taipei City, Taiwan) |
AI | The software is used for facilitating the detection, visualization, and characterization of thyroid nodule features on sonographic images | K180006 | Radiology | August 2018 |
| AmCAD-UV (AmCad BioMed Corporation, Taipei City, Taiwan) |
AI | The tool is used for classifying the ultrasonic color intensity data from signals of flow Doppler ultrasound images | K170069 | Radiology | April 2017 |
| Arterys Cardio DL (Arterys Inc., San Francisco, CA, USA) |
DL | The software is used for the analysis of cardiac MRI images | K163253 | Radiology Cardiology |
January 2017 |
| Arterys Oncology DL (Arterys Inc., San Francisco, CA, USA) |
DL | The software is used for measuring and tracking lesions and nodules from MRI and CT images | K173542 | Radiology Oncology |
January 2018 |
| Arterys MICA (Arterys Inc., San Francisco, CA, USA) |
AI | AI platform used for liver and lung cancer diagnosis from MRI and CT images | K182034 | Radiology Oncology |
October 2018 |
| BladderScan Prime PLUS System (Verathon Inc., Bothell, WA, USA) |
DL | The tool provides improved bladder volume measurement accuracy | K172356 | Radiology | Sepember 2017 |
| Bone VCAR (BVCAR) (GE Medical Systems SCS, Buc, France) |
DL | The tool is used for automated spine labeling (segments or whole spine) from CT images | K183204 | Radiology | April 2019 |
| Brainomix 360° e-CTA (Brainomix Limited, Oxford, UK) |
AI | The tool is used for automatically detecting LVO on CT angiography | K192692 | Radiology | May 2020 |
| BriefCase (Aidoc Medical, Ltd., Tel Aviv, Israel) |
DL | The tool is used for detecting acute abnormalities across the body, helping radiologists to prioritize life-threatening cases, expediting patient care | K180647 | Radiology Emergency Medicine |
August 2018 |
| cvi42 for cardiac CT/MRI (Circle Cardiovascular Imaging Inc., Calgary, AB, Canada) |
ML/DL | The software is used for assessing heart function, flow, and tissue attributes from CT/MRI images | K141480 | Radiology Cardiology |
August 2014 |
| ClariCT.AI (ClariPI Inc., Seoul, South-Korea) |
DL | The tool is used for processing and enhancing CT images reducing noise | K183460 | Radiology | Jun2019 |
| ClearRead CT (Riverain Technologies, LLC, Miamisburg, OH, USA) |
DL | The software is used to detect pulmonary nodules and abnormalities in CT | K161201 | Radiology Oncology |
September 2016 |
| cmTriage (CureMetrix, Inc., La Jolla, CA, USA) |
AI | cmTriage is a tool enabling radiologists to triage, sort, and prioritize mammography | K183285 | Radiology Oncology |
March 2019 |
| ContaCT (Viz.AI, San Francisco, CA, USA) |
AI | The software is used for detecting stroke from CT angiogram images of the brain | DEN170073 | Radiology Neurology |
February 2018 |
| Critical Care Suite (GE Medical Systems, LLC, Waukesha, WI, USA) |
AI | The platform is used for automatically detecting PNX from X-rays, triaging critical cases | K183182 | Radiology Emergency Medicine | August 2019 |
| CuraRad-ICH (CuraCloud Corp., Seattle, WA, USA) |
DL | The tool is used for triaging suspected intracranial hemorrhage | K192167 | Radiology | April 2020 |
| Deep Learning Image Reconstruction (GE Medical Systems, LLC, Waukesha, WI, USA) |
DL | The application is used for CT image reconstruction Follow-up—K201745 DL Image Reconstruction for Gemstone Spectral Imaging (December 2020) |
K183202 | Radiology | April 2019 |
| DV.Target (Deepvoxel Inc., Irvine, CA, USA) |
DL | The algorithm is used to automatically delineate OARs. Contours generated by DV.Target may be used as an input to clinical workflows in radiation therapy. | K202928 | Radiology | April 2021 |
| EchoMD Automated Ejection Fraction Software (Bay Labs, Inc., San Francisco, CA, USA) |
ML | This software is used for automated ECG analysis | K173780 | Radiology Cardiology |
June 2018 |
| FerriSmart Analysis System (Resonance Health Analysis Service Pty Ltd., Burswood, Australia) |
ML/CNN | The software is used for measuring liver iron concentration from R2-MRI images. The system is based on the previously approved (K043271, Jan2005) R2-MRI Analysis System | K182218 | Radiology Internal Medicine |
November 2018 |
| HealthCXR (Zebra Medical Vision Ltd., HaMerkaz, Israel) |
AI | The software is used for identifying and triaging pleural effusion in chest X-rays | K192320 | Radiology Emergency Medicine |
November 2019 |
| HealthMammo (Zebra Medical Vision Ltd., HaMerkaz, Israel) |
DL | The tool is used for supporting identifying and prioritizing suspicious mammograms | K200905 | Radiology Oncology |
June 2020 |
| HealthPNX (Zebra Medical Vision Ltd., HaMerkaz, Israel) |
AI | The tool increases the radiologist’s confidence in making PNX diagnosis from chest X-rays imaging output | K190362 | Radiology Emergency Medicine |
May 2019 |
| icobrain (icometrix NV, Leuven, Belgium) |
ML and DL | The software is used for interpreting MRI images from the brain for detecting neurological disorders | K181939 | Radiology Neurology |
November 2018 |
| Illumeo System (Philips Medical Systems Technologies, Ltd., Haifa, Israel) |
AI | The tool is used for acquiring, storing, distributing, processing, and displaying images | K173588 | Radiology | January 2018 |
| lnferRead Lung CT (Beijing Infervision Technology Co. Ltd., Beijing, China) |
AI | The tool is used for assisting radiologists fin detecting pulmonary nodules from CT (NCT04119960) |
K192880 | Radiology Oncology |
June 2020 |
| Infinitt PACS 7.0 (Infinitt Healthcare Co. Ltd., Seoul, South-Korea) |
AI | The software is used to analyze incoming tasks, identifying high-priority cases | K172803 | Radiology | Sepember 2017 |
| KOALA (IB Lab GmbH, Wien, Austria) |
DL | The algorithm is used to detect radiographic signs of knee osteoarthritis | K192109 | Radiology | November 2019 |
| Koios DS for Breast (Koios Medical, Inc., Chicago, IL, USA) |
AI | The software is used for analyzing ultrasound images for providing improved accuracy and efficiency in cancer diagnosis | K190442 | Radiology Oncology |
July 2019 |
| LiverMultiScan (Perspectum Diagnostics Ltd., Oxford, UK) |
ML | This platform is used to assess liver tissue to enable diagnostic and patient management decisions. | K190017 | Radiology | June 2019 |
| LVivo Software Application (DiA Imaging Analysis Ltd., Beer-Sheva, Israel) |
AI | The software provides an automated AI-based ejection fraction analysis, allowing a fast assessment of cardiac functions | K210053 | Radiology | January 2021 |
| LungQ (Thirona Corp., Nijmegen, Netherlands) |
AI | The software is used for automatically identifying lung abnormalities from CT images | K173821 | Radiology | June 2018 |
| MRCP+ V1.0 (Perspectum Diagnostics Ltd., Oxford, UK) |
AI | The software is used for quantitatively analyzing the biliary tree and pancreatic duct from MRCP images | K183133 | Radiology | January 2019 |
| MRCAT brain (Philips Medical Systems MR, Vantaa, Finland) |
AI | The tool is used for radiotherapy planning of primary and metastatic tumors using MRI | K193109 | Radiology | January 2020 |
| OsteoDetect (Imagen Technologies, Inc., New York, NY, USA) |
DL | The software is used for detecting signs of distal radius fracture from X-ray | DEN180005 | Radiology Emergency Medicine |
May 2018 |
| PixelShine (ALGOMEDICA, Palo Alto, CA, USA) |
DL | The software is used for improving the quality of scans obtained from any CT images, reducing noise | K161625 | Radiology | September 2016 |
| PowerLook Density Assessment Software (iCAD, Inc., Nashua, NH, USA) |
ML | The algorithm is used for assessing breast density in 2D and 3D mammography | K180125 | Radiology | April 2018 |
| ProFound™ AI Software (iCAD, Inc., Nashua, NH, USA) |
DL | The software is used for detecting both malignant soft tissue densities and calcifications from DBT images | K191994 | Radiology Oncology |
April 2019 |
| QuantX (Qlarity Imaging, Chicago, IL, USA) |
AI | The software is used for assessing and characterizing breast abnormalities from MRIdata | DEN170022 | Radiology Oncology |
July 2017 |
| qp-Prostate (Quibim S.L., Valencia, Spain) |
AI | The tool is used for analyzing prostate MRI images | K203582 | Radiology Oncology |
December 2020 |
| Rapid ASPECTS (iSchemaView, Inc., San Mateo, CA, USA) |
AI | The tool is used as assisted diagnostic software for lesions suspicious of cancer | K200760 | Radiology | May 2020 |
| RAPID-ICH (iSchemaView, Inc., San Mateo, CA, USA) |
AI | The tool is used to triage non-contrast CT (NCCT) cases for rapidly detecting suspicious intracranial hemorrhage | K193087 | Radiology | March 2020 |
| RayCare 3.1 (RaySearch Laboratories AB, Stockholm, Sweden) |
ML/DL | The software is used for improving workflow efficiency across different treatments in medical, radiation, and surgical oncology to support decisions in the clinic | K200487 | Radiology Oncology |
June 2020 |
| RayStation 10.1 (RaySearch Laboratories AB, Stockholm, Sweden) |
ML | The platform is used to automatically generate treatment plans | K210645 | Radiology Oncology |
June 2021 |
| RBknee (Radiobotics ApS, Copenaghen, Denmark) |
ML | The software is used for automatically identifying osteoarthritis in the knees based on X-ray images | K203696 | Radiology | August 2021 |
| Red DotTM (Behold.AI Technologies Ltd., London, UK) |
AI | The software is used for assessing PNX from chest X-ray images | K191556 | Radiology | January 2020 |
| StoneChecker (Imaging Biometrics, LLC, Elm Grove, WI, USA) |
AI | The software is used with standard CT scans in people with kidney stones for measuring stone parameters and to inform clinical decisions | K191530 | Radiology | June 2019 |
| StrokeViewer (NiCo-Lab B.V., Amsterdam, Netherlands) |
AI | This tool is used for the localization and quantification of stroke biomarkers from CT scans | K200873 | Radiology | October 2020 |
| SubtleMR (Subtle Medical, Inc., Menlo Park, CA, USA) |
CNN | The application is used for improving the quality of MRI images increasing resolution and reducing noise | K191688 | Radiology | September 2019 |
| SubtlePET (Subtle Medical, Inc., Menlo Park, CA, USA) |
DNN | The application is used for processing PET images | K182336 | Radiology | November 2018 |
| syngo.CT Cardiac Planning (Siemens Medical Solutions USA, Inc., Malvern, PA, USA) |
AI | The software is used forenhancing CT images; analysis of morphology and pathology of vascular and cardiac structures | K200515 | Radiology | March 2020 |
| TransparaTM (Screenpoint Medical B.V., Nijmegen, Netherlands) |
ML | The software provides a support solution for mammography, identifying suspicious areas in 2D and 3D mammograms | K192287 | Radiology Oncology |
December 2019 |
| Veolity (MeVis Medical Solutions AG, Bremen, Germany) |
ML | The software is used to recognize even the subtlest potential signs of lung cancer | K201501 | Radiology | February 2021 |
| Workflow Box including DCLExpertTM (Mirada Medical Ltd., Oxford, UK) |
AI | The software is used for autocontouring organs for cancer treatment planning | K181572 | Radiology | July 2018 |
| AI-ECG Platform (Shenzhen Carewell Electronics, Ltd., Shenzhen, China) |
AI | AI platform for assisting physicians in measuring and interpreting ECG | K180432 | Cardiology | November 2018 |
| AI-ECG Tracker (Shenzhen Carewell Electronics, Ltd., Shenzhen, China) |
AI | The tool is used for improving the detection efficiency of non-persistent arrhythmias (irregular heartbeats) | K200036 | Cardiology | March 2020 |
| BioFlux Device (Biotricity Inc., Redwood City, CA, USA) |
AI | The tool is used for detecting arrhythmias | K172311 | Cardiology | December 2017 |
| EchoGo Core (Ultromics Ltd., Oxford, UK) |
ML | The application is used to automatically evaluate cardiac functions from echocardiography, enabling physicians to diagnose heart failure and coronary artery disease | K191171 | Cardiology | November 2019 |
| Eko Analysis Software (Eko Devices Inc., Oakland, CA, USA) |
ANN | The software is used for detecting suspected murmurs in the heart sounds and atrial fibrillation from ECG data | K192004 | Cardiology | January 2020 |
| eMurmur ID (CSD Labs GmbH, Graz, Austria) |
ML | The software is used to understand, identify, and detect heart murmurs | K181988 | Cardiology | April 2019 |
| KardiaAI (AliveCor, Inc., Mountain View, CA, USA) |
AI | The tool is used for enhancing cardiac MRI to improve diagnosis of heart disorders | K181823 | Cardiology | November 2019 |
| KOSMOS (EchoNous Inc., Redmond, WA, USA) |
DL | This tool combining ultrasound with DL is used for clinical assessment of the heart, lungs, and abdomen | K193518 | Cardiology | March 2020 |
| Ventripoint Medical System Plus (VMS+) 3.0 (Ventripoint Diagnostics Ltd., Toronto, ON, Canada) |
AI | The tool is used for measuring whole heart function using conventional ultrasound (NCT01557582) |
K191493 | Cardiology | October 2019 |
| Altoida (Altoida, Inc., Washington, DC, USA) |
ML | The software is used for detecting AD up to 10 years prior to the onset. ML is used for classifying patients’ risk of MCI due to AD (NCT02843529) | FDA-ClassII | Neurology | August 2021 |
| BrainScope Ahead 100 (Brainscope Company, Inc., Bethesda, MD, USA) |
AI | The software is used for interpreting the structural condition of the patient’s brain after head injury from EEG data | DEN140025 | Neurology | November 2014 |
| Cognoa ASD Diagnosis Aid (Cognoa, Inc., Palo Alto, CA, USA) |
ML | The software is used for evaluating patients at risk of ASD | DEN200069 | Neurology | June 2021 |
| complete control system gen2 (Coapt, LLC, Chicago, IL, USA) |
AI/ML | The platform provides a human–bionic interface that learns and adapts to users, giving them unrivaled control of their prosthetic arms | K191083 | Neurology | April 2019 |
| EnsoSleep (EnsoData, Inc., Madison, WI, USA) |
AI | The application assists clinicians in the diagnosis of sleep disorders | K162627 | Neurology | March 2017 |
| QbTest/QbCheck (QbTech AB, Goteborg, Sweden) |
AI/ML | The tools are used for braingazing using eye-tracking technology to capture eye vergence and AI algorithms for classifying ADHD patients vs. non-ADHD | K040894 K143468 | Neurology Psychiatry |
June 2004 March 2016 |
| Clarus 700 (Carl Zeiss Meditec Inc., Dublin, CA, USA) |
DL | The algorithm is applied to diagnosing and monitoring retina disorders | K191194 | Ophthalmology | May 2019 |
| EyeArt (EyeNuk, Inc., Woodland Hills, CA, USA) |
AI | The software is used as a screening tool for detecting diabetic retinopathy | K200667 | Ophthalmology | March 2020 |
| IDx (Digital Diagnostics Inc. -IDx LLC., Coralville, IA, USA) |
AI | The software is used for detecting diabetic retinopathy | DEN180001 | Ophthalmology | January 2018 |
| DreaMed Advisor Pro (DreaMed Diabetes, Ltd., Petah Tikva, Israel) |
AI | The application is used for automatically determining the optimal therapy to maintain balanced glucose levels | DEN170043 | Endocrinology | June 2018 |
| Guardian Connect System (Medtronic Minimed, Northridge, CA, USA) |
AI | The application is used with diabetic patients for monitoring blood glucose content, predicting changes | P160007 | Endocrinology | March 2018 |
| APAS Independence (Clever Culture Systems AG, Bäch, Switzerland) |
AI/ML | The tool is used to automate culture plate imaging, analysis, and interpretation | K183648 | Microbiology | Sepember 2019 |
| NightOwl (Ectosense nv, Leuven, Belgium) |
AI | The algorithm is used for analyzing biophysical parameters for evaluating sleep-related breathing disorders of patients suspected of sleep apnea (NCT03774199; NCT04194073) | K191031 | Anesthesiology | March 2020 |
| NuVasive Pulse System (NuVasive, Inc., San Diego, CA. USA) |
AI | The tool is used during spinal surgery, neck dissection, and thoracic surgeries, improving surgical procedures | K180038 | Surgery | June 2018 |
| Sight OLO (Sight Diagnostics Ltd., Tel Aviv, Israel) |
AI | The algorithm is used for inspecting blood samples (NCT03595501) |
K190898 | Hematology | November 2019 |
| SOZO (ImpediMed Ltd., Carlsbad, CA, USA) |
AI | The tool is use for the clinical assessment of unilateral lymphedema, combining BIS with AI to create a rapid, non-invasive scan of a person’s body | K190529 | Gastroenterology Urology |
November 2019 |
| wheezo WheezeRate Detector (Respiri Ltd., Melbourne, Australia) |
ML | The tool is used for asthma management and remote monitoring | K202062 | Pneumology | March 2021 |
Abbreviation: AD—Alzheimer’s disease; ADHD—attention deficit hyperactivity disorder; AI—artificial intelligence; ANN—artificial neural network; ASD—autism spectrum disorder; BIS—bioimpedance spectroscopy; DL—deep learning; CNN—convolutional neural network; CT—computed tomography; DBT—digital breast tomosynthesis; EEG—electroencephalogram; ECG—electrocardiogram; LVO—large vessel occlusion; MCI—mild cognitive impairment: ML—machine learning; MRCP—magnetic resonance cholangiopancreatography; MRI—magnetic resonance imaging; OARs—organs-at-risk; OSA—obstructive sleep apnea; PET—positron emission tomography; PNX—pneumothorax.
References
- Available online: https://www.brookings.edu/techstream/what-investment-trends-reveal-about-the-global-ai-landscape/ (accessed on 14 October 2021).
- Available online: https://outsideinsight.com/insights/global-ai-investment-150-billion-2025/ (accessed on 14 October 2021).
- Hinton, G. Deep Learning-A Technology With the Potential to Transform Health Care. JAMA 2018, 320, 1101–1102.
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29.
- He, J.; Baxter, S.L.; Xu, J.; Xu, J.; Zhou, X.; Zhang, K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019, 25, 30–36.
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358.
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317–1318.
- Muhammad, L.J.; Algehyne, E.A.; Usman, S.S.; Ahmad, A.; Chakraborty, C.; Mohammed, I.A. Supervised Machine Learning Models for Prediction of COVID-19 Infection using Epidemiology Dataset. SN Comput. Sci. 2021, 2, 11.
- Mirri, S.; Delnevo, G.; Roccetti, M. Is a COVID-19 Second Wave Possible in Emilia-Romagna (Italy)? Forecasting a Future Outbreak with Particulate Pollution and Machine Learning. Computation 2020, 8, 74.
- Patel, L.; Shukla, T.; Huang, X.; Ussery, D.W.; Wang, S. Machine Learning Methods in Drug Discovery. Molecules 2020, 25, 5277.
- Reda, C.; Kaufmann, E.; Delahaye-Duriez, A. Machine learning applications in drug development. Comput. Struct. Biotechnol. J. 2020, 18, 241–252.
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360.
- Ramesh, N.; Tasdizen, T. Detection and segmentation in microscopy images. In Computer Vision for Microscopy Image Analysis; Academic Press Books; Elsevier: New York, NY, USA, 2021; pp. 43–71.
- Alloghani, M.; Al-Jumeily, D.; Mustafina, J.; Hussain, A.; Aljaaf, A.J. A Systematic Review on Supervised and Unsupervised Machine Learning Algorithms for Data Science. In Supervised and Unsupervised Learning for Data Science; Springer Nature: London, UK, 2020; pp. 3–21.
- Cleret de Langavant, L.; Bayen, E.; Yaffe, K. Unsupervised Machine Learning to Identify High Likelihood of Dementia in Population-Based Surveys: Development and Validation Study. J. Med. Internet Res. 2018, 20, e10493.
- Lu, C.; Ghoman, S.K.; Cutumisu, M.; Schmolzer, G.M. Unsupervised Machine Learning Algorithms Examine Healthcare Providers’ Perceptions and Longitudinal Performance in a Digital Neonatal Resuscitation Simulator. Front. Pediatr. 2020, 8, 544.
- Roohi, A.; Faust, K.; Djuric, U.; Diamandis, P. Unsupervised Machine Learning in Pathology: The Next Frontier. Surg. Pathol. Clin. 2020, 13, 349–358.
- Lopez, C.; Tucker, S.; Salameh, T.; Tucker, C. An unsupervised machine learning method for discovering patient clusters based on genetic signatures. J. Biomed. Inf. 2018, 85, 30–39.
- Ceriotti, M. Unsupervised machine learning in atomistic simulations, between predictions and understanding. J. Chem. Phys. 2019, 150, 150901.
- Ma, E.Y.; Kim, J.W.; Lee, Y.; Cho, S.W.; Kim, H.; Kim, J.K. Combined unsupervised-supervised machine learning for phenotyping complex diseases with its application to obstructive sleep apnea. Sci. Rep. 2021, 11, 4457.
- Omta, W.A.; van Heesbeen, R.G.; Shen, I.; de Nobel, J.; Robers, D.; van der Velden, L.M.; Medema, R.H.; Siebes, A.; Feelders, A.J.; Brinkkemper, S.; et al. Combining Supervised and Unsupervised Machine Learning Methods for Phenotypic Functional Genomics Screening. SLAS Discov. 2020, 25, 655–664.
- Neftci, E.O.; Averbeck, B.B. Reinforcement learning in artificial and biological systems. Nat. Mach. Intell. 2019, 1, 133–143.
- Yauney, G.; Shah, P. Reinforcement Learning with Action-Derived Rewards for Chemotherapy and Clinical Trial Dosing Regimen Selection. In Proceedings of the 3rd Machine Learning for Healthcare Conference, Palo Alto, CA, USA, 17–18 August 2018; pp. 161–226.
- Sirous, H.; Campiani, G.; Brogi, S.; Calderone, V.; Chemi, G. Computer-Driven Development of an in Silico Tool for Finding Selective Histone Deacetylase 1 Inhibitors. Molecules 2020, 25, 1952.
- Brogi, S.; Brindisi, M.; Joshi, B.P.; Sanna Coccone, S.; Parapini, S.; Basilico, N.; Novellino, E.; Campiani, G.; Gemma, S.; Butini, S. Exploring clotrimazole-based pharmacophore: 3D-QSAR studies and synthesis of novel antiplasmodial agents. Bioorg Med. Chem. Lett. 2015, 25, 5412–5418.
- Brogi, S.; Papazafiri, P.; Roussis, V.; Tafi, A. 3D-QSAR using pharmacophore-based alignment and virtual screening for discovery of novel MCF-7 cell line inhibitors. Eur. J. Med. Chem. 2013, 67, 344–351.
- Brogi, S.; Kladi, M.; Vagias, C.; Papazafiri, P.; Roussis, V.; Tafi, A. Pharmacophore modeling for qualitative prediction of antiestrogenic activity. J. Chem. Inf. Model. 2009, 49, 2489–2497.
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564.
- Lin, X.; Li, X.; Lin, X. A Review on Applications of Computational Methods in Drug Screening and Design. Molecules 2020, 25, 1375.
- Kohlbacher, S.M.; Langer, T.; Seidel, T. QPHAR: Quantitative pharmacophore activity relationship: Method and validation. J. Cheminform. 2021, 13, 57.
- Flori, L.; Petrarolo, G.; Brogi, S.; La Motta, C.; Testai, L.; Calderone, V. Identification of novel SIRT1 activators endowed with cardioprotective profile. Eur. J. Pharm. Sci. 2021, 165, 105930.
- Sirous, H.; Chemi, G.; Campiani, G.; Brogi, S. An integrated in silico screening strategy for identifying promising disruptors of p53-MDM2 interaction. Comput. Biol. Chem. 2019, 83, 107105.
- Battah, B.; Chemi, G.; Butini, S.; Campiani, G.; Brogi, S.; Delogu, G.; Gemma, S. A Repurposing Approach for Uncovering the Anti-Tubercular Activity of FDA-Approved Drugs with Potential Multi-Targeting Profiles. Molecules 2019, 24, 4373.
- Sirous, H.; Chemi, G.; Gemma, S.; Butini, S.; Debyser, Z.; Christ, F.; Saghaie, L.; Brogi, S.; Fassihi, A.; Campiani, G.; et al. Identification of Novel 3-Hydroxy-pyran-4-One Derivatives as Potent HIV-1 Integrase Inhibitors Using in silico Structure-Based Combinatorial Library Design Approach. Front. Chem. 2019, 7, 574.
- Batool, M.; Ahmad, B.; Choi, S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019, 20, 2783.
- Maia, E.H.B.; Assis, L.C.; de Oliveira, T.A.; da Silva, A.M.; Taranto, A.G. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front. Chem. 2020, 8, 343.
- Fischer, A.; Smiesko, M.; Sellner, M.; Lill, M.A. Decision Making in Structure-Based Drug Discovery: Visual Inspection of Docking Results. J. Med. Chem. 2021, 64, 2489–2500.
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061.
- Decherchi, S.; Grisoni, F.; Tiwary, P.; Cavalli, A. Editorial: Molecular Dynamics and Machine Learning in Drug Discovery. Front. Mol. Biosci. 2021, 8, 673773.
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico Methods for Drug Design and Discovery. Front. Chem. 2020, 8, 612.
- Zaccagnini, L.; Brogi, S.; Brindisi, M.; Gemma, S.; Chemi, G.; Legname, G.; Campiani, G.; Butini, S. Identification of novel fluorescent probes preventing PrP(Sc) replication in prion diseases. Eur. J. Med. Chem. 2017, 127, 859–873.
- Brogi, S. Computational Approaches for Drug Discovery. Molecules 2019, 24, 3061.
- Sirous, H.; Campiani, G.; Calderone, V.; Brogi, S. Discovery of novel hit compounds as potential HDAC1 inhibitors: The case of ligand and structure-based virtual screening. Comput. Biol. Med. 2021, 137, 104808.
- Vazquez, J.; Lopez, M.; Gibert, E.; Herrero, E.; Luque, F.J. Merging Ligand-Based and Structure-Based Methods in Drug Discovery: An Overview of Combined Virtual Screening Approaches. Molecules 2020, 25, 4723.
- Ferreira, L.L.G.; Andricopulo, A.D. Editorial: Chemoinformatics Approaches to Structure- and Ligand-Based Drug Design. Front. Pharm. 2018, 9, 1416.
- Ivanov, J.; Polshakov, D.; Kato-Weinstein, J.; Zhou, Q.; Li, Y.; Granet, R.; Garner, L.; Deng, Y.; Liu, C.; Albaiu, D.; et al. Quantitative Structure-Activity Relationship Machine Learning Models and their Applications for Identifying Viral 3CLpro- and RdRp-Targeting Compounds as Potential Therapeutics for COVID-19 and Related Viral Infections. ACS Omega 2020, 5, 27344–27358.
- Ancuceanu, R.; Dinu, M.; Neaga, I.; Laszlo, F.G.; Boda, D. Development of QSAR machine learning-based models to forecast the effect of substances on malignant melanoma cells. Oncol. Lett. 2019, 17, 4188–4196.
- Keyvanpour, M.R.; Shirzad, M.B. An Analysis of QSAR Research Based on Machine Learning Concepts. Curr. Drug Discov. Technol. 2021, 18, 17–30.
- Simoes, R.S.; Maltarollo, V.G.; Oliveira, P.R.; Honorio, K.M. Transfer and Multi-task Learning in QSAR Modeling: Advances and Challenges. Front. Pharm. 2018, 9, 74.
- Lill, M.A. Multi-dimensional QSAR in drug discovery. Drug Discov. Today 2007, 12, 1013–1017.
- Sadik, K.; Byadi, S.; Hachim, M.E.; Hamdani, N.E.; Podlipnik, Č.; Aboulmouhajir, A. Multi-QSAR approaches for investigating the relationship between chemical structure descriptors of Thiadiazole derivatives and their corrosion inhibition performance. J. Mol. Struct. 2021, 1240, 130571.
- Speck-Planche, A.; Cordeiro, M.N. Multi-Target QSAR Approaches for Modeling Protein Inhibitors. Simultaneous Prediction of Activities Against Biomacromolecules Present in Gram-Negative Bacteria. Curr. Top. Med. Chem. 2015, 15, 1801–1813.
- Solimeo, R.; Zhang, J.; Kim, M.; Sedykh, A.; Zhu, H. Predicting chemical ocular toxicity using a combinatorial QSAR approach. Chem. Res. Toxicol. 2012, 25, 2763–2769.
- Zhu, H.; Tropsha, A.; Fourches, D.; Varnek, A.; Papa, E.; Gramatica, P.; Oberg, T.; Dao, P.; Cherkasov, A.; Tetko, I.V. Combinatorial QSAR modeling of chemical toxicants tested against Tetrahymena pyriformis. J. Chem. Inf. Model. 2008, 48, 766–784.
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharm. 2018, 9, 1275.
- Melo-Filho, C.C.; Dantas, R.F.; Braga, R.C.; Neves, B.J.; Senger, M.R.; Valente, W.C.; Rezende-Neto, J.M.; Chaves, W.T.; Muratov, E.N.; Paveley, R.A.; et al. QSAR-Driven Discovery of Novel Chemical Scaffolds Active against Schistosoma mansoni. J. Chem. Inf. Model. 2016, 56, 1357–1372.
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477.
- Dara, S.; Dhamercherla, S.; Jadav, S.S.; Babu, C.M.; Ahsan, M.J. Machine Learning in Drug Discovery: A Review. Artif. Intell. Rev. 2021, 2021, 1–53.
- Vignaux, P.A.; Minerali, E.; Foil, D.H.; Puhl, A.C.; Ekins, S. Machine Learning for Discovery of GSK3beta Inhibitors. ACS Omega 2020, 5, 26551–26561.
- Huang, D.Z.; Kouznetsova, V.L.; Tsigelny, I.F. Deep-learning- and pharmacophore-based prediction of RAGE inhibitors. Phys. Biol. 2020, 17, 036003.
- Shi, C.; Dong, F.; Zhao, G.; Zhu, N.; Lao, X.; Zheng, H. Applications of machine-learning methods for the discovery of NDM-1 inhibitors. Chem. Biol. Drug Des. 2020, 96, 1232–1243.
- Tinivella, A.; Pinzi, L.; Rastelli, G. Prediction of activity and selectivity profiles of human Carbonic Anhydrase inhibitors using machine learning classification models. J. Cheminform. 2021, 13, 18.
- Ballester, P.J. Machine Learning for Molecular Modelling in Drug Design. Biomolecules 2019, 9, 216.
- Medina-Franco, J.L. Grand Challenges of Computer-Aided Drug Design: The Road Ahead. Front. Drug Discov. 2021, 1, 2.
- Rodriguez, S.; Hug, C.; Todorov, P.; Moret, N.; Boswell, S.A.; Evans, K.; Zhou, G.; Johnson, N.T.; Hyman, B.T.; Sorger, P.K.; et al. Machine learning identifies candidates for drug repurposing in Alzheimer’s disease. Nat. Commun. 2021, 12, 1033.
- Tanoli, Z.; Vaha-Koskela, M.; Aittokallio, T. Artificial intelligence, machine learning, and drug repurposing in cancer. Expert Opin. Drug Discov. 2021, 16, 977–989.
- Zhou, Y.; Wang, F.; Tang, J.; Nussinov, R.; Cheng, F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health 2020, 2, e667–e676.
- Xu, Y.; Verma, D.; Sheridan, R.P.; Liaw, A.; Ma, J.; Marshall, N.M.; McIntosh, J.; Sherer, E.C.; Svetnik, V.; Johnston, J.M. Deep Dive into Machine Learning Models for Protein Engineering. J. Chem. Inf. Model. 2020, 60, 2773–2790.
- Gao, W.; Mahajan, S.P.; Sulam, J.; Gray, J.J. Deep Learning in Protein Structural Modeling and Design. Patterns 2020, 1, 100142.
- Hameduh, T.; Haddad, Y.; Adam, V.; Heger, Z. Homology modeling in the time of collective and artificial intelligence. Comput. Struct. Biotechnol. J. 2020, 18, 3494–3506.
- Torrisi, M.; Pollastri, G.; Le, Q. Deep learning methods in protein structure prediction. Comput. Struct. Biotechnol. J. 2020, 18, 1301–1310.
- Lavecchia, A. Machine-learning approaches in drug discovery: Methods and applications. Drug Discov. Today 2015, 20, 318–331.
- Gawehn, E.; Hiss, J.A.; Schneider, G. Deep Learning in Drug Discovery. Mol. Inf. 2016, 35, 3–14.
- Kimber, T.B.; Chen, Y.; Volkamer, A. Deep Learning in Virtual Screening: Recent Applications and Developments. Int. J. Mol. Sci. 2021, 22, 4435.
- Melville, J.L.; Burke, E.K.; Hirst, J.D. Machine learning in virtual screening. Comb. Chem. High Throughput Screen. 2009, 12, 332–343.
- Terranova, N.; Venkatakrishnan, K.; Benincosa, L.J. Application of Machine Learning in Translational Medicine: Current Status and Future Opportunities. AAPS J. 2021, 23, 74.
- van der Graaf, P.H.; Benson, N. Systems pharmacology: Bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development. Pharm. Res. 2011, 28, 1460–1464.
- Stern, A.M.; Schurdak, M.E.; Bahar, I.; Berg, J.M.; Taylor, D.L. A Perspective on Implementing a Quantitative Systems Pharmacology Platform for Drug Discovery and the Advancement of Personalized Medicine. J. Biomol. Screen. 2016, 21, 521–534.
- Agoram, B.M.; Martin, S.W.; van der Graaf, P.H. The role of mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modelling in translational research of biologics. Drug Discov. Today 2007, 12, 1018–1024.
- Bradshaw, E.L.; Spilker, M.E.; Zang, R.; Bansal, L.; He, H.; Jones, R.D.O.; Le, K.; Penney, M.; Schuck, E.; Topp, B.; et al. Applications of Quantitative Systems Pharmacology in Model-Informed Drug Discovery: Perspective on Impact and Opportunities. CPT Pharmacomet. Syst. Pharm. 2019, 8, 777–791.
- Lazarou, G.; Chelliah, V.; Small, B.G.; Walker, M.; van der Graaf, P.H.; Kierzek, A.M. Integration of Omics Data Sources to Inform Mechanistic Modeling of Immune-Oncology Therapies: A Tutorial for Clinical Pharmacologists. Clin. Pharm. 2020, 107, 858–870.
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219.
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.W.; Karthikesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. NPJ Digit. Med. 2021, 4, 65.
- Briganti, G.; Le Moine, O. Artificial Intelligence in Medicine: Today and Tomorrow. Front. Med. 2020, 7, 27.
- Nichols, J.A.; Herbert Chan, H.W.; Baker, M.A.B. Machine learning: Applications of artificial intelligence to imaging and diagnosis. Biophys. Rev. 2019, 11, 111–118.
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.; van Ginneken, B.; Sanchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88.
- Benjamens, S.; Dhunnoo, P.; Mesko, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: An online database. NPJ Digit. Med. 2020, 3, 118.
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56.
- Muehlematter, U.J.; Daniore, P.; Vokinger, K.N. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–2020): A comparative analysis. Lancet Digit. Health 2021, 3, e195–e203.
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep Learning: A Primer for Radiologists. Radiographics 2017, 37, 2113–2131.
- Adadi, A.; Adadi, S.; Berrada, M. Gastroenterology Meets Machine Learning: Status Quo and Quo Vadis. Adv. Bioinform. 2019, 2019, 1870975.
- van der Sommen, F.; de Groof, J.; Struyvenberg, M.; van der Putten, J.; Boers, T.; Fockens, K.; Schoon, E.J.; Curvers, W.; de With, P.; Mori, Y.; et al. Machine learning in GI endoscopy: Practical guidance in how to interpret a novel field. Gut 2020, 69, 2035–2045.
- Auger, S.D.; Jacobs, B.M.; Dobson, R.; Marshall, C.R.; Noyce, A.J. Big data, machine learning and artificial intelligence: A neurologist’s guide. Pract. Neurol. 2020, 21, 4–11.
- Zhu, G.; Jiang, B.; Tong, L.; Xie, Y.; Zaharchuk, G.; Wintermark, M. Applications of Deep Learning to Neuro-Imaging Techniques. Front. Neurol. 2019, 10, 869.
- Tong, Y.; Lu, W.; Yu, Y.; Shen, Y. Application of machine learning in ophthalmic imaging modalities. Eye Vis. 2020, 7, 22.
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175.
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 300–313.
- Cuocolo, R.; Perillo, T.; De Rosa, E.; Ugga, L.; Petretta, M. Current applications of big data and machine learning in cardiology. J. Geriatr. Cardiol. 2019, 16, 601–607.
- Chan, S.; Reddy, V.; Myers, B.; Thibodeaux, Q.; Brownstone, N.; Liao, W. Machine Learning in Dermatology: Current Applications, Opportunities, and Limitations. Dermatol. Ther. 2020, 10, 365–386.
- Serag, A.; Ion-Margineanu, A.; Qureshi, H.; McMillan, R.; Saint Martin, M.J.; Diamond, J.; O’Reilly, P.; Hamilton, P. Translational AI and Deep Learning in Diagnostic Pathology. Front. Med. 2019, 6, 185.
- Hamamoto, R.; Suvarna, K.; Yamada, M.; Kobayashi, K.; Shinkai, N.; Miyake, M.; Takahashi, M.; Jinnai, S.; Shimoyama, R.; Sakai, A.; et al. Application of Artificial Intelligence Technology in Oncology: Towards the Establishment of Precision Medicine. Cancers 2020, 12, 3532.
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98.
- Cutillo, C.M.; Sharma, K.R.; Foschini, L.; Kundu, S.; Mackintosh, M.; Mandl, K.D.; MI in Healthcare Workshop Working Group. Machine intelligence in healthcare-perspectives on trustworthiness, explainability, usability, and transparency. NPJ Digit. Med. 2020, 3, 47.
- Shah, P.; Kendall, F.; Khozin, S.; Goosen, R.; Hu, J.; Laramie, J.; Ringel, M.; Schork, N. Artificial intelligence and machine learning in clinical development: A translational perspective. NPJ Digit. Med. 2019, 2, 69.
- Adlung, L.; Cohen, Y.; Mor, U.; Elinav, E. Machine learning in clinical decision making. Med 2021, 2, 642–665.
- Komatsu, M.; Sakai, A.; Dozen, A.; Shozu, K.; Yasutomi, S.; Machino, H.; Asada, K.; Kaneko, S.; Hamamoto, R. Towards Clinical Application of Artificial Intelligence in Ultrasound Imaging. Biomedicines 2021, 9, 720.
- Khemasuwan, D.; Sorensen, J.S.; Colt, H.G. Artificial intelligence in pulmonary medicine: Computer vision, predictive model and COVID-19. Eur. Respir. Rev. 2020, 29, 200181.
- Kaplan, A.; Cao, H.; FitzGerald, J.M.; Iannotti, N.; Yang, E.; Kocks, J.W.H.; Kostikas, K.; Price, D.; Reddel, H.K.; Tsiligianni, I.; et al. Artificial Intelligence/Machine Learning in Respiratory Medicine and Potential Role in Asthma and COPD Diagnosis. J. Allergy Clin. Immunol. Pr. 2021, 9, 2255–2261.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
17 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




