Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marianna Ranieri | + 1829 word(s) | 1829 | 2021-10-20 11:19:51 | | | |
| 2 | Catherine Yang | Meta information modification | 1829 | 2021-12-16 03:03:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ranieri, M. CaSR-Regulated microRNAs. Encyclopedia. Available online: https://encyclopedia.pub/entry/17158 (accessed on 11 January 2026).
Ranieri M. CaSR-Regulated microRNAs. Encyclopedia. Available at: https://encyclopedia.pub/entry/17158. Accessed January 11, 2026.
Ranieri, Marianna. "CaSR-Regulated microRNAs" Encyclopedia, https://encyclopedia.pub/entry/17158 (accessed January 11, 2026).
Ranieri, M. (2021, December 15). CaSR-Regulated microRNAs. In Encyclopedia. https://encyclopedia.pub/entry/17158
Ranieri, Marianna. "CaSR-Regulated microRNAs." Encyclopedia. Web. 15 December, 2021.
Copy Citation
The calcium sensing receptor (CaSR) is a unique G protein-coupled receptor (GPCR) activated by extracellular Ca2+ and by other physiological cations, aminoacids, and polyamines. CaSR is the main controller of the extracellular Ca2+ homeostatic system by regulating parathyroid hormone (PTH) secretion and, in turn, Ca2+ absorption and resorption. Recent advances highlight novel signaling pathways activated by CaSR signaling involving the regulation of microRNAs (miRNAs).
calcium sensing receptor (CaSR)
calcium
microRNAs
1. CaSR and AQP2 Interplay
A postulated mechanism for the process occurring in the collecting duct is that, during the vasopressin antidiuretic action promoting water reabsorption from the lumen, urinary Ca2+ concentration increases secondary to urine concentration. Increased Ca2+ levels, in turn, activate the CaSR located on the apical membrane of the principal cells. CaSR activation reduces the vasopressin-stimulated insertion of AQP2 into the plasma membrane and the rate of water reabsorption, consequently reducing the risk of Ca2+ supersaturation [1][2][3][4]. Maintenance and regulation of water balance is essential for all physiological processes and is critically dependent on water intake and water output in the kidney under the control of the antidiuretic hormone vasopressin. Dysregulation associated with water balance is responsible of several disorders, such as congenital nephrogenic diabetes insipidus (NDI), idiopathic syndrome of inappropriate antidiuretic hormone secretion (SIADH), nephrogenic syndrome of inappropriate antidiuresis (NSIAD), and autosomal dominant polycystic kidney disease (ADPKD) (revised in Ranieri et al., 2019) [5].
Already in the 1997, Sands and coworkers reported evidence of the presence of an apical “Calcium/polycation receptor proteins (CaRs)” in rat kidney terminal inner medullary collecting duct (tIMCD) that specifically reduces vasopressin-elicited osmotic water permeability when luminal calcium rises. This evidence provides support for a unique and new tIMCD apical membrane signaling mechanism linking calcium and water metabolism [6].
However, clinical evidence for an effect of luminal calcium on AQP2-mediated water reabsorption was provided for the first time, in humans (enuretic children), in a study of Valenti and collaborators, demonstrating that urinary AQP2 and calciuria correlate with the severity of enuresis [7]. Interestingly, hypercalciuric enuretic children receiving a low calcium diet to reduce hypercalciuria, had decreased overnight urine output (reduced nocturnal enuresis) paralleled by an increase in nighttime AQP2 excretion and osmolality [7]. Further evidence has been provided, more recently, in a bed rest study. Immobilization results in alterations of renal function, fluid redistribution, and bone loss, which couples to a rise of urinary calcium excretion. Under these conditions it was observed that bed rest induced an increase in blood hematocrit (reflecting water loss) which coincided with a reduction of urinary AQP2 likely paralleled by an increase in urinary calcium due to bone demineralization [8].
All these results strongly support the indication that urinary calcium can modulate the vasopressin-dependent urine concentration through a down-regulation of AQP2 trafficking.
In a previous study, we demonstrated that in cultured renal cells and microdissected collecting ducts, the inhibitory effect of CaSR signaling on AQP2 trafficking to the plasma membrane is associated with a significant decrease in cAMP-induced AQP2 phosphorylation at serine 256 (pS256) and AQP2 trafficking, resulting in a reduced osmotic water permeability response [9]. Specifically, calcimimetics activation of CaSR reduced AQP2 translocation to the plasma membrane in response to the cAMP elevation forskolin-induced. These data were also confirmed in HEK-293 cells transfected with two gain-of-function variants of CaSR, the CaSR-N124K mutation and the CaSR-R990G polymorphism, exploited to mimic “tonic” activation of CaSR [10]. The physiological consequence of the negative feedback on cAMP-induced AQP2-pS256 phosphorylation and trafficking stimulated by CaSR signaling is lowering the osmotic water permeability response both in cells and in isolated mouse collecting duct [9].
This theory that elevated concentration of calcium in urine counteract vasopressin action via the activation of CaSR expressed at luminal membrane of principal cells has been further validated in a mouse model double-knockout (dKO) for Pendrin/NaCl Cotransporter (NCC) [11], which display significant calcium wasting and severe volume depletion, despite high circulating vasopressin levels [12].
Due to severe hypercalciuria, a tonic activation of the luminal CaSR in the collecting duct is expected in this dKO mice model and, quite interestingly, those mice had a strong reduction in total AQP2 expression associated with a significantly higher expression of AQP2-pS261 and ubiquitinated AQP2. In addition, in dKO mice, exposure of inner medulla kidney slices to the proteasome inhibitor MG132 increased total AQP2 by 50%, indicating that the rate of AQP2 degradation via proteasome is significantly higher. It has been recently suggested that CaSR expressed at the apical membrane of collecting duct principal cells could mediate the effects of hypercalciuria in reducing vasopressin-elicited osmotic water permeability and urinary concentrating ability by the activation of autophagic degradation of AQP2. Indeed, proteomic analysis of inner medullary collecting ducts isolated from parathyroid hormone-treated rats revealed increased autophagic degradation of a specific set of proteins including AQP2 [13].
Interestingly, the functional link between CaSR and AQP2 degradation was supported by the observation that the reduced total AQP2 and higher levels of AQP2-pS261 found in dKO mice are paralleled by higher levels of p38 mitogen-activated protein kinase (p38-MAPK), an enzyme activated by CaSR signaling and known to phosphorylate AQP2 at Ser261 [14][15]. Of note, CaSR inhibition with the calcilytic NPS2143 reduced AQP2-pS261 levels in dKO mice, demonstrating that CaSR acts upstream of p38-MAPK and mediates the upregulation of AQP2-pS261. Moreover, inhibition of p38-MAPK caused a drastic decrease in AQP2-pS261, along with a nearly five-fold increase in total AQP2. Furthermore, in dKO mice, p38-MAPK inhibition results in a drastic reduction in ubiquitinated AQP2 that is paralleled by a strong increase in total AQP2 [11].
In addition to the effect on AQP2 trafficking, previous findings demonstrated that high external calcium reduces AQP2 expression both in the collecting duct cell line mpkCCD and in hypercalciuric rats [16][17]. Moreover, vitamin D-elicited hypercalcemia/hypercalciuria is associated with polyuria in humans. At the end, dihydrotachysterol (DHT) induces AQP2 water channel downregulation despite unaltered AQP2 mRNA expression in rats, suggesting a higher rate of AQP2 degradation attributed to activation of the calcium-sensitive protease calpain [18].
Ultimately, these data support a direct effect of luminal calcium on AQP2 expression in collecting duct principal cells and point to a role of calcium in regulating both AQP2 trafficking and expression.
Of note, regulation events of post-transcriptional gene expression can occur and be involved in several diseases, under the direct control of the small non-coding RNAs, the microRNAs (miRNAs) [19].
2. CaSR-Regulated miRNAs
MiRNAs are ubiquitous endogenous, short non-coding, most frequently of 19–25 nucleotides in length, single-stranded (ss)RNA transcripts that act as post-transcriptional regulators of gene expression by blocking protein translation and/or inducing messenger RNA (mRNA) degradation. miRNAs may act as transcriptional or splicing regulators within the nucleus [19], and be involved in genetic exchange with adjacent cells, through exosomes [20]. Many miRNAs display tissue-specific expression patterns and are involved in the development and maintenance of organ function. Approximately 60% of protein-coding genes are influenced by miRNAs [21] that play crucial roles in several biological processes, including control of cell cycle and differentiation, proliferation, and metabolism. As such, miRNA deregulation is being increasingly associated with several human pathologies [22]. Since their discovery in 1993 [23], numerous miRNAs have been identified in humans and other eukaryotic organisms, and their role as key regulators of gene expression is still being elucidated.
Only since 2012 have the miRNA activated by CaSR been indicated as key regulators of diverse proteins involved in different pathophysiological circumstances. Indeed, Hou and co-workers described the physiological function of claudins in the paracellular transport mechanisms with a focus on renal Ca2+ handling [24] (revised also in 2016 in [25]). In the thick ascending limb of Henle, paracellular Ca2+ reabsorption involves the functional interplay of three important claudin genes: claudin-14, -16, and -19, associated with human kidney diseases with hypercalciuria, nephrolithiasis, and bone mineral loss. A novel microRNA-based signaling pathway downstream of CaSR that directly regulates claudin-14 gene expression has been described indicating that claudin-14 is a key regulator for renal Ca2+ homeostasis. Through physical interaction, claudin-14 blocks the paracellular cation channel made of claudin-16 and -19, critical for Ca2+ reabsorption in the tick ascending limb. The molecular cascade of CaSR-microRNAs-claudins forms a regulatory loop to maintain proper Ca2+ homeostasis in the kidney [24][26]. Under normal dietary condition, claudin-14 proteins are suppressed by two microRNA molecules, miR-9 and miR-374. Both microRNAs directly target the 3′-UTR of claudin-14 mRNA; induce its mRNA decay and translational repression in a synergistic manner, causing claudin-14 to decline, leading to decreases in cation permeation [26][27][28][29]. These data indicate that the regulation of miRNA by CaSR signaling may occur on several layers within the kidney.
Moreover, the silencing of the CaSR has been demonstrated to induce tumors in colorectal cancer, associated with increased expression of miR135b and miR-146b, which are considered to be oncogenic [30]. In colon cancer cell lines other miRNAs—miR21, miR-145, and miR-135a—are inversely correlated with CaSR expression [31][32].
Furthermore, altered expression of miRNAs have been implicated in parathyroid function and may have an important role in the development of parathyroid tumors [33][34].
Our recent studies suggest that CaSR may regulate AQP2 expression also via miRNA [11] (Table 1).
Table 1. MicroRNA expression downstream CaSR signaling.
| miRNA | Target mRNA | Target Protein | Target Organ | References | |
|---|---|---|---|---|---|
| miR-9 miR-374 |
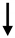 |
CLDN14 | Claudin-14 | Thick Ascending Limb cell, kidney | Hou J, Organogenesis 2012 [24]; Gong Y, Hou J, JASN 2014 [27]; Gong Y et al., JASN 2015 [28]; Hou J, Curr Opin Nephrol Hypert 2016 [25] |
| miR-21 miR-135a miR-135b |
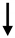 |
Tumor suppressors | Tumor suppressor proteins | Human colon carcinoma cell lines, colon | Singh N et al., Int J Cancer, 2013 [31][32] |
| miR-145 |  |
Oncogenes | Oncoproteins | Human colon carcinoma cell lines, colon | Singh N et al., Int J Cancer, 2013 [31][32] |
| miR-375 miR-429 miR-361 |
 |
PTH | Parathormone | Parathyroid | Shilo V et al., FASEB J 2015 [33] |
| miR-137 |  |
AQP2 | Aquaporin-2 | Collecting duct, kidney | Ranieri M et al., FASEB J 2018 [11] |
However, despite several studies having demonstrated that transcriptional and post-transcriptional regulation of AQP2 play crucial roles in AQP2 expression levels within the cell, along with a profound impact on water homeostasis [35][36], little is known about the role of miRNA in the regulation of AQP2 expression.
Several studies highlight an emerging role of miRNAs in AQP regulation (reviewed in [37]). Specifically, miRNAs have been identified as endogenous modulators of the expression of several AQPs [38][39][40][41][42][43][44][45][46][47][48]. Two AQP2-targeting miRNAs, miR-32 and miR-137, were reported to decrease AQP2 expression in kidney collecting duct cells independently of vasopressin regulation [41]. The authors demonstrated a significant decrease of AQP2 translation in mpkCCDc14 cells transfected with miR-32 or miR-137 providing novel insights into the regulation of AQP2 by RNA interference.
Specifically, in dKO mice, miR-137 was found to be about 1.7-fold higher compared to WT mice, which was in line with the reduced translation of AQP2 mRNA. Noteworthy, miR-137 transcript levels were increased by the calcimimetic NPS-R-568 in WT mice; furthermore, in dKO mice, miR-137 transcript levels were drastically reduced in response to CaSR or p38-MAPK inhibition with the calcilytic NPS2143 or SB203580, respectively, providing the first evidence that CaSR signaling directly acts upstream of the miR-137-AQP2 axis [11].
These findings represent the first demonstration that CaSR can regulate AQP2 expression via AQP2-targeting miRNA.
The discovery of miRNAs as endogenous modulators of AQPs offers a potential therapeutic approach for the regulation of AQP-related disorders [37].
References
- Procino, G.; Carmosino, M.; Tamma, G.; Gouraud, S.; Laera, A.; Riccardi, D.; Svelto, M.; Valenti, G. Extracellular calcium antagonizes forskolin-induced aquaporin 2 trafficking in collecting duct cells. Kidney Int. 2004, 66, 2245–2255.
- Procino, G.; Mastrofrancesco, L.; Mira, A.; Tamma, G.; Carmosino, M.; Emma, F.; Svelto, M.; Valenti, G. Aquaporin 2 and apical calcium-sensing receptor: New players in polyuric disorders associated with hypercalciuria. Semin. Nephrol. 2008, 28, 297–305.
- Procino, G.; Mastrofrancesco, L.; Tamma, G.; Lasorsa, D.R.; Ranieri, M.; Stringini, G.; Emma, F.; Svelto, M.; Valenti, G. Calcium-sensing receptor and aquaporin 2 interplay in hypercalciuria-associated renal concentrating defect in humans. An in vivo and in vitro study. PLoS ONE 2012, 7, e33145.
- Centrone, M.; Ranieri, M.; Di Mise, A.; Berlingerio, S.P.; Russo, A.; Deen, P.M.T.; Staub, O.; Valenti, G.; Tamma, G. AQP2 Abundance is Regulated by the E3-Ligase CHIP Via HSP70. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 515–531.
- Ranieri, M.; Di Mise, A.; Tamma, G.; Valenti, G. Vasopressin-aquaporin-2 pathway: Recent advances in understanding water balance disorders. F1000Research 2019, 8.
- Sands, J.M.; Naruse, M.; Baum, M.; Jo, I.; Hebert, S.C.; Brown, E.M.; Harris, H.W. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J. Clin. Investig. 1997, 99, 1399–1405.
- Valenti, G.; Laera, A.; Pace, G.; Aceto, G.; Lospalluti, M.L.; Penza, R.; Selvaggi, F.P.; Chiozza, M.L.; Svelto, M. Urinary aquaporin 2 and calciuria correlate with the severity of enuresis in children. J. Am. Soc. Nephrol. 2000, 11, 1873–1881.
- Tamma, G.; Di Mise, A.; Ranieri, M.; Svelto, M.; Pisot, R.; Bilancio, G.; Cavallo, P.; De Santo, N.G.; Cirillo, M.; Valenti, G. A decrease in aquaporin 2 excretion is associated with bed rest induced high calciuria. J. Transl. Med. 2014, 12, 133.
- Ranieri, M.; Tamma, G.; Di Mise, A.; Russo, A.; Centrone, M.; Svelto, M.; Calamita, G.; Valenti, G. Negative feedback from CaSR signaling to aquaporin-2 sensitizes vasopressin to extracellular Ca2+. J. Cell Sci. 2015, 128, 2350–2360.
- Ranieri, M.; Tamma, G.; Di Mise, A.; Vezzoli, G.; Soldati, L.; Svelto, M.; Valenti, G. Excessive signal transduction of gain-of-function variants of the calcium-sensing receptor (CaSR) are associated with increased ER to cytosol calcium gradient. PLoS ONE 2013, 8, e79113.
- Ranieri, M.; Zahedi, K.; Tamma, G.; Centrone, M.; Di Mise, A.; Soleimani, M.; Valenti, G. CaSR signaling down-regulates AQP2 expression via a novel microRNA pathway in pendrin and NaCl cotransporter knockout mice. FASEB J. 2018, 32, 2148–2159.
- Soleimani, M.; Barone, S.; Xu, J.; Shull, G.E.; Siddiqui, F.; Zahedi, K.; Amlal, H. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. P. Natl. Acad. Sci. USA 2012, 109, 13368–13373.
- Khositseth, S.; Charngkaew, K.; Boonkrai, C.; Somparn, P.; Uawithya, P.; Chomanee, N.; Payne, D.M.; Fenton, R.A.; Pisitkun, T. Hypercalcemia induces targeted autophagic degradation of aquaporin-2 at the onset of nephrogenic diabetes insipidus. Kidney Int 2017, 91, 1070–1087.
- Nedvetsky, P.I.; Tabor, V.; Tamma, G.; Beulshausen, S.; Skroblin, P.; Kirschner, A.; Mutig, K.; Boltzen, M.; Petrucci, O.; Vossenkämper, A.; et al. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J. Am. Soc. Nephrol. 2010, 10, 1645–1656.
- Trepiccione, F.; Pisitkun, T.; Hoffert, J.D.; Poulsen, S.B.; Capasso, G.; Nielsen, S.; Knepper, M.A.; Fenton, R.A.; Christensen, B.M. Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int. 2014, 86, 757–767.
- Bustamante, M.; Hasler, U.; Leroy, V.; de Seigneux, S.; Dimitrov, M.; Mordasini, D.; Rousselot, M.; Martin, P.-Y.; Féraille, E. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J. Am. Soc. Nephrol. 2008, 1, 109–116.
- Sands, J.M.; Flores, F.X.; Kato, a.; Baum, M.a.; Brown, E.M.; Ward, D.T.; Hebert, S.C.; Harris, H.W. Vasopressin-elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Am. J. Physiol. 1998, 274, F978–F985.
- Puliyanda, D.P.; Ward, D.T.; Baum, M.A.; Hammond, T.G.; Harris, H.W., Jr. Calpain-mediated AQP2 proteolysis in inner medullary collecting duct. Biochem. Biophys. Res. Commun. 2003, 303, 52–58.
- Hwang, H.W.; Wentzel, E.A.; Mendell, J.T. A hexanucleotide element directs microRNA nuclear import. Science 2007, 315, 97–100.
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105.
- Simpson, K.; Wonnacott, A.; Fraser, D.J.; Bowen, T. MicroRNAs in Diabetic Nephropathy: From Biomarkers to Therapy. Curr. Diabetes Rep. 2016, 16, 35.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854.
- Hou, J. Lecture: New light on the role of claudins in the kidney. Organogenesis 2012, 8, 1–9.
- Hou, J. Claudins and mineral metabolism. Curr. Opin. Nephrol. Hypertens. 2016, 25, 308–313.
- Gong, Y.; Renigunta, V.; Himmerkus, N.; Zhang, J.; Renigunta, A.; Bleich, M.; Hou, J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. Embo J. 2012, 31, 1999–2012.
- Gong, Y.; Hou, J. Claudin-14 underlies Ca++-sensing receptor-mediated Ca++ metabolism via NFAT-microRNA-based mechanisms. J. Am. Soc. Nephrol. 2014, 25, 745–760.
- Gong, Y.; Himmerkus, N.; Plain, A.; Bleich, M.; Hou, J. Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. J. Am. Soc. Nephrol. 2015, 26, 663–676.
- Negri, A.L. Role of claudins in renal calcium handling. Nefrologia 2015, 35, 347–352.
- Fetahu, I.S.; Tennakoon, S.; Lines, K.E.; Groschel, C.; Aggarwal, A.; Mesteri, I.; Baumgartner-Parzer, S.; Mader, R.M.; Thakker, R.V.; Kallay, E. miR-135b- and miR-146b-dependent silencing of calcium-sensing receptor expression in colorectal tumors. Int. J. Cancer 2016, 138, 137–145.
- Singh, N.; Chakrabarty, S. Induction of CaSR expression circumvents the molecular features of malignant CaSR null colon cancer cells. Int. J. Cancer 2013, 133, 2307–2314.
- Singh, N.; Liu, G.; Chakrabarty, S. Isolation and characterization of calcium sensing receptor null cells: A highly malignant and drug resistant phenotype of colon cancer. Int. J. Cancer 2013, 132, 1996–2005.
- Shilo, V.; Ben-Dov, I.Z.; Nechama, M.; Silver, J.; Naveh-Many, T. Parathyroid-specific deletion of dicer-dependent microRNAs abrogates the response of the parathyroid to acute and chronic hypocalcemia and uremia. FASEB J. 2015, 29, 3964–3976.
- Vaira, V.; Verdelli, C.; Forno, I.; Corbetta, S. MicroRNAs in parathyroid physiopathology. Mol. Cell. Endocrinol. 2017, 456, 9–15.
- Hasler, U.; Leroy, V.; Martin, P.Y.; Feraille, E. Aquaporin-2 abundance in the renal collecting duct: New insights from cultured cell models. Am. J. Physiol Ren. Physiol 2009, 297, F10–F18.
- Moeller, H.B.; Olesen, E.T.B.; Fenton, R.A. Regulation of the water channel aquaporin-2 by posttranslational modification. Am. J. Physiol. Ren. Physiol. 2011, 300, F1062–F1073.
- Gomes, A.; da Silva, I.V.; Rodrigues, C.M.P.; Castro, R.E.; Soveral, G. The Emerging Role of microRNAs in Aquaporin Regulation. Front. Chem. 2018, 6, 238.
- Chao, G.; Wang, Y.; Zhang, S.; Yang, W.; Ni, Z.; Zheng, X. MicroRNA-29a increased the intestinal membrane permeability of colonic epithelial cells in irritable bowel syndrome rats. Oncotarget 2017, 8, 85828–85837.
- Chen, G.; Shi, Y.; Liu, M.; Sun, J. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 175.
- Huebert, R.C.; Jagavelu, K.; Hendrickson, H.I.; Vasdev, M.M.; Arab, J.P.; Splinter, P.L.; Trussoni, C.E.; Larusso, N.F.; Shah, V.H. Aquaporin-1 promotes angiogenesis, fibrosis, and portal hypertension through mechanisms dependent on osmotically sensitive microRNAs. Am. J. Pathol. 2011, 179, 1851–1860.
- Kim, J.E.; Jung, H.J.; Lee, Y.J.; Kwon, T.H. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am. J. Physiol. Ren. Physiol. 2015, 308, F749–F764.
- Li, H.; Shi, H.; Gao, M.; Ma, N.; Sun, R. Long non-coding RNA CASC2 improved acute lung injury by regulating miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell Biosci. 2018, 8, 15.
- Luo, L.; Yang, R.; Zhao, S.; Chen, Y.; Hong, S.; Wang, K.; Wang, T.; Cheng, J.; Zhang, T.; Chen, D. Decreased miR-320 expression is associated with breast cancer progression, cell migration, and invasiveness via targeting Aquaporin 1. Acta Biochim. Et Biophys. Sin. 2018, 50, 473–480.
- Sepramaniam, S.; Armugam, A.; Lim, K.Y.; Karolina, D.S.; Swaminathan, P.; Tan, J.R.; Jeyaseelan, K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J. Biol. Chem 2010, 285, 29223–29230.
- Tang, R.; Pei, L.; Bai, T.; Wang, J. Down-regulation of microRNA-126-5p contributes to overexpression of VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol. Lett. 2016, 38, 1277–1284.
- Wang, Y.; Huang, J.; Ma, Y.; Tang, G.; Liu, Y.; Chen, X.; Zhang, Z.; Zeng, L.; Wang, Y.; Ouyang, Y.B.; et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J. Cereb. Blood Flow Metab. 2015, 35, 1977–1984.
- Xiong, W.; Ran, J.; Jiang, R.; Guo, P.; Shi, X.; Li, H.; Lv, X.; Li, J.; Chen, D. miRNA-320a inhibits glioma cell invasion and migration by directly targeting aquaporin 4. Oncol. Rep. 2018, 39, 1939–1947.
- Zheng, L.; Cheng, W.; Wang, X.; Yang, Z.; Zhou, X.; Pan, C. Overexpression of MicroRNA-145 Ameliorates Astrocyte Injury by Targeting Aquaporin 4 in Cerebral Ischemic Stroke. Biomed. Res. Int. 2017, 2017, 9530951.
More
Information
Subjects:
Physiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
16 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




