Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md Sultan Mahmud | + 3564 word(s) | 3564 | 2021-11-29 03:26:02 | | | |
| 2 | Bruce Ren | Meta information modification | 3564 | 2021-12-15 02:46:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mahmud, M.S. Antimicrobial/Antiviral Potential of Cannabinoids and Cannabis sativa. Encyclopedia. Available online: https://encyclopedia.pub/entry/17106 (accessed on 14 January 2026).
Mahmud MS. Antimicrobial/Antiviral Potential of Cannabinoids and Cannabis sativa. Encyclopedia. Available at: https://encyclopedia.pub/entry/17106. Accessed January 14, 2026.
Mahmud, Md Sultan. "Antimicrobial/Antiviral Potential of Cannabinoids and Cannabis sativa" Encyclopedia, https://encyclopedia.pub/entry/17106 (accessed January 14, 2026).
Mahmud, M.S. (2021, December 14). Antimicrobial/Antiviral Potential of Cannabinoids and Cannabis sativa. In Encyclopedia. https://encyclopedia.pub/entry/17106
Mahmud, Md Sultan. "Antimicrobial/Antiviral Potential of Cannabinoids and Cannabis sativa." Encyclopedia. Web. 14 December, 2021.
Copy Citation
Antimicrobial resistance has emerged as a global health crisis and, therefore, new drug discovery is a paramount need. Cannabis sativa contains hundreds of chemical constituents produced by secondary metabolism, exerting outstanding antimicrobial, antiviral, and therapeutic properties.
antibiotic resistance
antimicrobial
cannabinoid
cannabis
COVID-19
food-borne
plant pathogen
1. Introduction
The term ‘antimicrobial agent’ refers to specific synthetic or natural substances such as drugs, chemicals, or extracts that have the ability to either kill or inhibit the growth of microbes, including bacteria, fungi and algae [1]. Antibiotics have played a tremendous role in attenuating mortality and morbidity of humans since the antibiotic era started at the early of the last century [2][3]. The introduction of antibiotics into therapeutics has extended the average human life expectancy by around 23 years in just 100 years [4]. However, because of widespread misuse of antibiotics, bacteria have developed mechanisms to escape from antimicrobial agents. Although antibiotic resistance is a natural phenomenon [5] (it was observed before the extensive use of penicillin [6]), its pace has been accelerated due to overuse, inappropriate prescribing and extensive agricultural use [7]. Today, antimicrobial resistance is one of the greatest challenges for global health, and the World Health Organization (WHO) has declared it one of the top threats for humanity [8]. In the United States, more than 2.8 million people are infected by antibiotic-resistant bacteria, with over 35,000 deaths every year. An estimated USD $4.6 billion is spent to fight only six multidrug-resistant pathogens [9]. Globally, drug resistant infections cause half a million deaths each year, and the toll is suspected to exceed 10 million by 2050 [10]. Many first-line antibiotics are predicted to be ineffective by 2025 and, consequently, the ‘post antibiotic era’ will start soon, or may already has started [9][11]. Though the discovery of new antibiotics is critical, concerning the pace of antibiotic resistance, unfortunately, a huge innovation gap has been created in antibiotic drug discovery after the end of its ‘golden era’ between 1950 and 1970 [12]. It is almost 50 years since the last new antibiotic was discovered, and research funding to find new antibiotics has been drastically reduced in both the pharmaceutical and academia domain, which considering such investment nonprofitable during an economic crisis [13][14]. In 2017, the WHO published a global priority pathogen list comprising 12 species of bacteria categorized by critical, high, and medium antibiotic resistance, with the aim of ensuring quick R&D responses, guiding strategic directions and achieving new antibiotics for urgent public health needs (Figure 1) [15]. The United States Centers for Disease Control and Prevention’s (CDC) 2019 AR Threats Report listed 18 germs, including bacteria and fungi, on three levels of human health concern: urgent, serious, and concerning, as a measure of estimation of antibiotic resistance burden in the USA [9]. Today, the world is witnessing how an emerging infectious disease such as the COVID-19 pandemic, caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), can result from a lack of appropriate medicines, in addition to many other causes. The pandemic led to more than 4.8 million documented deaths globally in the 23 months up to 6 October 2021 [16].
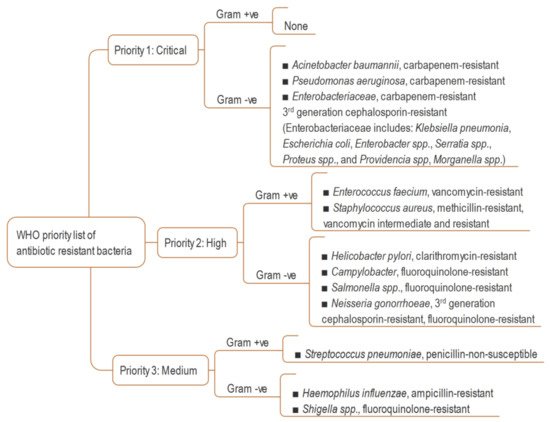
Figure 1. WHO global priority list of resistant bacteria [15].
In the history of the treatment of infectious diseases, cannabis has been used for thousands of years without knowledge of the scientific background of its effects [17][18]. A substantial amount of research has documented that C. sativa possesses hundreds of secondary metabolites including cannabinoids, terpenes and phenolic compounds [19] which have pharmacological properties in anticonvulsant therapy, appetite stimulation, neurodegenerative diseases, pain treatment, skin pathologies and infectious diseases [20]. Cannabinoids and terpenes, or essential oils (EO) enriched with these, are well known to confer anti-inflammatory effects in mammals during infectious diseases [21][22][23]. So far, 545–550 known compounds, of which about 177 phytocannabinoids, about 200 terpenes and nearly same number of phenolics, have been identified from C. sativa [20][24][25][26]. Bonini et al. reviewed the pharmacological potential of cannabinoids, stating that preclinical and clinical studies of cannabinoid compounds are beneficial for treatment of pain, colitis, spasticity, nausea and vomiting, anorexia, sleep disorders, anxiety, epilepsy, and Alzheimer’s disease [24]. Since cannabinoids can modulate the immune response through binding CB1 and CB2 receptors (a G-protein-coupled receptor densely located in the immune tissue, nervous tissue and brain), their role in infectious diseases has been discussed critically in many scientific publications [27][28][29][30][31][32]. However, the antimicrobial activity of cannabinoids, extracts and EOs from C. sativa is not unexpected, as many secondary metabolites of plants exhibit bioactivity against numerous pathogenic bacteria and fungi [33][34][35]. There is also fragmentary evidence in the literatures that cannabis compounds have efficacy against some viruses [25][32].
2. Antibacterial Activity of Cannabinoids and C. sativa
2.1. Historical Overview
The antibacterial efficacy of C. sativa was scientifically revealed in a dissertation by Krejci in 1950 [36] and preliminary results were published later stating that extracts were effective against only Gram-positive bacteria (GPB) [37][38]. Independently, the microbial inhibitory property of seeds of hemp was observed by Ferenczy in 1956. The diffused compounds from whole seeds produced an inhibitory zone against GPB in culture medium [39]. Later, resinous organs of the plant, such as the seeds and leaves, exhibited a considerable amount of antibacterial activity against GPB in an acidic culture medium, but were found ineffective against gram negative bacteria (GNB), yeasts and molds [40]. It was observed that the antibacterial activity depended on the intensity of the hashish reaction, which indicated the activity might come from psychoactive Δ9-tetrahydrocannabinol (THC), though other cannabinoids from C. sativa had not been identified at that time [40]. The following sections include some subsections of the WHO priority list, as well as some non-listed pathogenic bacteria.
2.2. Antibacterial Activities of Cannabinoids against Pathogens in the WHO’s Priority List
Cannabinoids and C. sativa extracts have substantial activity against several resistant bacteria in the WHO’s current priority list (Table 1). All major cannabinoids, including cannabidiol (CBD), THC, cannabigerol (CBG), cannabichromene (CBC), cannabinol (CBN), their derivatives like cannabidiolic acid (CBDA), cannabichromenic acid (CBCA), and even extracts and EOs, inhibit MRSA including the epidemic-causing EMRSA 15 and EMRSA 16. Methicillin-resistant Staphylococcus aureus (MRSA) are resistant to all known beta-lactam antibiotics [41], and even to linezolid, daptomycin and vancomycin [42]. Extensive work has been published recently by Farha et al., enlightening the antibiotic potency of major cannabinoids against MRSA regarding their efficacy to inhibit biofilms and persister cells [43]. Biofilms represent a subpopulation of bacteria that secure themselves against adverse situations, and persister cells, which are dormant and non-dividing, are common sources of antibiotic tolerance to MRSA [44][45]. When a biofilm forms, bacterial cells acquire 10–1000 times more resistance to antibiotics [46]. Biofilms and persisters of MRSA are considered important virulence factors, especially when formed on necrotic tissues and medical devices [43]. All five major cannabinoids can obstruct the formation of biofilms, destroy preformed biofilms and eradicate stationary phase cells of MRSA. MRSA persisters, which are highly resistant to gentamicin, ciprofloxacin, and vancomycin [47] can be killed by cannabinoids, and notably by CBG, at a concentration of 5 µg/mL [43], whereas oxacillin and vancomycin are ineffective [48]. The MIC90 of CBG against MRSA strains is favorable compared to conventional antibiotics [43]. The efficacy of CBG against biofilms and persisters of MRSA was found to be MIC 2 µg/mL in vivo, in a murine systemic infection model. CBG was found to be hemolytic at only 32 µg/mL, many-fold higher than MIC [43].
Table 1. Activity of cannabinoids and C. sativa against the resistant pathogens enlisted in WHO’s current priority list.
| Pathogen | Compound/Extract/EO | Activity | Reference Antibiotic | Ref | |
|---|---|---|---|---|---|
| Antibiotic | Activity | ||||
| Gram+ve | |||||
| Enterococcus faecium | EO, α-humulene, α-pinene, β-pinene, myrcene | MIC 0.75–1.87 (%v/v) MBC 1.39–2.83 (%v/v) |
[49] | ||
| E. faecium | EO, α-humulene, α-pinene, β-pinene, myrcene | MIC 1–4 µg/mL | Ciprofloxacin | MIC 8 µg/mL | [50] |
| EMRSA 15 and EMRSA 16 | CBD, THC, CBG, CBC, CBN | MIC 0.5–2.0 µg/mL | [51] | ||
| MRSA | 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol and 8-hydroxycannabinolic acid A | IC50 6.7 µM | Ciprofloxacin | IC50 0.4 µM | [52] |
| MRSA | CVDVM | MIC 15.6 µM | [53] | ||
| MRSA | CBCA | MIC 3.9 µM | [53] | ||
| MRSA | CBD | MIC 1 µg/mL | Tobramycin, Meropenem, Ofloxacin | MIC 1, 16, 64 µg/mL (respectively) | [54] |
| MRSA | CBD | MBEC 2–4 µg/mL | [55] | ||
| MRSA | CBD analogs | MIC 0.25–64.0 µg/mL | Vancomycin, Daptomycin, Mupirocin | MIC 0.125–2.0 µg/mL | [55] |
| MRSA | CBD, CBN, CBC, CBDV and Δ1 & 9-THC | IC50 5.8–10.6 µM | Ciprofloxacin | IC50 9.33 µM | [56] |
| MRSA | CBDA | MIC 4 µg/mL | Tobramycin, Meropenem, Ofloxacin | MIC 1, 16, 64 µg/mL (respectively) | [54] |
| MRSA | CBG | MIC 2 µg/mL and MBEC 4 µg/mL |
[43] | ||
| MRSA | EO | IC50 0.82–4.22 µg/mL | [57] | ||
| MRSA, VISA, VRSA, E. faecium | CBD | MIC 1–2 µg/mL | Vancomycin, Daptomycin, Trimethoprim, Mupirocin, Clindamycin | MIC 0.125 to >64 µg/mL | [55] |
| Streptococcus pneumoniae | CBD | MIC 1–4 µg/mL | Vancomycin, Daptomycin, Trimethoprim, Mupirocin, Clindamycin | MIC 0.25 to >64 µg/mL | [55] |
| VRE | CBCA | MIC 7.8 µM | [53] | ||
| Gram -ve | |||||
| Escherichia coli | Aqueous extract | MIC 7.14 mg/mL | Ciprofloxacin | MIC < 0.12 mg/mL | [58] |
| E. coli | N-p-trans-coumaroyl-tyramine | IC50 0.8 µg/mL | Ciprofloxacin | IC50 0.01 µg/mL | [59] |
| E. coli | Seed extract | MIC 25 µg/mL | [60] | ||
| E. coli and Salmonella typhimurium | Seed extract | Growth inhibition at 1 mg/mL | [61] | ||
| E. coli, and Pseudomonas aeruginosa | EO | MIC 1.2 mg/mL | MIC 0.062–1.0 mg/mL | [62] | |
| Enterobacter aerogenes | Seed extract | MIC 2.5 mg/mL | [61] | ||
| Neisseria gonorrhoeae | CBD | MIC 1–2 µg/mL | Vancomycin, Levofloxacin, Meropenem, Gentamicin |
MIC 0.002–4.0 µg/mL | [55] |
| N. gonorrhoeae | CBD analogs | MIC 0.03–16.0 µg/mL | Mupirocin Colistin | MIC 1–32 µg/mL | [55] |
| P. aeruginosa | Aqueous extract | MIC 7.14 mg/mL | Ciprofloxacin | MIC 1.23 mg/mL | [58] |
| P. aeruginosa | Whole plant extract | MIC 12.5 µg/mL | [60] | ||
The rapid bactericidal activity of CBD was observed (<3 h) at 2 µg/mL [55], and the effect resembled that of the natural nonionic detergents, saponins [54]. CBD and CBDA showed no toxicity to human keratinocyte cells at up to seven and four-fold higher concentration of their respective MIC against MRSA (Table 1) [54]. CBD could potentiate bacitracin activity, reducing its MIC 64-fold against resistant bacteria, including MRSA [63]. The combination affected morphological changes of the pathogen, impaired cell division and induced membrane irregularities. No synergistic or antagonist effect was seen on MRSA resulting from CBD with conventional antibiotics including vancomycin, methicillin, clindamycin, tobramycin, teicoplanin, ofloxacin and meropenem [54]. Because of the hydrophobic nature of CBD, it cannot attack enough of the bacterial membrane to enhance the uptake of antibiotic drugs and does not interfere the mechanism of action of last-resort antibiotics.
In an in vivo study, CBCA showed more potent and faster bactericidal activity than vancomycin with lower a toxicity level to the mammalian cell lines A549 and HepG2. CBCA and cannabidivarin methyl ester (CBDVM) rendered minimum toxicity concentration (MTC), greater than 100 µM on both cell lines, which is far higher than their respective MIC against MRSA (Table 1). Additionally, compared to vancomycin, the compound exhibited more biocidal activity with higher a bacterial load. Rapid bactericidal activity of CBCA could reduce treatment time and provide less opportunity for emergence of bacterial resistance. A time-kill assay showed considerable reduction of CBCA activity after 8 h of exposition to MRSA. The activity of CBCA was observed against both the exponential and stationary phases of MRSA and was independent of their cellular metabolism [53]. The killing activity of many antibiotics is attributed to their effect on dividing bacteria cells, which is crucially interrupted by the stationary phase of MRSA, resulting in higher morbidity in nosocomial infections [64]. Synergistic effects of phytocannabinoids and terpenoids are reported in the treatment of infections related to MRSA and fungi [65]. The penetration of bacteria cell membranes differs among cannabinoids, which results in the non-identical effects of these compounds [54].
In contrast to pure active compounds, C. sativa extracts and EOs sometimes have even greater activity against resistant pathogens as a result of probable synergism. Drug-resistant clinical isolates, including MRSA, vancomycin-resistant Staphylococcus aureus (VRSA) and vancomycin-intermediate Staphylococcus aureus (VISA) demonstrated susceptibility to alcoholic C. sativa extracts [66][67]. A profound inhibitory efficacy was achieved when an ethanolic extract of C. sativa leaves was combined with a Thuja orientalis leaf extract in a 1:1 ratio. The synergism was obtained due to the antibacterial effect of the phenolic compounds quercetin, gallic acid and catechin present in the leaf extract [66].
Gram-negative organisms generally exhibit more resistance to antibiotics due to their distinctive structure. They are dominant killers in intensive care units showing resistance to wide-spectrum antibiotics including third-generation cephalosporins and carbapenems [68]. They differ in structure from GPB since they have an outer membrane containing lipopolysaccharide (LPS)/endotoxin, which provides the pathogen intrinsic resistance against antibacterial agents [69]. This acts as an important barrier and provides protection by resisting the penetration of toxic antibiotics and innate host immune molecules [70].
However, GNB, whose outer membrane is permeable, are susceptible to cannabinoids [43]. All the five major cannabinoids showed synergism against clinically isolated multidrug-resistant GNB, including Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Escherichia coli when used with polymyxin B at sublethal concentration [43][55]. The activity against K. pneumoniae was increased for EO exhibiting full synergism with addition of ciprofloxacin [62]. Naringenin with EO was found to be bactericidal against drug resistant Helicobacter pylori [71]. Aqueous and solvent extracts of leaf, stem and roots also displayed substantial activity against K. pneumoniae, A. baumannii and Haemophilus influenzae [72].
CBD has strong inhibitory efficacy on release of membrane vesicles (MV) from E. coli VCS257 and can boost bactericidal power of vancomycin against E. coli, to which it shows resistance [73]. MVs are nanosized spheres composed of lipid membranes derived from the outer membrane of bacteria that can cause an extra layer of protection against antibiotics [74][75]. EO exhibits synergistic effect against E. coli, and P. aeruginosa in combination with ciprofloxacin [62]. P. aeruginosa is resistant to antibiotics including beta-lactams, aminoglycosides and quinolones [76]. The efficacy of solvent extracts of C. sativa against P. aeruginosa in terms of inhibitory zone is comparable with gentamicin [60], ampicillin [77] and ciprofloxacin [62]. Notably, the level of sensitivity of the extracts in qualitative tests is not equipollent since their polarity and solubility change their diffusivity through media [78][79]. However, in many other investigations, the activity of C. sativa was shown against P. aeruginosa [72][80][81][82][83][84], E. coli [72][60][77][81][85][86][87][88][89][90][91], Salmonella species [85][89][92][93], Shigella species [85][91], K. pneumoniae [91], Acinetobacter calcoaceticus [88], Morganella morganii [72] and Serratia marcescens [93].
The ability of cannabinoids to modulate physiological and pathophysiological activities can hinder bacterial conjugation by targeting plasmid DNA [94]. Conjugation is one of the major processes of acquiring antibiotic resistance and involves replication and transfer of an extra piece of bacterial DNA plasmid into a recipient bacterium [95]. Plasmids contain genes to express resistance to antibiotics. Δ9-THC, CBN and CBD impaired plasmid transfer activity near to zero for pKM 101 and TP 114 [94]. Tetrahydrocannabinolic acid (THCA) reduced plasmid curing activity by 30% in E. coli K12 F’lac strain [96]. Plasmid curing is a process by which the plasmid is eliminated, and the bacteria become susceptible. THCA and some cannabispiro compounds were inhibited transformation of plasmid DNA (pBR322), elimination (F’lac) and transfer (R144) of plasmid from E. coli to E. coli, and even killing plasmid carrying bacteria despite possessing a higher MIC value [97].
Apart from phytocannabinoids, some endocannabinoids (EC) and endocannabinoid-like (EC-like) natural endogenous compounds have good potency against MRSA biofilms. Anamide and arachidonoyl serine, an EC and EC-like natural endogenous compound respectively, did not kill the bacteria in vitro, but inhibited biofilm formation and preformed biofilms of MRSA, altered biofilm-associated virulence factors, and could modify MRSA cell surface characteristics [98]. The compounds also exhibited synergy with different antibiotics including ampicillin, methicillin and gentamicin under both planktonic growth conditions and biofilm formation [99]. Besides, their combination with methicillin impaired slime formation of MRSA [99]. The slime layer is not easily be washed off and can be expressed as a virulence factor [100][101].
2.3. Antibacterial Activities of Cannabinoids against Pathogenic Bacteria Not on the WHO Priority List
C. sativa has broad-spectrum antibacterial efficacy against a number of pathogenic bacteria (Table 2) that are not listed in WHO’s current priority list.
Table 2. Activity of cannabinoids and C. sativa against pathogens other than those on the WHO’s priority list (* collected from foods or food environments).
| Pathogen | Compound/Extract/EO | Activity | Reference Antibiotic | Ref | |
|---|---|---|---|---|---|
| Antibiotic | Activity | ||||
| Gram+ve | |||||
| Bacillus subtilis and Staphylococcus aureus | Leaf extract | MIC 1.56 mg/mL | [90] | ||
| B. subtilis, S. aureus and Micrococcus luteus | EO | MIC 1.2–4.7 mg/mL | Ciprofloxacin | MIC 0.015–0.031 mg/mL | [62] |
| B. subtilis, S. aureus, Mycobacterium smegmatis | CBC, its homologs and isomers | MIC 0.39–3.12 µg/mL | [102] | ||
| Clostridium species *, Enterococcus hirae *, Streptococcus salivarius * | EO, α-humulene, α-pinene, β-pinene, myrcene | MIC ≥ 0.8 (%v/v) | [49] | ||
| Enterococcus *, Staphylococcus *, and Bacillus species * | EO | MIC ≥ 0.5 µg/mL | Ampicillin, Ciprofloxacin | MIC ≥ 0.25 µg/mL | [50] |
| Listeria monocytogenes strains * | EO | MIC/MBC 2.5–5.0 μL/mL | [103] | ||
| L. monocytogenes * | EO | MIC ≥ 1 µg/mL | Ampicillin | MIC ≥ 0.25 µg/mL | [50] |
| L. monocytogenes * | EO, α-pinene, Myrcene | MBC ≥ 1024 µg/mL | [104] | ||
| Lancefield Group A Streptococcus sp. | Leaf extract | MIC 20 mg/mL MBC 30 mg/mL |
[105] | ||
| MRSA biofilms * | Seed extract | MIC 1 mg/mL | [61] | ||
| MSSA | CBCA | MIC 7.8 µM | [53] | ||
| MSSA, VISE, Staphylococcus epidermidis, Staphylococcus pyogenes, Enterococcus faecalis, Cutibacterium acnes, Clostridioides difficile | CBD | MIC 0.5–4.0 µg/mL | Vancomycin, Daptomycin, Trimethoprim, Mupirocin, Clindamycin, Levofloxacin, Meropenem, Gentamicin, Erythromycin, Tetracycline, Mupirocin | MIC 0.03–64.0 µg/mL | [55] |
| Mycobacterium intracellulare | CBG | IC50 15 µg/mL | [106] | ||
| S. aureus | 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol, 8-hydroxycannabinolic acid A | IC50 3.5 µM | Ciprofloxacin | IC50 0.4 µM | [52] |
| S. aureus | Aqueous extract | MIC 3.57 mg/mL | Ciprofloxacin | MIC 0.62 µg/mL | [58] |
| S. aureus | Methanol extract | MIC 25 µg/mL | [60] | ||
| S. aureus (including multi drug resistant S. aureus 104) | EO | MIC 8 mg/mL | [71] | ||
| S. aureus (mature and pre-formed biofilms) | EO | MBEC 24 mg/mL | [71] | ||
| S. aureus and E. faecalis | Seed extract | MIC 1 mg/mL | [61] | ||
| S. aureus biofilm * | EO | MIC 0.5 mg/mL | [61] | ||
| S. aureus planktonic cells * | EO | MIC 1 mg/mL | [61] | ||
| S. aureus * | EO | MIC 1.25–5.0 µg/mL | [103] | ||
| S. aureus * | EO | MIC 1–4 µg/mL | Ciprofloxacin | MIC 0.5–16.0 µg/mL | [50] |
| S. aureus, S. epidermidis | CBD, CBDA | MIC 1–4 µg/mL | Torbamycin, Meropenem, Ofloxacin | MIC 0.06–0.5 µg/mL | [54] |
| SA-1199B (MDR), RN4220 (Macrolide-resistant), XU212 (Tetracycline-resistant) |
CBD, CBC, THC, CBG, CBN, Carboxylated versions, Abnormal cannabinoids | MIC 0.5–4.0 µg/mL | [51] | ||
| Staphylococcus species | THC, CBD | MIC 1–5 µg/mL | [107] | ||
| Staphylococcus, Lactococcus and Bacillus species | CBD, CBN, CBC, CBDV and Δ1 & 9-THC | IC50 2.6–9.2 µM | Ciprofloxacin | IC50 0.003–2.4 µM | [56] |
| Gram-ve | |||||
| Moraxella catarrhalis, Neisseria meningitidis and Legionella pneumophila | CBD | MIC 0.25–1.0 µg/mL | Vancomycin, Levofloxacin, Meropenem, Gentamicin | MIC 0.03–32 µg/mL | [55] |
| Pectobacterium carotovorum subsp. carotovorum * | EO, α-humulene, α-pinene, β-pinene, myrcene | MIC ≥ 1.24 (%v/v) | [49] | ||
| Pseudomonas fluorescens and Xanthobacter flavus | CBD, CBN, CBC, CBDV and Δ1 & 9-THC | IC50 3.1–9.3 µM | Ciprofloxacin | IC50 0.15–2.3 µM | [56] |
| Pseudomonas species | EO(s) and Terpenes | MIC 1.05–1.97 (%v/v) | [49] | ||
CBD has bacitracin activity, reducing its MIC 64-fold against Listeria monocytogenes and Enterococcus faecalis [63]. It can increase the effectiveness of kanamycin against Staphylococcus aureus without affecting MV release [73]. The EO exhibited bactericidal activity against clinically isolated methicillin-resistant Staphylococcus pseudintermedius (MRSP) from dogs suffering from pyoderma [108]. A combination of ciprofloxacin with EO significantly decreased MIC against Bacillus subtilis, S. aureus and Micrococcus luteus due to partial and full synergism [62]. The inhibition pattern of seed extract against S. aureus biofilms is similar to that of vancomycin, and the efficacy was found to be dose-dependent [109]. The bactericidal activity of solvent extracts against penicillin resistant S. aureus was recorded by Kabelik [18][110]. Acidic fractions are responsible for the antimicrobial properties of crude extract of leaves [111]. Leaf extract out-performs chloramphenicol in terms of inhibition zone against the strep-throat-causing Lancefield Group A Streptococcus sp., and its activity is comparable with penicillin and amoxicillin [10], which are commercially used as beta-lactam antibiotics for strep-throat treatment.
Moreover, a considerable number of diffusion tests showed medium to higher activity against S. aureus [60][77][80][83][85][86][88][91][93][111][112], B. subtilis [60][88][89][91][93][111], Bacillus cereus [86][89][93], Bacillus pumilus [111], E. faecalis [86][92][93][113], Micrococcus flavus [111], M. luteus [88][93], Brevibacterium linens, Brochothrix thermosphacta [88] and Methicillin-resistant coagulase-negative Staphylococci (MRCoNS) [67]. The findings indicate that C. sativa can be targeted as a natural source for developing antibacterial drugs.
Like other antibiotics, a plant’s secondary metabolites encounter a barrier at the outer membrane of GNB, and limited efficacy is observed [114]. Nevertheless, many studies show C. sativa having a moderate to large inhibitory zone for Yersinia enterocolitica [88][92][113], Vibrio cholerae [82], Citrobacter freundii CCM 7187 [93], Erwinia carotovora [115], Bordetella bronchioseptica, Proteus vulgaris [111], Aeromonas hydrophyla, Beneckea natriegens, and Flavobacterium suaveolens [88].
It can be assumed that the bioactivity of C. sativa extracts and EOs fundamentally come from compounds such as cannabinoids, phenolics and terpenes [62][61][103]. The anntimicrobial profile of low-level THC content of C. sativa (industrial hemp) is partially related to CBD [50], CBDA [109], phenolics including flavonoids, caffeoyltyramine, cannabisin and polyphenols [58][61] and terpenes including α-pinene, α-humulene, β-pinene, β-caryophyllene, (E) caryophyllene, caryophyllene oxide and myrcene [62][49][50][108][103][104].
3. Antifungal Activity
Both superficial and systemic fungal infections have increased due to the emergence of many immunological dysfunctions in people [116]. The management of fungal infections suffers from the unavailability of drugs, toxicity, resistance and relapse of conditions [117]. Therefore, finding new antifungal drugs to combat fungal infections is a priority. In agreement with the set threshold by Kuete and Dabur to ascribe the antimicrobial and antifungal properties of plant juices [118][119], C. sativa extract, EO and their phytoconstituents possess significant activity against a number of pathogenic fungi and algae (Table 3).
Table 3. Activity of cannabinoids and C. sativa against fungi.
| Pathogen | Compound/Extract/EO | Activity | Reference Antibiotic | Ref | |
|---|---|---|---|---|---|
| Antibiotic | Activity | ||||
| Candida albicans | Extract | MIC 0.25 mg/mL | [120] | ||
| C. albicans | Extract | MIC 1.42 mg/mL | Fluconazole | MIC 2 mg/mL | [58] |
| C. albicans | 4-terpenyl cannabinolate | MIC 8.5 µg/mL | [121] | ||
| C. albicans | 8-hydroxycannabinol | IC50 4.6 µM | Amphotericin B | IC50 0.3 µM | [52] |
| C. albicans | Cannabis and ginger blend | MIC 4.69 mg/mL | [122] | ||
| C. albicans | CBDV | IC50 11.9 mM | Nystatin | IC50 1.50 mM | [56] |
| C. albicans | CBNA | IC50 8.5 µg/mL | [121] | ||
| Candida krusei | Cannabinoids | IC50 53.4–60.5 µM | amphotericin B | IC50 0.7 µM | [52] |
| Candida neoformans | β-caryophyllene/oxide | IC50 1.18–19.4 µg/mL | [57] | ||
| Candida species | β-caryophyllene | MIC 1.45–10.0 µg/mL | [57] | ||
| Plasmodium falciparum | Cannabinoids | IC50 4.0–6.7 µM | Chloroquine | IC50 0.1–0.5 µM | [52] |
| P. falciparum | CBNA | IC50 2.4–2.7 µg/mL | [121] | ||
| Trichophyton and Arthroderma species | EO | MIC 0.312–6.3 µg/mL | Griseofulvin | MIC 1.26 to >8.0 µg/mL | [123] |
Candida albicans, a prevalent opportunistic pathogenic fungus to humans, which is resistant to fluconazole, exhibited higher susceptibility to C. sativa extracts, EO and other compounds. Moreover, EO of C. sativa has a full synergistic effect with fluconazole, resulting in a 16-fold reduction of MIC against Candida spp. [62]. C. albicans is part of a natural microflora that forms asymptomatic colonies on the skin and inside the body and can proliferate if the host has an immunosuppressed condition and cause superficial mucosal and dermal infections [124][125]. Activity against Candida species [60][82][83][111][113] Fusarium spp. [77], Candida neoformans [82] and Aspergillus [77][111][126] are documented. Antifungal activity is cultivar-dependent [123] and also related to the active compounds’ chemical structures [84]. The findings indicate that more intensive study on the fungicidal activity of C. sativa phytoextracts is required for the treatment of fungal infections, especially for external use.
References
- Burnett-Boothroyd, S.; McCarthy, B. Antimicrobial treatments of textiles for hygiene and infection control applications: An industrial perspective. In Textiles for Hygiene and Infection Control; Elsevier: Amsterdam, The Netherlands, 2011; pp. 196–209.
- Shahid, M.; Sobia, F.; Sahai, A.; Tripathi, T.; Singh, A.; Shahzad, A.; Khan, H.M. Umesh Plant Natural Products as a Potential Source for Antibacterial Agents: Recent Trends. Anti-Infect. Agents Med. Chem. 2009, 8, 211–225.
- Ashkenazi, S. Beginning and possibly the end of the antibiotic era. J. Paediatr. Child Health 2012, 49, E179–E182.
- Hutchings, M.I.; Truman, A.; Wilkinson, B. Editorial overview: Antimicrobials: Tackling AMR in the 21st century. Curr. Opin. Microbiol. 2019, 51, 3–5.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Abraham, E.P.; Chain, E. An Enzyme from Bacteria able to Destry penicillin. Nature 1940, 146, 837.
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283.
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 13 October 2020).
- CDC 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest_threats.html (accessed on 9 August 2021).
- O’Neill, J. Review on Antimicrobial Resistance. Available online: https://amr-review.org/background.html (accessed on 1 July 2021).
- Ledingham, K.; Hinchliffe, S.; Jackson, M.; Thomas, F.; Tomson, G. Antibiotic resistance: Using a cultural contexts of health approach to address a global health challenge. WHO Reg. Off. Eur. 2019, 29, 166.
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134.
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154.
- Piddock, L. The crisis of no new antibiotics—What is the way forward? Lancet Infect. Dis. 2012, 12, 249–253.
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017.
- John Hopkins University. Available online: https://coronavirus.jhu.edu/ (accessed on 3 July 2021).
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157.
- Kabelik, J.; Krejci, Z.; Santavy, F. Hemp as a medicament. Bull. Narc. 1960, 12, 5–23.
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150.
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31.
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349.
- Downer, E.J. Anti-inflammatory Potential of Terpenes Present in Cannabis sativa L. ACS Chem. Neurosci. 2020, 11, 659–662.
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290.
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315.
- Paland, N.; Pechkovsky, A.; Aswad, M.; Hamza, H.; Popov, T.; Shahar, E.; Louria-Hayon, I. The Immunopathology of COVID-19 and the Cannabis Paradigm. Front. Immunol. 2021, 12, 631233.
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60.
- Tahamtan, A.; Tavakoli-Yaraki, M.; Rygiel, T.P.; Mokhtari-Azad, T.; Salimi, V. Effects of Cannabinoids and their Receptors on Viral Infections. J. Med. Virol. 2016, 88, 1–12.
- Beji, C.; Loucif, H.; Telittchenko, R.; Olagnier, D.; Dagenais-Lussier, X.; Van Grevenynghe, J. Cannabinoid-Induced Immunomodulation during Viral Infections: A Focus on Mitochondria. Viruses 2020, 12, 875.
- Reiss, C.S. Cannabinoids and Viral Infections. Pharmaceuticals 2010, 3, 1873–1886.
- Klein, T.W.; Friedman, H.; Specter, S. Marijuana, immunity and infection. J. Neuroimmunol. 1998, 83, 102–115.
- Cabral, G.A. Marijuana and Cannabinoids: Effects on infections, immunity, and AIDS. In Cannabis Therapeutics in HIV/AIDS; Russo, E.B., Ed.; Routledge: New York, USA, 2002; pp. 61–86. ISBN 9780203049105.
- Tagne, A.M.; Pacchetti, B.; Sodergren, M.; Cosentino, M.; Marino, F. Cannabidiol for Viral Diseases: Hype or Hope? Cannabis Cannabinoid Res. 2020, 5, 121–131.
- Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and Medicinal Chemistry. Curr. Med. Chem. 2011, 18, 1085–1099.
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256.
- Marcu, J.P. An Overview of Major and Minor Phytocannabinoids; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 1, ISBN 9780128003763.
- Krejci, Z. Hemp as a Medicament. Ph.D. Thesis, Faculty of Natural Sciences, Brno, Czech Republic, 1950.
- Krejci, Z. Antibacterial action of Canabis indica. Lek. List. 1952, 7, 500–503.
- Krejci, Z. Hanf (Cannabis sativa) -Antibiotisches Heilmittel. 2. Mitteilung: Methodik und Ergebnisse der bakteriologischen Untersuchungen und vorläufige klinische Erfahrungen. Pharmazie 1959, 14, 155–166.
- Ferenczy, L. Antbacterial substances in seeds. Nature 1956, 178, 639–640.
- Ferenczy, L.; Gracza, L.; Jakobey, I. An antibacterial preparatum from hemp (Cannabis sativa L.). Naturwissenschaften 1958, 45, 188.
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12.
- Nannini, E.; Murray, B.E.; Arias, C.A. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 2010, 10, 516–521.
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346.
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Genet. 2006, 5, 48–56.
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188.
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39.
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 1–7.
- Severin, A.; Tabei, K.; Tenover, F.; Chung, M.; Clarke, N.; Tomasz, A. High Level Oxacillin and Vancomycin Resistance and Altered Cell Wall Composition in Staphylococcus aureus Carrying the Staphylococcal mecA and the Enterococcal vanA Gene Complex. J. Biol. Chem. 2004, 279, 3398–3407.
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419.
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302.
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430.
- Radwan, M.M.; ElSohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically Active Cannabinoids from High-Potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911.
- Galletta, M.; Reekie, T.; Nagalingam, G.; Bottomley, A.; Harry, E.; Kassiou, M.; Triccas, J. Rapid Antibacterial Activity of Cannabichromenic Acid against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 523.
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900.
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 1–18.
- Nalli, Y.; Arora, P.; Riyaz-Ul-Hassan, S.; Ali, A. Chemical investigation of Cannabis sativa leading to the discovery of a prenylspirodinone with anti-microbial potential. Tetrahedron Lett. 2018, 59, 2470–2472.
- Wanas, A.S.; Radwan, M.M.; Mehmedic, Z.; Jacob, M.; Khan, I.A.; Elsohly, M.A. Antifungal activity of the volatiles of high potency Cannabis sativa L. Against Cryptococcus neoformans. Rec. Nat. Prod. 2015, 10, 214–220.
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461.
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; ElSohly, M.A. Chemical and Biological Studies of Cannabis sativa Roots. Med. Cannabis Cannabinoids 2018, 1, 104–111.
- Ali, E.M.M.; Almagboul, A.Z.I.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial Activity of Cannabis sativa L. Chin. Med. 2012, 03, 61–64.
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Di Gioia, D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT 2020, 124, 109149.
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400.
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci. Rep. 2020, 10, 1–12.
- Mascio, C.T.M.; Alder, J.D.; Silverman, J.A. Bactericidal Action of Daptomycin against Stationary-Phase and Nondividing Staphylococcus aureus Cells. Antimicrob. Agents Chemother. 2007, 51, 4255–4260.
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364.
- Chakraborty, S.; Afaq, N.; Singh, N.; Majumdar, S. Antimicrobial activity of Cannabis sativa, Thuja orientalis and Psidium guajava leaf extracts against methicillin-resistant Staphylococcus aureus. J. Integr. Med. 2018, 16, 350–357.
- Nashra, A.; Sujatha, R.; Sameer, D.; Bagoliwal, A.; Mishra, V.; Kumar, A.; Majid, A. Comparative Evaluation of Antibacterial Efficacy of Cannabis Sativa, Allium Sativum, Allium Cepa, Thuja Orientalis and Psidium Guajava against Drug Resistance Pathogens. Int. J. Health Sci. Res. 2018, 8, 89–97.
- Torres, J.A.; Villegas, M.V.; Quinn, J.P. Current concepts in antibiotic-resistant Gram-negative bacteria. Expert Rev. Anti-Infect. Ther. 2007, 5, 833–843.
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? Antibiotikaresistenz: Was ist so besonders an den Gram-negativen. GMS Hyg. Infect. Control 2017, 12, 1–24.
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. mBio 2016, 7, e01541-16.
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266.
- Shah, S.B.; Sartaj, L.; Hussain, S.; Ullah, N.; Idrees, M.; Shaheen, A.; Javed, M.S.; Aslam, M.K. In-vitro evaluation of antimicrobial, antioxidant, alpha-amylase inhibition and cytotoxicity properties of Cannabis sativa. Adv. Tradit. Med. 2019, 20, 181–187.
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.; et al. Cannabidiol Is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324.
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. et Biophys. Acta (BBA)—Proteins Proteom. 2009, 1794, 808–816.
- Nagakubo, T.; Nomura, N.; Toyofuku, M. Cracking Open Bacterial Membrane Vesicles. Front. Microbiol. 2019, 10, 3026.
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2018, 37, 177–192.
- Anjum, M.; Arooj, Z.-E.-; Azam, S.; Rehman, P.; Khadim, J.; Anjum, M. Evaluation of antimicrobial activity and ethnobotanical study of Cannabis sativa L. Pure Appl. Biol. 2018, 7, 706–713.
- Kourmouli, A.; Valenti, M.; Van Rijn, E.; Beaumont, H.J.E.; Kalantzi, O.-I.; Schmidt-Ott, A.; Biskos, G. Can disc diffusion susceptibility tests assess the antimicrobial activity of engineered nanoparticles? J. Nanoparticle Res. 2018, 20, 2–7.
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126.
- Mathur, P.; Singh, A.; Srivastava, V.R.; Singh, D.; Mishra, Y. Antimicrobial activity of indigenous wildly growing plants: Potential source of green antibiotics. Afr. J. Microbiol. Res. 2013, 7, 3807–3815.
- Naveed, M.; Khan, T.A.; Ali, I.; Hassan, A.; Ali, H.; Ud, Z.; Hassan, Z.; Tabassum, S.; Majid, A.; Rehman, M.U. In vitro antibacterial activity of Cannabis sativa leaf extracts to some selective pathogenicbacterial strains. Int. J. Biosci. (IJB) 2014, 4, 65–70.
- Lone, T.A.; Lone, R.A. Extraction of cannabinoids from Cannabis sativa L plant and its potential antimicrobial activity. Univers. J. Med. Dent. 2012, 1, 51–55.
- Mkpenie, V.N.; Essien, E.E.; Udoh, I.I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2012, 11, 300–307.
- Elsohly, H.N.; Turner, C.E.; Clark, A.M.; Elsohly, M.A. Synthesis and Antimicrobial Activities of Certain Cannabichromene and Cannabigerol Related Compounds. J. Pharm. Sci. 1982, 71, 1319–1323.
- Ullah, S.; Jan, G.; Gul, F.; Khan, S.; Husna, H.; Sher, J.; Abidullah, S. Phytochemistry and antibacterial activities of some selected plants of war affected area of bajaur agency, pakistan. J. Pharmacogn. Phytochem. 2018, 7, 416–417.
- Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes. Molecules 2020, 25, 691.
- Kim, S.-Y.; Kang, D.-H.; Kim, J.-K.; Ha, Y.-G.; Hwang, J.Y.; Kim, T.; Lee, S.-H. Antimicrobial Activity of Plant Extracts Against Salmonella typhimurium, Escherichia coli O157:H7, and Listeria monocytogenes on Fresh Lettuce. J. Food Sci. 2010, 76, M41–M46.
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262.
- Nasrullah, S.; Rahman, K.; Ikram, M.; Nisar, M.; Khan, I. Screening of antibacterial activity of medicinal plants. Int. J. Pharm. Sci. Rev. Res. 2012, 14, 25–29.
- Sharma, C.; Kaur, S.; Chaudhry, S.; Aman, R. Antimicrobial Potential of Three Common Weeds of Kurukshetra: An in vitro Study. Res. J. Microbiol. 2015, 10, 280–287.
- Ali, M.; Romman, M.; Parvez, R.; Shuaib, M.; Bahadur, S.; Khalil, A.A.K.; Khan, M.; Haq, F.; Jan, S.; Hayat, S.S.S.; et al. Anti-bacterial activity of Cannabis sativa Linn. leaf extracts against different pathogenic bacterial strains. Biosci. Res. 2020, 17, 2730–2735.
- Zheljazkov, V.D.; Sikora, V.; Dincheva, I.; Kačániová, M.; Astatkie, T.; Semerdjieva, I.B.; Latkovic, D. Industrial, CBD, and Wild Hemp: How Different Are Their Essential Oil Profile and Antimicrobial Activity? Molecules 2020, 25, 4631.
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Molecules 2017, 22, 700.
- Oyedemi, B.M. Antiplasmid and Antimicrobial Activities of Synthetic and Natural Products from Selected Medicinal Plants; UCL School of Pharmacy London: London, UK, 2014.
- Raleigh, E.; Low, K. Conjugation. Brenner’s Encycl. Genet. Second Ed. 2013, 1, 144–151.
- Spengler, G.; Molnar, A.; Schelz, Z.; Amaral, L.; Sharples, D.; Molnar, J. The Mechanism of Plasmid Curing in Bacteria. Curr. Drug Targets 2006, 7, 823–841.
- Molnár, J.; Csiszár, K.; Nishioka, I.; Shoyama, Y. The effects of cannabispiro compounds and tetrahydrocannabidiolic acid on the plasmid transfer and maintenance in Escherichia coli. Acta Microbiol. Hung. 1986, 33, 221–231.
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018, 8, 17696.
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Potential combinations of endocannabinoid/endocannabinoid-like compounds and antibiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2020, 15, e0231583.
- Veringa, E.M.; Ferguson, D.A.; Lambe, D.W.; Verhoef, J. The role of glycocalyx in surface phagocytosis of Baeteroides spp., in the presence and absence of clindamycin. J. Antimicrob. Chemother. 1989, 23, 711–720.
- Nazir, R.; Rehman, S.; Nisa, M.; Baba, U. ali Exploring bacterial diversity: From cell to sequence. In Freshwater Microbiology: Perspectives of Bacterial Dynamics in Lake Ecosystems; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 263–306. ISBN 9780128174951.
- Turner, C.E.; Elsohly, M.A. Biological Activity of Cannabichromene, its Homologs and Isomers. J. Clin. Pharmacol. 1981, 21, 283S–291S.
- Pellegrini, M.; Palmieri, S.; Ricci, A.; Serio, A.; Paparella, A.; Sterzo, C.L. In vitro antioxidant and antimicrobial activity of Cannabis sativa L. cv ‘Futura 75’ essential oil. Nat. Prod. Res. 2020, 1–5.
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes Virulence by Cannabis sativa L. Essential Oil. Front. Cell. Infect. Microbiol. 2018, 8, 293.
- Anumudu, C.K.; Akpaka, M.N.; Anumudu, I.C. Antimicrobial activity of Cannabis sativa extracts on Lancefield Group A Streptococcus species associated with streptococcal pharyngitis (strep throat). Afr. J. Biol. Sci. 2020, 2, 9.
- Radwan, M.M.; Ross, S.A.; Slade, D.; Ahmed, S.A.; Zulfiqar, F.; ElSohly, M.A. Isolation and Characterization of New Cannabis Constituents from a High Potency Variety. Planta Med. 2008, 74, 267–272.
- Van Klingeren, B.; Ham, M.T. Antibacterial activity of Δ9-tetrahydrocannabinol and cannabidiol. Antonie van Leeuwenhoek 1976, 42, 9–12.
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; De Filippis, A.; Fiorito, F.; De Martino, L.; Fratini, F. Antimicrobial Activity of Some Essential Oils against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus pseudintermedius-Associated Pyoderma in Dogs. Animals 2020, 10, 1782.
- Radošević, A.; Kupinić, M.; Grlić, L.; Kupini, M. Antibiotic Activity of Various Types of Cannabis Resin. Nature 1962, 195, 1007–1009.
- Kabelik, V.J. Hanf (Cannabis sativa)—Antibiotisches Heilmittel. 1. Mitteilung: Hanf in der Alt- und Volksmedizin. Pharmazie 1958, 12, 439–443.
- Wasim, K.; Haq, I.; Ashraf, M. Antimicrobial studies of the leaf of Cannabis sativa L. Pak. J. Pharm. Sci. 1995, 8, 29–38.
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J. Med. Plants Res. 2008, 2, 98–110.
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kačániová, M.; Astatkie, T.; Dincheva, I. Grinding and Fractionation during Distillation Alter Hemp Essential Oil Profile and Its Antimicrobial Activity. Molecules 2020, 25, 3943.
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13.
- Viswanath, H.; Bhat, K.A.; Bhat, N.; Wani, T.; Mughal, M.N. Antibacterial Efficacy of Aqueous Plant Extracts against Storage Soft Rot of Potato Caused by Erwinia carotovora. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2630–2639.
- Nola, I.; Kostović, K.; Oremović, L.; Soldo-Belić, A.; Lugović, L. Candida infections today—How big is the problem? Acta Dermatovenerol. Croat. 2003, 11, 171–177.
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254.
- Dabur, R.; Singh, H.; Chhillar, A.; Ali, M.; Sharma, G. Antifungal potential of Indian medicinal plants. Fitoterapia 2004, 75, 389–391.
- Kuete, V. Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Med. 2010, 76, 1479–1491.
- Aboul-ela, M.A.; Bahaa, N.; Din, E. Antimicrobial Evaluation of Extracts from some Yemeni Plants. Alexander J. Pharm. Sci. 1995, 9, 35–37.
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Zulfiqar, F.; ElSohly, M.A. Cannabinoid Ester Constituents from High-Potency Cannabis sativa. J. Nat. Prod. 2008, 71, 536–542.
- Žitek, T.; Leitgeb, M.; Golle, A.; Dariš, B.; Knez, Ž.; Hrnčič, M.K. The Influence of Hemp Extract in Combination with Ginger on the Metabolic Activity of Metastatic Cells and Microorganisms. Molecules 2020, 25, 4992.
- Orlando, G.; Adorisio, S.; Delfino, D.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; D’Antonio, M.; Zengin, G.; Acquaviva, A.; et al. Comparative Investigation of Composition, Antifungal, and Anti-Inflammatory Effects of the Essential Oil from Three Industrial Hemp Varieties from Italian Cultivation. Antibiotics 2021, 10, 334.
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92.
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis ofCandida albicansbiofilm. Pathog. Dis. 2016, 74, ftw018.
- Khan, I.H.; Javaid, A. Antifungal activity of leaf extract of Cannabis sativa against Aspergillus flavipes. Pak. J. Weed Sci. Res. 2020, 26, 447–453.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
15 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




