
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rúben Fernandes | + 1148 word(s) | 1148 | 2021-11-02 06:42:20 | | | |
| 2 | Nora Tang | + 1819 word(s) | 2967 | 2021-12-15 09:07:43 | | |
Video Upload Options
Lifestyle interventions must be made with considerable involvement of clinicians, and it should be considered that not all patients will respond in the same manner. Individuals with a high risk of diabetic progression will present compensatory metabolic mechanisms, translated into metabolic biomarkers that will therefore show potential predictive value to differentiate between progressors/non-progressors in T2D. Specific novel biomarkers are being proposed to entrap prediabetes and target progressors to achieve better outcomes.
1. Introduction
According to the International Diabetes Federation, diabetes affects more than 463 million people, with type 2 diabetes (T2D) being the most common, accounting for around 90% of all diabetes worldwide in 2019 [1]. T2D was responsible for more than 4.2 million deaths in 2019, and is also a trigger for other non-communicable diseases, putting considerable pressure on national health systems [1]. T2D is associated with severe comorbidities, such as cardiovascular diseases (ischemic heart disease, myocardial infarction, peripheral arterial disease, heart failure, and stable angina being the most prevalent) [2], kidney diseases (such as glomerulosclerosis and glomerular hypertrophy inflammation/fibrosis, which ultimately lead to diabetic kidney disease) [3], and liver diseases (nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), liver failure, cirrhosis, and hepatocellular carcinoma) [4], while it also increases the possibility of developing several types of cancer (such as breast cancer, bladder cancer, pancreatic cancer, non-Hodgkin lymphoma, etc.) [5]. Outcomes of such comorbidities can be reduced with early intervention in the development of type 2 diabetes. In recent decades, there has been a massive effort and investment to find biomarkers that can detect T2D early and support the implementation of prophylactic measures.
One of the most important clinical symptoms of diabetes mellitus is hyperglycemia. Thus, monitoring blood glucose levels via a glycated hemoglobin assessment remains the most common screening method. However, when glucose levels are elevated, the disease is already active. Significant investments in research have allowed the identification of biomarkers that can be used to describe the progression from a subclinical to a clinical stage, and some biomarkers have been described as having potential predictive value to differentiate between progressors and non-progressors.
The critical threshold is prediabetes. Prediabetes is an asymptomatic disorder of the normoglycemia–hyperglycemia transitional state, when plasma glucose is above normal range but below clinical diabetes. Prediabetic subjects present either impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or both, as well as an increased risk of developing type 2 diabetes. Such metabolic alterations are already mentioned as being responsible for microvascular problems (such as retinopathy, nephropathy, and neuropathy—persistent complications among the hyperglycemic community) [6]. Whether prediabetes justifies clinical identification and intervention is still continuously debated among international professional organizations, and overall criteria remain without consensus. However, the importance of targeting prediabetes is relevant considering that the risk of developing diabetes can decrease by 40 to 70% with lifestyle alterations in prediabetic patients [7]. The main problem associated with prediabetes is that it may lead to overdiagnosis and, therefore, overtreatment. The pharmacotherapy associated with prediabetes can include antidiabetic drugs such as biguanides (e.g., metformin) or thiazolidinediones (e.g., rosiglitazone), and others, such as GLP-1 analogs or α-glucosidase inhibitors. In addition to pharmacotherapy, bariatric surgery (such as gastric bypass or sleeve gastrectomy) has already been studied in prediabetic patients, with positive results, such as the reversion of IGT to normal values in 98% of individuals [7].
At a prediabetic stage, several metabolic imbalances are already established, occurring before the clinical manifestations. Identifying these imbalances with adequate and precise biomarkers can facilitate early intervention. In America, one in every three individuals have prediabetes, and 11% will develop diabetes [8]. Worldwide, prediabetes is increasing, and the expectation is that, by 2030, the number of people with prediabetes will increase to more than 470 million. Each year, 5–10% will progress to diabetes and develop diabetic comorbidities, such as hypertension [9].
2. Development and Findings
A total of 145 total cumulative records were retrieved from PubMed, 13 of which were duplicates and, hence, immediately excluded. The title and abstract were examined for the remaining 132 records, following concordance assessment of the inclusion criteria and objectives, resulting in an additional exclusion of 103 records. Thus, 29 studies were identified as being eligible and relevant. All manuscripts were carefully studied, and biomarkers were identified and counted. The analysis of the results identified two approaches to novel prediabetic biomarkers: metabolomics [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30], and microRNA studies [31][32][33][34][35][36][37][38]. The results are shown in Figure 1 . Further ahead, Table 1 and Table 2 summarize the most relevant biomarkers’ descriptions, outcomes, advantages, and disadvantages.
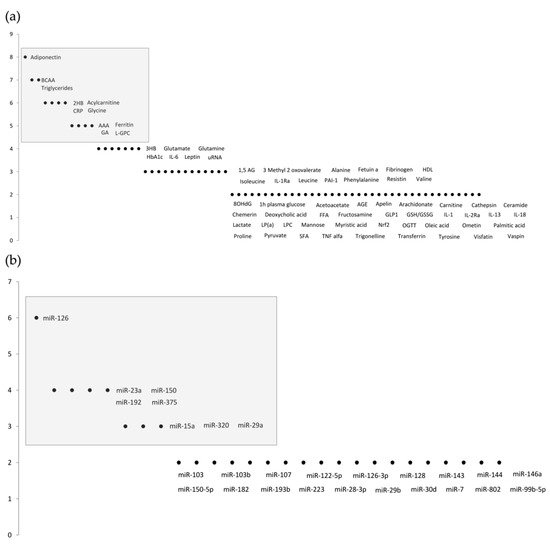
| Biomarker | Description/Outcomes | Advantages/Disadvantages | References |
|---|---|---|---|
| 2-Hydroxybutyrate (2HB) | 2HB is a metabolite of alpha-ketobutyrate synthesis produced in the threonine and methionine catabolism and glutathione anabolism; it is a predictive marker of hyperglycemia and beta-cell dysfunction; Elevated levels of 2HB are associated with insulin resistance, oxidative stress, lipid oxidation, and diabetic state aggravation. Decreased levels of 2HB were observed 6 months after bariatric surgery as a representative improvement of the pathology. | 2HB has proven to be a biomarker independent of sex, age, BMI, and collection site; however, it is still in a premature investigation stage. | [39][40][41][42] |
| Aromatic Amino Acids (AAAs) | AAAs, tyrosine, and phenylalanine are amino acids with an integrated aromatic ring. Phenylalanine is a precursor of tyrosine, and tyrosine is a precursor of catecholamines. Both tyrosine and phenylalanine are glucogenic and ketogenic amino acids. Increased levels of tyrosine and phenylalanine were observed in obesity-related insulin resistance, and predicted the development of T2D. After diabetic treatment with glipizide and metformin, AAA levels changed in accordance with the patient’s insulin resistance status. |
Different expression patterns of amino acids can be predictive of prediabetes in various cohorts. Additionally, significance can be altered after variable adjustment of body mass index (BMI), age, sex, race/ethnicity, and FPG levels. | [18][43][44] |
| Adiponectin | Adiponectin is a hormone secreted from the adipose tissue with insulin sensitivity, antidiabetic, anti-inflammatory, and anti-atherogenic properties. Adiponectin stimulates a broad spectrum of metabolic actions via ceramidase activation; it is directly correlated with insulin sensitivity, and inversely correlated with T2D development risk. Lower adiponectin levels were observed 10 years prior to T2D diagnosis. | A biomarker independent of ethnic differences, it can be affected by sex-specific mechanisms nevertheless. Certain studies do not corroborate the lower adiponectin levels in prediabetics compared with healthy individuals. |
[45][46][47][48] |
| Acylcarnitine | Acylcarnitines result from the conjugations of acyl-coenzyme A with carnitine conjugation for the transport of fatty acids through the inner mitochondrial membrane for beta-oxidation. They are associated with the NF-κB pathway, and can promote insulin resistance and inflammation. Acylcarnitine has shown to be higher in prediabetes due to the dysregulation of mitochondrial fatty acid oxidation. A panel of acylcarnitines was observed to be associated with T2D development in a 6-year follow-up. | Some acylcarnitines did not show any association with body fat or waist–hip ratio, fat mass, or fat distribution. Overall, they are independent biomarkers of traditional risk factors. | [49][50][51][52] |
| Branched-Chain Amino Acids (BCAAs) | BCAAs such as leucine, isoleucine, and valine are the most abundant and essential amino acids present in a regular diet. Accumulation of BCAAs activates via mTOR and, consequently, S6 kinase, which leads to serine phosphorylation of the substrate-1 (IRS–1) insulin receptor, causing insulin resistance. High levels of BCAAs are associated with obesity, insulin resistance, impaired glucose tolerance, and T2D. BCAA levels normalize after bariatric surgery. | Phenotypic and genetic factors can influence BCAA levels, which can reveal associations with both sex and BMI. There is still some debate on whether BCAAs are the cause or the effect and, as such, whether they should be considered a biomarker. | [53][54][55] |
| C-Reactive Protein (CRP) | CRP is an inflammatory biomarker of hepatic origin associated with the acute phase response; it responds to transcription factors released by macrophages and adipocytes. Higher CRP levels were found in patients with prediabetes and insulin resistance, rendering it a sensitive biomarker for early T2D diagnosis. These results may be a consequence of the low state of chronic inflammation grade found before the onset of type 2 diabetes. |
Association between CRP and prediabetes is independent of age, sex, ethnicity, alcohol consumption, smoking, hypertension, BMI, and total cholesterol. It is still in an early investigation stage for prediabetes signaling. | [56][57][58][59] |
| Ferritin | Ferritin is a protein (acute phase reactant) involved in iron storage, which is able to release iron in a controlled manner. Iron contributes to insulin resistance via many pathways, such as β-cell oxidative stress and β-cell apoptosis through ROS formation. Iron metabolism seems to be correlated with T2D status: uncontrolled T2D is associated with iron deficiency. High ferritin levels translate to an increased risk of developing T2D. Dietary restriction and chelation may prevent T2D progression. | The threshold level is still uncertain, and may vary according to age and sex. Ferritin levels are predictive of diabetes progression independently of a comprehensive range of risk factors, such as physical activity, smoking, and family history. | [60][61][62][63] |
| Glycated Albumin (GA) | Albumin is the most commonly studied soluble protein, and is highly susceptible to post-translational modifications (PTMs). One frequent modification is glycation, resulting in GA. GA plays a vital role in diabetic pathophysiology; it is inversely correlated with obesity and positively correlated with diabetes. The increase observed in diabetes is associated with secondary comorbidities. GA can act as an antigen, elicit the immune response, and form complexes that can accumulate in the arteries and kidneys, leading to nephropathy and atherosclerosis. | Accurate assessment for short-term glycemic control. The enzymatic method is sensitive, fast, and less susceptible to pre-analytical variables. Values of GA are not reliable in individuals with abnormal albumin metabolism. | [22][64][65][66][67] |
| Glycine | Glycine is a nonessential stable amino acid, able to be synthesized by the body from serine. Glycine is a precursor of protein metabolism, and can act as a neurotransmitter and as a co-ligand for N-methyl-D-aspartate glutamate receptors to control insulin secretion and liver glucose output, functioning on both the pancreas and the brain. Lower glycine levels are associated with an increased risk of prediabetes, type 2 diabetes, and obesity, and are also correlated with insulin resistance and glucose intolerance. | Glycine levels are not dependent exclusively on glycemic status, and may vary in individuals with abnormal amino acid metabolisms or metabolic syndrome. | [10][18][23][68] |
| Linoleoyl-glycerophosphocholine (LGPC) | Linoleoyl-glycerophosphocholine (LGPC) is a metabolite of the phospholipase A2 hepatic enzyme and lecithin-cholesterol acyltransferase. Known for its anti-inflammatory properties, it acts as a non-competitive enzyme inhibitor of phospholipase A2, usually increasing during the inflammatory state. This metabolite’s plasma concentration is associated with an increased risk of developing insulin resistance, impaired glucose tolerance, and diabetes. | Independent of age, sex, body mass index, familial diabetes, fasting glucose, waist circumference, blood pressure, glycosylated hemoglobin, triglycerides, and high-density lipoprotein cholesterol. | [21][69] |
| Triglycerides | Triglycerides are the most common lipids present in the body, and are composed of three fatty acids and a glycerol molecule. They are often an indication of conditions such as obesity and metabolic dysfunction. High levels of triglycerides are associated with diabetic progression, beta-cell dysfunction, and impaired insulin secretion. Studies have demonstrated that the product of triglycerides and glucose is able to discriminate prediabetes and diabetes, and triglyceride levels can be improved with physical activity and, therefore, improve glycemic status. | Triglycerides have already been implemented in clinical practice. In prediabetic individuals, high levels of triglycerides are a predictive factor for T2D progression. Studies found variations between different ethnicities. | [70][71][72] |
| miRNAs | Description/Outcomes | References |
|---|---|---|
| miRNA-15a | miRNA-15a is associated with several biological processes, such as angiogenesis and insulin production; it is also involved in the activation of TGFβR1, CTGF, and p53 proteins. Lower miRNA-15a levels were found in individuals who developed T2D in a 10-year follow-up. The association between miRNA-15a and diabetic progression was still significant after variable adjustment for age, sex, BMI, and hypertension status. |
[73][74] |
| miRNA-23a | miRNA-23a indirectly targets SMAD4—a critical pathway in the regulation of insulin-dependent glucose transport activity. NEK7 is also a target of miRNA-23a and, in animal models, a low level of NLRP3 induced pyroptosis, mitigating the hepatic and renal complications of T2D. The levels of miRNA-23a are lower in prediabetic and T2D patients compared with healthy individuals. Levels of miRNA-23a can also distinguish prediabetic and T2D patients. |
[75][76] |
| miRNA-29a | miRNA-29a was observed to improve pancreatic beta-cell function in in vitro studies. Likewise, upregulation of miRNA-29a is implicated in diabetic progression by IGT and decreased insulin secretion. Higher expression of miRNA-29a is an independent predictor of T2D, IFG, and IR. Additionally, it is significantly correlated with stress hormone levels. |
[77][78] |
| miRNA-126 | One of the most studied miRNAs in prediabetic conditions, it is highly correlated with VEGF, and with the promotion of angiogenesis. Anti-miRNA-126 targets SPRED-1 via Ras/ERK/VEGF and PI3K/Akt/eNOS, inhibiting the proliferation and migration of endothelial progenitor cells and promoting apoptosis. Low levels of miRNA-126 are strongly correlated with the progression of the disease. |
[79][80] |
| miRNA-150 | Previous miRNA-150 studies demonstrated its regulatory function in beta-cell formation, hematopoietic stem cell differentiation, and obesity-induced inflammation and insulin resistance by controlling adipose tissue and beta-cell function. In the CORDIOPREV study, prediabetic progressors were evaluated in a 5-year follow-up; miRNA-150 levels were higher in plasma several years before the diagnosis of T2D. |
[81][82] |
| miRNA-192 | miRNA-192 is involved in IFG and IGT, triglyceride levels, and the fatty liver index. Moreover, miRNA-192 inhibited the proliferation of pancreatic beta-cell lines and insulin secretion. Levels of miRNA-192 are found to be higher in diabetic subjects. Interestingly, vitamin D supplementation modulates miRNA-192 levels, improving the hyperglycemic status in prediabetic patients. |
[83][84][85] |
| miRNA-320 | Expression of miRNA-320 is associated with VEGF, IGF1, and FGF. The VEGFa/miRNA-320 axis modulates proliferation, apoptosis, and angiogenesis of endothelial cells, and has been reported to be an active player in diabetic progression. miRNA-320 is positively correlated with prediabetic incidence, and improves diabetic progression via adipoR1 after duodenal–jejunal bypass. |
[86][87][88] |
| miRNA-375 | miRNA-375 is a pancreatic-islet-specific miRNA involved in regulating insulin secretion and maintaining average pancreatic alpha and beta-cell mass. miRNA-375 levels are higher and independently associated in prediabetic and diabetic individuals. Deregulation of miRNA-375 was observed years before the onset of T2D in the CORDIOPREV trial. |
[89][90][91] |
Metabolomics is a high-throughput technique that enables the identification and quantification of small molecules present in biological samples such as blood, urine, and tissue. Metabolomics is increasingly used to address metabolic dysregulation associated with prediabetes ( Figure 2 ). The method used in metabolomics combines analytical chemistry and data analysis with spectroscopic/spectrometric techniques (such as mass spectrometry or nuclear magnetic resonance) and separation techniques (such as gas chromatography, high-performance liquid chromatography (HPLC), ultra-HPLC, etc.) in a profitable, high-yield manner.
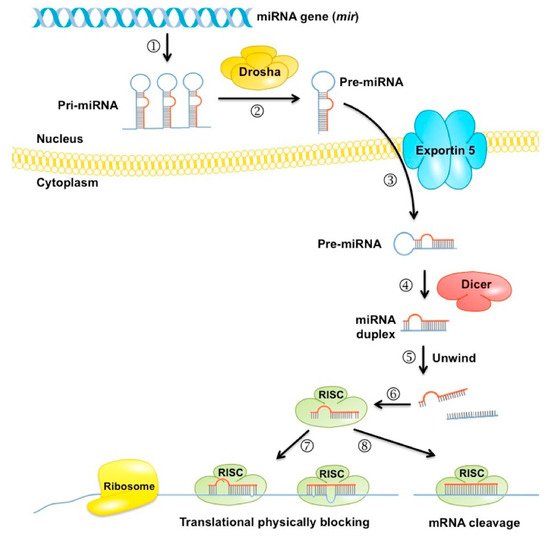
miRNAs are small, non-coding RNAs involved in post-transcriptional gene expression ( Figure 3 ); they can modulate important biological mechanisms such as growth and proliferation, differentiation, and cell death. Research on miRNAs is more recent than metabolomics research, leading to the belief that there is still much to be uncovered. miRNAs are becoming increasingly prominent in many pathologies, including prediabetic studies. Interestingly enough, different miRNA profiles were found in healthy, prediabetic, and diabetic individuals [38].
miRNAs associated with diabetic progression have different types of correlation according to their miRNA-specific function. Moreover, miRNAs can predict diabetic complications such as cardiovascular diseases, chronic renal disease, or retinopathy. They display consistent and reproducible circulating levels, and are stable and resistant to RNase activity—essential characteristics in biomarker assessment. Previous studies concluded that diabetes-related miRNA does not change dramatically in the prediabetic stage [38]. Moreover, due to a wide range of prediabetic-associated miRNAs, choosing a set of representative prediabetic biomarkers is challenging [92][93][94].
3. Conclusions
Diabetes is still one of the most challenging health problems worldwide. Programs for prevention and awareness of diabetes have proven to be insufficient to stop this pandemic; hence, clinical intervention could be the answer to avoid diabetic progression by targeting prediabetes. The growing attention to novel glycemic biomarkers is attributable to the limitations demonstrated by both HbA1c and OGTT.
From the present study, our interpretation is that these biomarkers are the ones that, so far, are at a more advanced research stage and, thus, are more promising for clinical implementation. However, many other biomarkers have been the target of research in diabetes (such as ophthalmate or galectin-3) with positive results, demonstrating the continuous effort of the academic community to find, comprehend, and interpret new and reliable molecules for the assessment of the (pre)diabetic pathology.
We believe that a biomarker multiplex is the most effective solution to achieve better sensitivity and specificity in predicting progressors in T2D. Such an achievement would improve patients’ health and decrease the national system’s burden regarding diabetes. Moreover, low-cost, effective interventions in the form of lifestyle changes would be sufficient to diminish drug/surgery-based clinical interventions.
References
- IDF. Diabetes Atlas. Int. Diabetes Fed. 2019, 1, 10–15.
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Rodriguez, M.P.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113.
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045.
- Tolman, K.G.; Fonseca, V.; Dalpiaz, A.; Tan, M.H. Spectrum of Liver Disease in Type 2 Diabetes and Management of Patients with Diabetes and Liver Disease. Diabetes Care 2007, 30, 734–743.
- Collins, K.K. The Diabetes-Cancer Link. Diabetes Spectr. 2014, 27, 276–280.
- Buysschaert, M.; Bergman, M. Definition of Prediabetes. Med. Clin. N. Am. 2011, 95, 289–297.
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303.
- Keck, J.W.; Thomas, A.R.; Hieronymus, L.; Roper, K.L. Prediabetes Knowledge, Attitudes, and Practices at an Academic Family Medicine Practice. J. Am. Board Fam. Med. 2019, 32, 505–512.
- Alderman, M.H. Prediabetes: An unexplored cardiovascular disease risk factor. J. Hypertens. 2021, 39, 42–43.
- Long, J.; Yang, Z.; Wang, L.; Han, Y.; Peng, C.; Yan, C.; Yan, D. Metabolite biomarkers of type 2 diabetes mellitus and pre-diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 1–17.
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233.
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharm. 2019, 70, 809–824.
- Thorens, B.; Rodriguez, A.; Cruciani-Guglielmacci, C.; Wigger, L.; Ibberson, M.; Magnan, C. Use of preclinical models to identify markers of type 2 diabetes susceptibility and novel regulators of insulin secretion—A step towards precision medicine. Mol. Metab. 2019, 27, S147–S154.
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467.
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2019, 2019, 1–17.
- Diwaker, A.; Kishore, D.; Singh, V.; Mahapatra, S.P. The Novel Biomarkers in Diabetes. J. Assoc. Physicians India 2019, 67, 65–69.
- Jagannathan, R.; Buysschaert, M.; Medina, J.L.; Katz, K.; Musleh, S.; Dorcely, B.; Bergman, M. The 1-h post-load plasma glucose as a novel biomarker for diagnosing dysglycemia. Acta Diabetol. 2018, 55, 519–529.
- Gar, C.; Rottenkolber, M.; Prehn, C.; Adamski, J.; Seissler, J.; Lechner, A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 2017, 55, 21–32.
- Maghsoudi, A.S.; Vakhshiteh, F.; Torabi, R.; Hassani, S.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.; Abdollahi, M. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens. Bioelectron. 2018, 99, 122–135.
- Liggi, S.; Griffin, J.L. Metabolomics applied to diabetes—Lessons from human population studies. Int. J. Biochem. Cell Biol. 2017, 93, 136–147.
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 345–361.
- Bhat, S.; Jagadeeshaprasad, M.G.; Venkatasubramani, V.; Kulkarni, M.J. Abundance matters: Role of albumin in diabetes, a proteomics perspective. Expert Rev. Proteom. 2017, 14, 677–689.
- Yan-Do, R.; MacDonald, P. Impaired “Glycine”-mia in Type 2 Diabetes and Potential Mechanisms Contributing to Glucose Homeostasis. Endocrinology 2017, 158, 1064–1073.
- Larsen, M.P.; Torekov, S.S. Glucagon-Like Peptide 1: A Predictor of Type 2 Diabetes? J. Diabetes Res. 2017, 2017, 1–13.
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846.
- Ribeiro, R.T.; Macedo, M.P.; Raposo, J.F. HbA1c, Fructosamine, and Glycated Albumin in the Detection of Dysglycaemic Conditions. Curr. Diabetes Rev. 2015, 12, 14–19.
- Urpi-Sarda, M.; Almanza-Aguilera, E.; Tulipani, S.; Tinahones, F.J.; Salas-Salvadó, J.; Andres-Lacueva, C. Metabolomics for Biomarkers of Type 2 Diabetes Mellitus: Advances and Nutritional Intervention Trends. Curr. Cardiovasc. Risk Rep. 2015, 9, 1–12.
- Dunmore, S.J.; Brown, J.E.P. The role of adipokines in β-cell failure of type 2 diabetes. J. Endocrinol. 2012, 216, T37–T45.
- Lyons, T.J.; Basu, A. Biomarkers in diabetes: Hemoglobin A1c, vascular and tissue markers. Transl. Res. 2012, 159, 303–312.
- Gjesing, A.P.; Pedersen, O. ‘Omics’-driven discoveries in prevention and treatment of type 2 diabetes. Eur. J. Clin. Investig. 2012, 42, 579–588.
- González-Sánchez, L.E.; Ortega-Camarillo, C.; Contreras-Ramos, A.; Barajas-Nava, L.A. miRNAs as biomarkers for diagnosis of type 2 diabetes: A systematic review. J. Diabetes 2021, 13, 792–816.
- Athira, S.; Misra, P.; Bhatia, K.; Sibin, M. Identification of circulatory miRNAs as candidate biomarkers in prediabetes—A systematic review and bioinformatics analysis. Gene Rep. 2020, 21, 100954.
- Pielok, A.; Marycz, K. Non-Coding RNAs as Potential Novel Biomarkers for Early Diagnosis of Hepatic Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 4182.
- Vasu, S.; Kumano, K.; Darden, C.M.; Rahman, I.; Lawrence, M.C.; Naziruddin, B. MicroRNA Signatures as Future Biomarkers for Diagnosis of Diabetes States. Cells 2019, 8, 1533.
- Zhang, W.; Zheng, J.; Hu, X.; Chen, L. Dysregulated expression of long noncoding RNAs serves as diagnostic biomarkers of type 2 diabetes mellitus. Endocrine 2019, 65, 494–503.
- Vaishya, S.; Sarwade, R.D.; Seshadri, V. MicroRNA, Proteins, and Metabolites as Novel Biomarkers for Prediabetes, Diabetes, and Related Complications. Front. Endocrinol. 2018, 9, 180.
- Ashoori, M.R.; Rahmati-Yamchi, M.; Ostadrahimi, A.; Aval, S.F.; Zarghami, N. MicroRNAs and adipocytokines: Promising biomarkers for pharmacological targets in diabetes mellitus and its complications. Biomed. Pharmacother. 2017, 93, 1326–1336.
- Raffort, J.; Hinault, C.; Dumortier, O.; Van Obberghen, E. Circulating microRNAs and diabetes: Potential applications in medical practice. Diabetologia 2015, 58, 1978–1992.
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. α-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883.
- Landaas, S. The formation of 2-hydroxybutyric acid in experimental animals. Clin. Chim. Acta 1975, 58, 23–32.
- Cobb, J.; Eckhart, A.; Perichon, R.; Wulff, J.; Mitchell, M.; Adam, K.-P.; Wolfert, R.; Button, E.; Lawton, K.; Elverson, R.; et al. A Novel Test for IGT Utilizing Metabolite Markers of Glucose Tolerance. J. Diabetes Sci. Technol. 2015, 9, 69–76.
- Tricò, D.; Prinsen, H.; Giannini, C.; De Graaf, R.; Juchem, C.; Li, F.; Caprio, S.; Santoro, N.; Herzog, R.I. Elevated α-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. J. Clin. Endocrinol. Metab. 2017, 102, 2473–2481.
- Owei, I.; Umekwe, N.; Stentz, F.; Wan, J.; Dagogo-Jack, S. Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism 2019, 98, 76–83.
- Walford, G.A.; Davis, J.; Warner, A.S.; Ackerman, R.J.; Billings, L.K.; Chamarthi, B.; Fanelli, R.R.; Hernandez, A.M.; Huang, C.; Khan, S.Q.; et al. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism 2013, 62, 1772–1778.
- Saltevo, J.; Kautiainen, H.; Vanhala, M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend. Med. 2009, 6, 463–470.
- Jiang, Y.; Owei, I.; Wan, J.; Ebenibo, S.; Dagogo-Jack, S. Adiponectin levels predict prediabetes risk: The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res. Care 2016, 4, e000194.
- Stefan, N.; Sun, Q.; Fritsche, A.; Machann, J.; Schick, F.; Gerst, F.; Jeppesen, C.; Joost, H.-G.; Hu, F.B.; Boeing, H.; et al. Impact of the Adipokine Adiponectin and the Hepatokine Fetuin-A on the Development of Type 2 Diabetes: Prospective Cohort- and Cross-Sectional Phenotyping Studies. PLoS ONE 2014, 9, e92238.
- Lai, H.; Lin, N.; Xing, Z.; Weng, H.; Zhang, H. Association between the level of circulating adiponectin and prediabetes: A meta-analysis. J. Diabetes Investig. 2015, 6, 416–429.
- Zhang, X.; Zhang, C.; Chen, L.; Han, X.; Ji, L. Human serum acylcarnitine profiles in different glucose tolerance states. Diabetes Res. Clin. Pract. 2014, 104, 376–382.
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum Levels of Acylcarnitines Are Altered in Prediabetic Conditions. PLoS ONE 2013, 8, e82459.
- Adams, S.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.; Garvey, W.T. Plasma Acylcarnitine Profiles Suggest Incomplete Long-Chain Fatty Acid β-Oxidation and Altered Tricarboxylic Acid Cycle Activity in Type 2 Diabetic African-American Women. J. Nutr. 2009, 139, 1073–1081.
- Sun, L.; Liang, L.; Gao, X.; Zhang, H.; Yao, P.; Hu, Y.; Ma, Y.; Wang, F.; Jin, Q.; Li, H.; et al. Early Prediction of Developing Type 2 Diabetes by Plasma Acylcarnitines: A Population-Based Study. Diabetes Care 2016, 39, 1563–1570.
- Ruiz-Canela, M.; Guasch-Ferré, M.; Toledo, E.; Clish, C.B.; Razquin, C.; Liang, L.; Wang, D.D.; Corella, D.; Estruch, R.; Hernáez, Á.; et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: Case-cohort study within the PREDIMED Trial. Diabetologia 2018, 61, 1560–1571.
- Chen, X.; Yang, W. Branched-chain amino acids and the association with type 2 diabetes. J. Diabetes Investig. 2015, 6, 369–370.
- Yemelyanov, A. Branched Chain Amino Acids and Risk of Type 2 Diabetes Mellitus: A Literature Review. Master’s Thesis, Georgia State University, Atlanta, GA, USA, 2021.
- Festa, A.; Hanley, A.J.; Tracy, R.P.; D’Agostino, R.; Haffner, S.M. Inflammation in the Prediabetic State Is Related to Increased Insulin Resistance Rather Than Decreased Insulin Secretion. Circulation 2003, 108, 1822–1830.
- Van Woudenbergh, G.J.; Kuijsten, A.; Sijbrands, E.J.G.; Hofman, A.; Witteman, J.C.M.; Feskens, E.J.M. Glycemic Index and Glycemic Load and Their Association with C-Reactive Protein and Incident Type 2 Diabetes. J. Nutr. Metab. 2011, 2011, 1–7.
- Sabanayagam, C.; Shankar, A.; Lim, S.C.; Lee, J.; Tai, E.S.; Wong, T.Y. Serum C-reactive protein level and prediabetes in two Asian populations. Diabetologia 2011, 54, 767–775.
- Grossmann, V.; Schmitt, V.H.; Zeller, T.; Panova-Noeva, M.; Schulz, A.; Laubert-Reh, D.; Juenger, C.; Schnabel, R.B.; Abt, T.G.; Laskowski, R.; et al. Profile of the Immune and Inflammatory Response in Individuals with Prediabetes and Type 2 Diabetes. Diabetes Care 2015, 38, 1356–1364.
- Sharifi, F.; Nasab, N.M.; Zadeh, H.J. Elevated serum ferritin concentrations in prediabetic subjects. Diabetes Vasc. Dis. Res. 2008, 5, 15–18.
- Huang, J.; Jones, D.; Luo, B.; Sanderson, M.; Soto, J.; Abel, E.D.; Cooksey, R.C.; McClain, N.A. Iron Overload and Diabetes Risk: A Shift from Glucose to Fatty Acid Oxidation and Increased Hepatic Glucose Production in a Mouse Model of Hereditary Hemochromatosis. Diabetes 2010, 60, 80–87.
- Kunutsor, S.K.; Apekey, T.A.; Walley, J.; Kain, K. Ferritin levels and risk of type 2 diabetes mellitus: An updated systematic review and meta-analysis of prospective evidence. Diabetes/Metab. Res. Rev. 2013, 29, 308–318.
- Forouhi, N.G.; Harding, A.H.; Allison, M.; Sandhu, M.S.; Welch, A.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Elevated serum ferritin levels predict new-onset type 2 diabetes: Results from the EPIC-Norfolk prospective study. Diabetology 2007, 50, 949–956.
- Lee, J.-E. Alternative biomarkers for assessing glycemic control in diabetes: Fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 74–78.
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and Pitfalls of Fructosamine and Glycated Albumin in the Diagnosis and Treatment of Diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176.
- Parrinello, C.M.; Selvin, E. Beyond HbA1c and Glucose: The Role of Nontraditional Glycemic Markers in Diabetes Diagnosis, Prognosis, and Management. Curr. Diabetes Rep. 2014, 14, 1–10.
- Selvin, E.; Rawlings, A.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: A prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014, 2, 279–288.
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615.
- Pérez-Matos, M.C.; Morales, M.; Toloza, F.J.K.; Ricardo-Silgado, M.L.; Mantilla-Rivas, J.O.; Pinzón-Cortes, J.A.; Perez-Mayorga, M.; Jiménez, E.; Guevara, E.; Mendivil, C.O. The Phospholipid Linoleoylglycerophosphocholine as a Biomarker of Directly Measured Insulin Resistance. Diabetes Metab. J. 2017, 41, 466–473.
- Ahn, N.; Baumeister, S.E.; Amann, U.; Rathmann, W.; Peters, A.; Huth, C.; Thorand, B.; Meisinger, C. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci. Rep. 2019, 9, 1–11.
- Hamasaki, H.; Noda, M.; Moriyama, S.; Yoshikawa, R.; Katsuyama, H.; Sako, A.; Mishima, S.; Kakei, M.; Ezaki, O.; Yanai, H. Daily Physical Activity Assessed by a Triaxial Accelerometer Is Beneficially Associated with Waist Circumference, Serum Triglycerides, and Insulin Resistance in Japanese Patients with Prediabetes or Untreated Early Type 2 Diabetes. J. Diabetes Res. 2015, 2015, 1–6.
- Shimodaira, M.; Niwa, T.; Nakajima, K.; Kobayashi, M.; Hanyu, N.; Nakayama, T. Serum Triglyceride Levels Correlated with the Rate of Change in Insulin Secretion Over Two Years in Prediabetic Subjects. Ann. Nutr. Metab. 2014, 64, 38–43.
- Al-Kafaji, G.; Al-Mahroos, G.; Alsayed, N.A.; Hasan, Z.A.; Nawaz, S.; Bakhiet, M. Peripheral blood microRNA-15a is a potential biomarker for type 2 diabetes mellitus and pre-diabetes. Mol. Med. Rep. 2015, 12, 7485–7490.
- Rawal, S.; Munasinghe, P.E.; Nagesh, P.T.; Lew, J.K.S.; Jones, G.T.; Williams, M.; Davis, P.; Bunton, D.; Galvin, I.F.; Manning, P.; et al. Down-regulation of miR-15a/b accelerates fibrotic remodelling in the Type 2 diabetic human and mouse heart. Clin. Sci. 2017, 131, 847–863.
- Chang, H.; Chang, H.; Cheng, T.; Lee, G.D.; Chen, X.; Qi, K. Micro-ribonucleic acid-23a-3p prevents the onset of type 2 diabetes mellitus by suppressing the activation of nucleotide-binding oligomerization-like receptor family pyrin domain containing 3 inflammatory bodies-caused pyroptosis through negatively regulating NIMA-related kinase 7. J. Diabetes Investig. 2021, 12, 334–345.
- Yang, Z.; Chen, H.; Si, H.; Li, X.; Ding, X.; Sheng, Q.; Chen, P.; Zhang, H. Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta Diabetol. 2014, 51, 823–831.
- Bagge, A.; Clausen, T.R.; Larsen, S.; Ladefoged, M.; Rosenstierne, M.W.; Larsen, L.; Vang, O.; Nielsen, J.H.; Dalgaard, L. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem. Biophys. Res. Commun. 2012, 426, 266–272.
- Liang, Y.-Z.; Dong, J.; Zhang, J.; Wang, S.; He, Y.; Yan, Y.-X. Identification of Neuroendocrine Stress Response-Related Circulating MicroRNAs as Biomarkers for Type 2 Diabetes Mellitus and Insulin Resistance. Front. Endocrinol. 2018, 9, 132.
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The Role of Circulating MicroRNA-126 (miR-126): A Novel Biomarker for Screening Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577.
- Zhang, T.; Lv, C.; Li, L.; Chen, S.; Liu, S.; Wang, C.; Su, B. Plasma miR-126 Is a Potential Biomarker for Early Prediction of Type 2 Diabetes Mellitus in Susceptible Individuals. BioMed Res. Int. 2013, 2013, 761617.
- Jiménez-Lucena, R.; Camargo, A.; Alcala-Diaz, J.F.; Romero-Baldonado, C.; Luque, R.M.; Van Ommen, B.; Delgado-Lista, J.; Ordovás, J.M.; Pérez-Martínez, P.; Rangel-Zuñiga, O.A.; et al. A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: From the CORDIOPREV study. Exp. Mol. Med. 2018, 50, 1–12.
- Ying, W.; Tseng, A.; Chang, R.C.-A.; Wang, H.; Lin, Y.-L.; Kanameni, S.; Brehm, T.; Morin, A.; Jones, B.; Splawn, T.; et al. miR-150 regulates obesity-associated insulin resistance by controlling B cell functions. Sci. Rep. 2016, 6, 20176.
- Parrizas, M.; Brugnara, L.; Esteban, Y.; Gonzalez-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; Garcia-Roves, P.M.; et al. Circulating miR-192 and miR-193b Are Markers of Prediabetes and Are Modulated by an Exercise Intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415.
- Lopez, Y.N.; Pittas, A.G.; Pratley, R.E.; Seyhan, A.A. Circulating levels of miR-7, miR-152 and miR-192 respond to vitamin D supplementation in adults with prediabetes and correlate with improvements in glycemic control. J. Nutr. Biochem. 2017, 49, 117–122.
- Pan, W.; Zhang, Y.; Zeng, C.; Xu, F.; Yan, J.; Weng, J. miR-192 is upregulated in T1DM, regulates pancreatic β-cell development and inhibits insulin secretion through suppressing GLP-1 expression. Exp. Ther. Med. 2018, 16, 2717–2724.
- Wei, G.; Yi, S.; Yong, D.; Shaozhuang, L.; Guangyong, Z.; Sanyuan, H. miR-320 mediates diabetes amelioration after duodenal-jejunal bypass via targeting adipoR1. Surg. Obes. Relat. Dis. 2018, 14, 960–971.
- Gao, J.; Ailifeire, M.; Wang, C.; Luo, L.; Zhang, J.; Yuan, L.; Zhang, L.; Li, X.; Wang, M. miR-320/VEGFA axis affects high glucose-induced metabolic memory during human umbilical vein endothelial cell dysfunction in diabetes pathology. Microvasc. Res. 2019, 127, 103913.
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int. J. Biol. Sci. 2021, 17, 402–416.
- Poy, M.N.; Hausser, J.; Trajkovski, M.; Braun, M.; Collins, S.; Rorsman, P.; Zavolan, M.; Stoffel, M. miR-375 maintains normal pancreatic-and-cell mass. Proc. Natl. Acad. Sci. USA 2009, 106, 5813–5818.
- Li, Y.; Xu, X.; Liang, Y.; Liu, S.; Xiao, H.; Li, F.; Cheng, H.; Fu, Z. miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression. Int. J. Clin. Exp. Pathol. 2010, 3, 254–264.
- Al-Muhtaresh, H.A.; Al-Kafaji, G. Evaluation of Two-Diabetes Related microRNAs Suitability as Earlier Blood Biomarkers for Detecting Prediabetes and type 2 Diabetes Mellitus. J. Clin. Med. 2018, 7, 12.
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2010, 48, 61–69.
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006.
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum MicroRNAs Are Promising Novel Biomarkers. PLoS ONE 2008, 3, e3148.




