Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qiuxia Huang | + 3429 word(s) | 3429 | 2021-12-13 03:00:33 | | | |
| 2 | Bruce Ren | Meta information modification | 3429 | 2021-12-14 02:31:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Huang, Q. Periodontal Bifunctional Biomaterials. Encyclopedia. Available online: https://encyclopedia.pub/entry/17065 (accessed on 08 February 2026).
Huang Q. Periodontal Bifunctional Biomaterials. Encyclopedia. Available at: https://encyclopedia.pub/entry/17065. Accessed February 08, 2026.
Huang, Qiuxia. "Periodontal Bifunctional Biomaterials" Encyclopedia, https://encyclopedia.pub/entry/17065 (accessed February 08, 2026).
Huang, Q. (2021, December 13). Periodontal Bifunctional Biomaterials. In Encyclopedia. https://encyclopedia.pub/entry/17065
Huang, Qiuxia. "Periodontal Bifunctional Biomaterials." Encyclopedia. Web. 13 December, 2021.
Copy Citation
Periodontitis is a chronic infectious disease that destroys periodontal supportive tissues and eventually causes tooth loss. It is attributed to microbial and immune factors. The goal of periodontal therapy is to achieve complete alveolar bone regeneration while keeping inflammation well-controlled. To reach this goal, many single or composite biomaterials have been developed that can produce any two of the antibacterial, anti-inflammatory, and periodontal tissue regeneration effects, such as antibacterial and bone regeneration effects. They are called bifunctional biomaterials.
periodontitis
antibacterial
bone regeneration
biomaterials
1. Introduction
Periodontitis is a common chronic infectious disease, and severe periodontitis affects 10.8% of the population in the world [1]. It is the primary cause of tooth loss in adults [2]. It causes irreversible damage to periodontal soft and hard tissues, including alveolar bone. Furthermore, it is closely related to many systemic diseases, including cardiovascular disease, diabetes, etc. [3]. The etiology of periodontitis is complex, involving host response, genetic, environmental and microbial factors [4]. A recent study suggested that vitamin D levels might be related to periodontitis [5] and a pilot study shed light on further exploring the link between metabolic syndrome and periodontitis [6]. Overall, the generally accepted pathogenesis is an imbalance of oral symbiotic microbial groups, along with host immune defense [4]. Moreover, the accumulation of bacteria related to periodontitis hinders tissue healing and regeneration processes [7][8].
Many efforts have been made to improve periodontal treatment outcome. Typical treatment of periodontitis focuses on pathological process and reaches a satisfactory outcome [9][10][11]. However, effectively regenerating periodontal tissues remains a challenge [12]. The purpose of periodontal therapy is to realize periodontal tissue regeneration after controlling inflammation via infection clearance [13]. To reach this goal, bioactive materials with antibacterial and osteogenic properties are in constant evolution. They are classified as bioactive agents, guided tissue regeneration/guided bone regeneration (GTR/GBR) membranes, tissue engineering scaffolds and drug delivery systems according to different patterns of action. Bioactive agents and drug delivery systems may be applied under the gingiva. GTR/GBR membranes are barriers covering periodontal defect and protecting the osteogenesis space from epithelial invasion. Tissue engineering scaffolds are applied to fill the periodontal defect.
2. Biomaterials
Periodontal biomaterials fight against pathogenic bacteria and promote alveolar bone reconstruction in lesions or bone defects. These biomaterials have different forms, such as agents, membranes, particles, pastes and gels. All these materials are used alone or in combination, with different performances, advantages and limitations in periodontal treatments. The summary of periodontal biomaterials is shown in Table 1.
2.1. Bioactive Agents
Periodontitis is a complex chronic disease, and comprehensive agents with different functions are of great demand for treatment [14]. Bioactive agents are either biological or synthetic, with both antibacterial and osteogenic activity. As potential therapeutic agents, they are prospective to improve the outcome of periodontal treatment.
In vitro and in vivo experiments, and even clinical trials have proven bioactive agents’ effects. In vitro experiments showed that they presented antibacterial activity against periodontal pathogens such as Porphyromonas gingivalis (P. gingivalis) and Streptococcus mutans (S. mutans). In addition, they promoted proliferation or osteogenic differentiation of cells responsible for periodontal regeneration, including human periodontal ligament fibroblasts (hPDLFCs), human periodontal ligament cells (hPDLCs) and bone marrow mesenchymal stem cells (BMSCs) [8][11][14][15]. In a mouse periodontitis model, psoralen and angelicin were able to reduce bone loss [14]. Particularly, 0.75% boric acid gel (BA) was placed under the gingiva as an adjunct to mechanical therapy in a clinical trial [15]. The results demonstrated that BA could generate some benefits, regarding clinical and radiographic indices. Furthermore, some agents could regulate inflammation and immune response, showing antioxidant activity [11][14][15].
Although bioactive agents have some advantages including safety, bifunction and convenient access, there are some limitations. For example, the efficacy of specific ingredients in some agents remains a mystery. To promote clinical application, further research is required to investigate the mechanisms and action modes in periodontal healing and regeneration processes. Further research is also necessary to be carried out in vivo.
2.2. GTR/GBR Membranes
The GTR/GBR membrane functions as a cell barrier to prevent the invasion of epithelium and connective tissues, facilitating the regeneration of periodontal ligament, cementum and alveolar bone [16]. At present, commercially available GTR/GBR membranes possess poor biocompatibility, poor stability and mechanical properties, fast degradation rate, and limited antibacterial and regeneration ability [16][17][18][19]. In the process of bone healing, infection caused by the colonization of pathogens at the defect site is one of the main causes of GBR failure [20]. To overcome these drawbacks, composite GTR/GBR membranes with different antibacterial and osteogenesis components have been designed, and present different performances [18][21][22]. They have great potential for periodontal treatment and may become promising alternative materials for GTR/GBR.
The antibacterial components of the GTR/GBR membranes mainly include antibiotics [23][24][25][26], nanoparticles of metals and their compounds [9][17][18][21][22][27], antimicrobial peptides (AMP) [28], and others. In clinic, antibiotics are commonly applied to resist the infection caused by oral pathogens and maintain a good healing environment. When encapsulated in inorganic nano carriers, their toxicity can be reduced [26]. In the microenvironment, a slow release of antibiotics can be maintained, as well as local effective concentration [20]. At present, minocycline [20], azithromycin [29], amoxicillin [23][30], doxycycline (DOX) [24], ornidazole [25] and metronidazole [26] are the main antibiotics used in the composite membranes. However, bacterial resistance of antibiotics has urged scientists to consider new antibacterial components [29]. Due to excellent antibacterial performance and little resistance, nanoparticles of metals and their compounds such as silver nanoparticles (AgNPs) and AMP have caught people’s attention [9][17][18][21][22][27]. Both of them rarely induce bacterial resistance due to multiple mechanisms. For example, they release metal ions, destroy the cell wall and membrane, penetrate the membrane and then disrupt intracellular activity including the synthesis of DNA, RNA and protein, etc., and stimulate the generation of activated oxygen [31][32][33]. Other antibacterial ingredients include polymers such as chitosan (CS) [28][34][35], traditional Chinese medicines such as curcumin [36] and inorganic materials such as bioactive glass [35].
The osteogenic components of GTR/GBR membranes mainly include conventional drugs, metal ions, inorganic materials and proteins, etc. They directly or indirectly regenerate bone in the following ways: (1) promoting migration, adhesion, proliferation and osteogenic differentiation of bone progenitor cells, (2) improving the expression level of osteogenic related genes and proteins, (3) promoting mineral deposition. Additionally, minocycline could reduce bone resorption [20]. Apart from bioactive ingredients, surface topography of the membranes which is beneficial to adhesion and spread of osteogenic-related cells could also enhance new bone formation. Lian et al. fabricated a composite membrane which was based on poly(lactic-co-glycolic acid)/gelatin (PG) fiber matrix and contained copper-loaded mesoporous silica nanoparticles (Cu@MSNs). It was suggested that the osteogenic ability of the membrane was better than that of frequently used membranes in clinics [37].
Apart from osteogenic properties, some composite membranes also had other abilities. For example, they showed anti-inflammatory performance [17][23][27][29][38] and had the ability to promote angiogenesis [20][37]. It is necessary to control inflammation since periodontal tissues are difficult to repair in an inflammatory microenvironment [39]. In addition, blood vessels can bring oxygen and blood to the lesion, which are important for bone tissue reconstruction [20]. Therefore, these multifunctional composite membranes may create a more favorable environment for periodontal tissue healing. To achieve both hard and soft tissue regeneration, asymmetric GBR films with dense and loose layers were synthesized [20][24][36][37]. The dense layer prevented fibrous connective tissue from invading the defect space, promoting fibroblast adhesion so as to enhance the formation of soft tissue above the new bone [36]. While the loose layer, which directly contacted with the bone defect space, was conducive to osteoblast adhesion, penetration and blood clot stability, thus guiding bone regeneration [37]. Moreover, a polylactide copolymer membrane showed comparable performance in mucosal healing and bone formation compared with a validated membrane for keratinized oral mucosa healing [40]. This may be attributed to the multiple properties of lactic acid, including tissue regeneration and antiseptic aspects.
Accurate matching and personalized customization are highlights of GTR/GBR membranes. Conventional GTR/GBR membranes need to be tailored according to various defects before implantation, resulting in inaccurate matching. In addition, different individuals have different antibacterial and osteogenic stages in the process of periodontal tissue healing. Considering these facts, Xu et al. designed an injectable hydrogel composite membrane [17]. It was able to automatically match the bone defect area by liquid and solid phase transformation. Therefore, it greatly improved operability and matching accuracy of the bone defect. Under the irradiation of blue light, the compound achieved early debridement of bacteria and produced Cu2+ with osteogenic properties. Under near-infrared irradiation, the composite produced a photothermal effect, and enhanced bone regeneration along with Cu2+. Through dual-light noninvasive regulation, the complex could transform antibacterial and osteogenic effects for patients at different healing stages. In conclusion, it could be a promising customized GTR strategy according to different defects and patients.
Currently, stability of the wound is considered to be crucial to periodontal regeneration as opposed to physical obstruction. A noninferiority study supported this view [41]. Platelet-rich fibrin (L-PRF) is a biological mediator with antibacterial effect against P. gingivalis but without a stable barrier effect [42]. Combined with inorganic bovine bone graft (IBB), L-PRF shows noninferior efficacy regarding clinical attachment level gain compared with collagen membrane in unfavorable infrabony defects. The effect of L-PRF contributed to maintenance of bone graft and fibrin, along with released polypeptide growth factors that stimulated regeneration. However, the contribution of antimicrobial properties of L-PRF was not mentioned here.
Compared with the traditional barrier membrane, novel GTR/GBR membranes have many improved properties and limitations. These properties include good biocompatibility [20], better mechanical property [37], controllable biodegradability, drug encapsulation ability, satisfactory antibacterial and osteogenesis ability [20], manageability as well as accurate matching with the defects [17]. The limitations are as follows. (1) Long degradation time. It was suggested that membranes generally need to maintain the barrier function for four–six weeks to ensure the repair of periodontal tissues in clinic [43][44]. However, according to available data, degradation time of newly developed membranes varies from about two months to more than six months [9][17][20][21][25][26][36][38]. In these circumstances, the membrane occupies the repair space of periodontal tissue and may affect complete restoration. (2) Long-term degradation and toxicity of elements. Studies have shown that the biocompatibility of the composite membranes was affected by high concentration of some elements, such as Cu2+ [37], TiO2 [17], Co2+ [38], nMgO [18], ornidazole [25] and metronidazole [26]. Therefore, it is necessary to further verify their long-term metabolic effect and toxicity.
2.3. Tissue Engineering Scaffolds
Tissue engineering scaffolds play an important role in maintaining space, storing growth factors and supporting cell attachment and proliferation after being grafted to the bone defect [39]. Three key factors of tissue engineering are the scaffold, cells and signals [16]. Ideal bone graft materials should have osteoconductivity, osteoinduction and osteogenesis properties. They allow migration of osteogenic cells, differentiation of stem cells and new bone formation, respectively. However, existing bone graft materials are osteoconductive, but lack sufficient osteoinductivity, stable bone regeneration and antimicrobial properties. Moreover, xenogenic and allogeneic bone grafts even create a risk of infection [45]. To make some improvements, tissue engineering scaffolds are developed in combination with growth factors or stem cells, as well as antibacterial components. These scaffolds have improved osteoinduction, osteogenesis and antibacterial activity.
Antibiosis is a new development of tissue engineering scaffolds. Apart from antibiotics, CS and bioglasses were incorporated into the scaffolds [39][46][47]. They significantly inhibited the growth of pathogens in planktonic culture and biofilm in a dose-dependent manner. For example, DOX released from CS enhanced calcium phosphate cement (CPCC) could reduce the colony-forming unit (CFU) of Staphylococcus aureus (S. aureus) and P. gingivalis by four orders of magnitude in biofilm [46].
Growth factor or stem cells improve osteoinduction and osteogenesis activity of tissue engineering scaffolds. These scaffolds had a porous structure, which is beneficial for growth factor loading, cell attachment, growth and proliferation [48]. Recombinant amelogenin (rhAm), an active ingredient to promote periodontal regeneration, was incorporated into a mesoporous hydroxyapatite/CS composite scaffold [39]. In the scaffold, sustained release of rhAm promoted osteogenic differentiation of hPDLCs. Animal experiments showed that the scaffold could promote the formation of cementoid tissue. Qiu et al. combined human periodontal ligament stem cell (hPDLSCs) beads with DOX to construct anti-infective bone regeneration scaffolds [46]. hPDLSCs could be released slowly from the beads and enhanced osteogenic differentiation. In addition, the released DOX not only enhanced calcium phosphate cement, but also inhibited bone resorption by inhibiting matrix metalloproteinases. Human periapical cyst mesenchymal stem cells (hPCy-MSCs), which were isolated from periapical cysts, were seeded on mineral-doped porous scaffolds [48]. The scaffolds enabled the reuse of biological waste and had the potential to regenerate bone and dental tissues. Apart from osteogenic ability, some elements were able to promote angiogenesis as well [47].
Tissue engineering scaffolds have antibacterial activity, improved osteoinduction and osteogenesis abilities, as well as excellent mechanical property in vitro. They are expected to control inflammation via combating infections and enhance bone regeneration. To confirm the results, further research is needed to investigate their long-term in vivo performances. Moreover, attention should be paid to the usage of some potentially toxic substances [47].
Table 1. The summary of periodontal biomaterials.
| Biomaterials | Characteristics | Performances | Advantages | Limitations | Refs. | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| Bioactive agents | Biological or synthetic agents with both antibacterial and osteogenic effect | Antibacterial effect on Porphyromonas gingivalis (P. gingivalis) and Streptococcus mutans (S. mutans) Promotion of osteogenic differentiation of human periodontal ligament fibroblasts (hPDLFCs) and human periodontal ligament cells (hPDLCs) |
Reduction in bone loss in animal model Probing depth (PD) reduction, clinical attachment level (CAL) gain and percentage of radiographic defect depth reduction (DDR%) in a clinical trial |
Safety, abundant sources | Unknown active ingredient and mechanism | [8][11][14][15][49] |
| Guided tissue regeneration/guided bone regeneration (GTR/GBR) membranes | Composite membranes based on natural or synthetic polymers, combined with antibacterial and osteogenic components They act as cell barriers to prevent the invasion of epithelium and connective tissues, facilitating alveolar bone regeneration | Antibacterial effect on P. gingivalis, S. mutans, Fusobacterium nucleatum (F. nucleatum), Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Streptococcus sanguis, Micrococcus luteus, Salmonella typhimurium, Peptostreptococcus anaerobius (Pa), Actinobacillus actinomycetes (Aa) and Enterococcus faecalis (E. faecalis) Promotion of mineralization, proliferation, osteogenic differentiation of osteoblasts, fibroblasts, hPDLCs, bone marrow mesenchymal stem cells (BMSCs), Mg63 cells, MC3T3 cells, human periodontal ligament fibroblasts (HPDLFs), mesenchymal stem cells (MSCs), human periodontal ligament stem cells (hPDLSCs) and dental pulp stem cells (DPSCs) Induction of M2 phenotype polarization of macrophages |
Reduction in bone loss, promotion of mucosal healing, new bone formation and fibril matrix deposition in animal model CAL gain, radiographic defect bone level (DBL) gain, reduction in PD and gingival recession (GR) in a clinical trial | Good biocompatibility, better mechanical property compared with traditional membrane, controllable biodegradability, drug encapsulation and release ability, manageability as well as accurate matching with the defects | Long degradation time, unknown long-term metabolic effect and toxicity of elements | [9][17][18][20][21][22][23][24][25][26][27][28][29][30][31][34][35][36][37][38][40][41][42] |
| Tissue engineering scaffolds | Scaffolds are composed of natural or synthetic polymers, antibacterial components, proteins or cells They are grafted to bone defect to maintain space, store growth factors, support cell attachment and proliferation |
Antibacterial effect on F. nucleatum, P. gingivalis, S. aureus and Aggregatibacter actinomycetemcomitans (A.actinomycetemcomitans) Promotion of osteogenic differentiation of hPDLCs, hPDLSCs, promotion of osteogenic and odontogenic differentiation of human periapical cyst mesenchymal stem cells (hPCy-MSCs) and promotion of preosteogenic responses of MC3T3-E1 cells |
Promotion of cementoid tissue formation in animal model | Good mechanical property, loading and sustained release of active ingredients, antibacterial property, improved osteoinduction and osteogenesis performances | Potential toxicity of certain ingredient, lack of research on osteogenic ability in vivo | [39][46][47][48] |
| Drug delivery systems | Natural or synthetic polymers are loaded with antibacterial and osteogenic components Along with systems’ degradation, active components experience burst release followed by sustained release | Antibacterial effect on A. atinomycetemcomitans, Prevotella nigricans (P. nigrescens), E. coli, S. aureus, P. gingivalis, E. faecalis, S. mutans and Streptococcus sanguinis (S. sanguinis) Promotion of proliferation, migration, attachment and osteogenic differentiation of human bone marrow-derived osteoblasts (HOB), BMSCs, osteoblasts and PDLSCs, promotion of mineralization |
Reduction in bone loss, increase in new bone formation and improvement of gingival index in animal model | Stability, stimulus responsiveness, effective controlled release mode, long-lasting effects | Potential toxicity of certain ingredients | [11][13][50][51][52][53][54] |
2.4. Drug Delivery Systems
Medication is an adjuvant to consolidate periodontal treatment and prevent the progression or recurrence of periodontal disease. Conventional periodontal medication has many limitations, including single effects, short-acting, drug resistance, side effects and inconvenient operation [55][56]. Furthermore, it does not show significant clinical and anti-microbial effects [57]. Currently, local drug delivery systems for the periodontal region are constructed by loading different substances with biocompatible carriers. These systems improve bioavailability of drugs, avoid side effects of systemic medication and achieving targeted, continuous and controllable release. Therefore, they are expected to be an ideal therapy [29][36].
The performance of drug delivery systems is related to type and proportion of the loaded components and surface of the carriers. In these systems, antibacterial components are not only antibiotics [51][54], but also metal nanoparticles such as silver [11][23][24][25][26][27][28][29][30][31][34][35][36][37][38][39][43][44][46][52][53][57][58] and polymer nanoparticles such as CS (nCS) [53]. Osteogenic components include drugs such as lovastatin and minocycline [51][54], metal nanoparticles such as silver and calcium [52][59], and polymer nanoparticles such as poly(lactic-co-glycolic acid) (nPLGA) and nCS [53], etc. Different ratios of active ingredients produce different effects. Xue et al. found that the performance of the mixture was the best when the nPLGA to nCS ratio was 3:7, and the content of silver nanoparticles (nAg) was 50 µg/mL [53]. In addition to the active components, surface modification of the carrier has direct contact with antibacterial properties. It kills bacteria by changing their membrane permeability without increasing drug resistance [59]. As the result, the systems have antibacterial activity against many kinds of bacteria and nearly achieve complete bone regeneration [13].
The mechanism of drug release from polymer includes diffusion and polymer degradation [54]. Thus, most systems present initial burst release and subsequent stable slow release for about 5–30 days. Initial burst release of antibiotics above minimal inhibitory concentration (MIC) is necessary since the control of infection and inflammation is more evident in the early stages of wound healing [29][50]. The subsequent stable release prevented the growth of residual bacteria [30]. In addition, infection must be controlled before bone regeneration. Therefore, Lee et al. designed a sequential release system which allowed the outside tetracycline release at the beginning, followed by the inside lovastatin [54]. This release strategy may increase the utilization of both antibacterial and osteogenesis ingredients. Furthermore, prolonged release time of lovastatin had a significant effect on enhancing periodontal bone regeneration.
To improve drug targeting and achieve on-demand delivery in specific microenvironments, stimuli-responsive release has become a new trend. There are a lot of alkaline phosphatases (ALP) in the periodontal pocket of periodontitis patients, which can decompose polyphosphate esters (PPEs). Considering this, Li et al. added PPE and minocycline hydrochloride (MH) to CS to prepare an enzyme-responsive drug release film [51]. Within 12 days, the accumulative release of MH reached 80% under the action of ALP. In addition, MH provided effective antibacterial activity for the membrane. Compared with the control group, more human gingival fibroblasts (HGFs) and rat osteoblasts attached to the membrane, and the osteogenesis of rat osteoblasts was promoted. In vivo experiments confirmed that the membrane could improve gingival index and reduce alveolar bone loss. In addition, PEGPD@SDF-1 thermosensitive hydrogel loaded with antimicrobial peptide and stromal cell-derived factor-1 (SDF-1) was synthesized to control drug release according to the severity of infection (Figure 1) [13]. In the hydrogel, drugs were released in response to gingipain secreted by P. gingivalis and realized antibacterial properties as well as regeneration. With thermosensitive injectable characteristics, the hydrogel could accurately fit the defect with tiny or irregular shapes and avoid invasive operations. Continuous secretion of gingival crevicular fluid and oral movement make the periodontal environment unstable. Nevertheless, hydrogels with certain adhesion properties may remain stable and present constant function in the periodontal pocket. Furthermore, the porous structure of the hydrogels in accordance with bone compartment facilitated the penetration, growth and differentiation of stem cells.
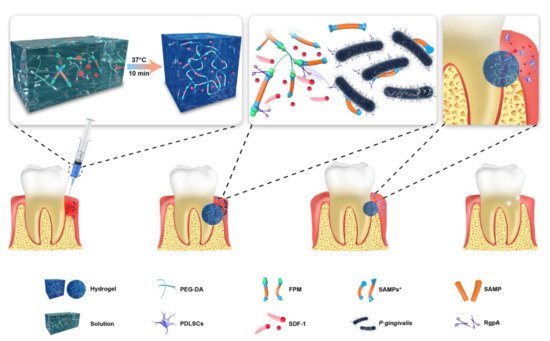
Figure 1. Schematic illustration of the composition and application of PEGPD@SDF-1 hydrogel. The hydrogel is cross-linked by FPM and PEG-DA and loaded with SDF-1. The transition from solution to hydrogel is completed at 37 °C in 10 min. FPM contains SAMP in the center and two anchor peptides with specific splicing sites in the lateral. The RgpA released by P. gingivalis initiates splicing of the specific sites in FPM. Then, SAMPs* and SDF-1 are released to resist P. gingivalis and promote osteogenesis, respectively. Reprinted with permission from reference [13]. Copyright 2021 American Chemical Society.
Drug delivery systems have many advantages as well as limitations. Compared with conventional medication, they have an effective controlled release mode, long-lasting effects, good osteogenic potential and antibacterial activity [54]. In addition, more researchers have applied components with strong antibacterial effect, low bacterial resistance and weak cytotoxicity [13]. However, some ingredients are still toxic to cells at high concentrations [50][53][54]. Therefore, attention should be paid to adjusting their concentrations to achieve a balance between biological activity and biocompatibility. Furthermore, a drug release time suitable for both infection control and osteogenesis still needs to be determined.
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260.
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944.
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35.
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511.
- Costantini, E.; Sinjari, B.; Piscopo, F.; Porreca, A.; Reale, M.; Caputi, S.; Murmura, G. Evaluation of Salivary Cytokines and Vitamin D Levels in Periodontopathic Patients. Int. J. Mol. Sci. 2020, 21, 2669.
- Patini, R.; Gallenzi, P.; Spagnuolo, G.; Cordaro, M.; Cantiani, M.; Amalfitano, A.; Arcovito, A.; Calla, C.; Mingrone, G.; Nocca, G. Correlation Between Metabolic Syndrome, Periodontitis and Reactive Oxygen Species Production. A Pilot Study. Open Dent. J. 2017, 11, 621–627.
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. 3 D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J. Mech. Behav. Biomed. Mater. 2020, 109, 103822.
- Zhang, R.; Yang, J.; Wu, J.; Xiao, L.; Miao, L.; Qi, X.; Li, Y.; Sun, W. Berberine promotes osteogenic differentiation of mesenchymal stem cells with therapeutic potential in periodontal regeneration. Eur. J. Pharmacol. 2019, 851, 144–150.
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222.
- El Sayed, N.; Baeumer, A.; El Sayed, S.; Wieland, L.; Weber, D.; Eickholz, P.; Pretzl, B. Twenty years later: Oral health-related quality of life and standard of treatment in patients with chronic periodontitis. J. Periodontol. 2019, 90, 323–330.
- Kibar, H.; Arslan, Y.E.; Ceylan, A.; Karaca, B.; Haliscelik, O.; Kiran, F. Weissella cibaria EIR/P2-derived exopolysaccharide: A novel alternative to conventional biomaterials targeting periodontal regeneration. Int. J. Biol. Macromol. 2020, 165, 2900–2908.
- Caffesse, R.G.; Echeverria, J.J. Treatment trends in periodontics. Periodontol 2000 2019, 79, 7–14.
- Liu, S.; Wang, Y.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates in Situ Periodontal Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 36880–36893.
- Li, X.; Yu, C.; Hu, Y.; Xia, X.; Liao, Y.; Zhang, J.; Chen, H.; Lu, W.; Zhou, W.; Song, Z. New Application of Psoralen and Angelicin on Periodontitis with Anti-bacterial, Anti-inflammatory, and Osteogenesis Effects. Front. Cell Infect. Microbiol. 2018, 8, 178.
- Kanoriya, D.; Singhal, S.; Garg, V.; Pradeep, A.R.; Garg, S.; Kumar, A. Clinical efficacy of subgingivally-delivered 0.75% boric acid gel as an adjunct to mechanotherapy in chronic periodontitis: A randomized, controlled clinical trial. J. Investig. Clin. Dent. 2018, 9, e12271.
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration-a materials perspective. Dent. Mater. 2012, 28, 703–721.
- Xu, Y.; Zhao, S.; Weng, Z.; Zhang, W.; Wan, X.; Cui, T.; Ye, J.; Liao, L.; Wang, X. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl Mater. Interfaces 2020, 12, 54497–54506.
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428.
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197.
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline-loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822.
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2021, 28, 51–62.
- Wu, T.; Huang, L.; Sun, J.; Sun, J.; Yan, Q.; Duan, B.; Zhang, L.; Shi, B. Multifunctional chitin-based barrier membrane with antibacterial and osteogenic activities for the treatment of periodontal disease. Carbohydr. Polym. 2021, 269, 118276.
- Ho, M.H.; Claudia, J.C.; Tai, W.C.; Huang, K.Y.; Lai, C.H.; Chang, C.H.; Chang, Y.C.; Wu, Y.C.; Kuo, M.Y.; Chang, P.C. The treatment response of barrier membrane with amoxicillin-loaded nanofibers in experimental periodontitis. J. Periodontol. 2020, 92, 886–895.
- Lian, M.; Sun, B.; Qiao, Z.; Zhao, K.; Zhou, X.; Zhang, Q.; Zou, D.; He, C.; Zhang, X. Bi-layered electrospun nanofibrous membrane with osteogenic and antibacterial properties for guided bone regeneration. Colloids Surf. B Biointerfaces 2019, 176, 219–229.
- Zhang, H.; Ma, H.; Zhang, R.; Wang, K.; Liu, J. Construction and characterization of antibacterial PLGA/wool keratin/ornidazole composite membranes for periodontal guided tissue regeneration. J. Biomater. Appl. 2020, 34, 1267–1281.
- Wang, Y.; Jiang, Y.; Zhang, Y.; Wen, S.; Wang, Y.; Zhang, H. Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 134–139.
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl Mater. Interfaces 2019, 11, 37381–37396.
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials 2018, 8, 327.
- Mathew, A.; Vaquette, C.; Hashimi, S.; Rathnayake, I.; Huygens, F.; Hutmacher, D.W.; Ivanovski, S. Antimicrobial and Immunomodulatory Surface-Functionalized Electrospun Membranes for Bone Regeneration. Adv. Healthc. Mater. 2017, 6, 1601345.
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 966–976.
- Vale, A.C.; Pereira, P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial free-standing polysaccharide composite films inspired by the sea. Int. J. Biol. Macromol. 2019, 133, 933–944.
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992.
- Fanoro, O.T.; Oluwafemi, O.S. Bactericidal Antibacterial Mechanism of Plant Synthesized Silver, Gold and Bimetallic Nanoparticles. Pharmaceutics 2020, 12, 1044.
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/beta-TCP electrospun composite for guided bone regeneration. Prog. Biomater. 2018, 7, 225–237.
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 055004.
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200.
- Lian, M.; Han, Y.; Sun, B.; Xu, L.; Wang, X.; Ni, B.; Jiang, W.; Qiao, Z.; Dai, K.; Zhang, X. A multifunctional electrowritten bi-layered scaffold for guided bone regeneration. Acta Biomater. 2020, 118, 83–99.
- Lin, W.C.; Yao, C.; Huang, T.Y.; Cheng, S.J.; Tang, C.M. Long-term in vitro degradation behavior and biocompatibility of polycaprolactone/cobalt-substituted hydroxyapatite composite for bone tissue engineering. Dent. Mater. 2019, 35, 751–762.
- Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous Hydroxyapatite/Chitosan Loaded with Recombinant-Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell Infect. Microbiol. 2020, 10, 180.
- Vacaras, S.; Baciut, G.; Gheban, D.; Bran, S.; Colosi, H.; Toader, S.; Opris, D.; Kretschmer, W.; Manea, A.; Armencea, G.; et al. Engaging a polylactide copolymer in oral tissue regeneration: First validation of Suprathel((R)) for guided epithelial and osseous healing. J. Med. Life 2021, 14, 181–197.
- Rexhepi, I.; Paolantonio, M.; Romano, L.; Serroni, M.; Santamaria, P.; Secondi, L.; Paolantonio, G.; Sinjari, B.; De Ninis, P.; Femminella, B. Efficacy of inorganic bovine bone combined with leukocyte and platelet-rich fibrin or collagen membranes for treating unfavorable periodontal infrabony defects: Randomized non-inferiority trial. J. Periodontol. 2021, 92, 1576–1587.
- Castro, A.B.; Herrero, E.R.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188.
- Kuo, S.M.; Chang, S.J.; Chen, T.W.; Kuan, T.C. Guided tissue regeneration for using a chitosan membrane: An experimental study in rats. J. Biomed. Mater. Res. A 2006, 76, 408–415.
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576.
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096.
- Qiu, G.; Huang, M.; Liu, J.; Wang, P.; Schneider, A.; Ren, K.; Oates, T.W.; Weir, M.D.; Xu, H.H.K.; Zhao, L. Antibacterial calcium phosphate cement with human periodontal ligament stem cell-microbeads to enhance bone regeneration and combat infection. J. Tissue Eng. Regen. Med. 2021, 15, 232–243.
- Liu, J.; Rawlinson, S.C.F.; Hill, R.G.; Fortune, F. Fluoride incorporation in high phosphate containing bioactive glasses and in vitro osteogenic, angiogenic and antibacterial effects. Dent. Mater. 2016, 32, e221–e237.
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.D.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials 2019, 12, 597.
- Xu, Y.; Zheng, B.; He, J.; Cui, Z.; Liu, Y. Silver nanoparticles promote osteogenic differentiation of human periodontal ligament fibroblasts by regulating the RhoA-TAZ axis. Cell Biol. Int. 2019, 43, 910–920.
- Koch, F.; Ekat, K.; Kilian, D.; Hettich, T.; Germershaus, O.; Lang, H.; Peters, K.; Kreikemeyer, B. A Versatile Biocompatible Antibiotic Delivery System Based on Self-Assembling Peptides with Antimicrobial and Regenerative Potential. Adv. Healthc. Mater. 2019, 8, e1900167.
- Li, N.; Jiang, L.; Jin, H.; Wu, Y.; Liu, Y.; Huang, W.; Wei, L.; Zhou, Q.; Chen, F.; Gao, Y.; et al. An enzyme-responsive membrane for antibiotic drug release and local periodontal treatment. Colloids Surf. B Biointerfaces 2019, 183, 110454.
- Fan, W.; Liu, D.; Li, Y.; Sun, Q.; Fan, B. AgCa-PLGA submicron particles inhibit the growth and colonization of E. Faecalis and P. gingivalis on dentin through infiltration into dentinal tubules. Int. J. Pharm. 2018, 552, 206–216.
- Xue, Y.; Hong, X.; Gao, J.; Shen, R.; Ye, Z. Preparation and biological characterization of the mixture of poly(lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomed. 2019, 14, 483–498.
- Lee, B.S.; Lee, C.C.; Wang, Y.P.; Chen, H.J.; Lai, C.H.; Hsieh, W.L.; Chen, Y.W. Controlled-release of tetracycline and lovastatin by poly(D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016, 11, 285–297.
- Tan, O.L.; Safii, S.H.; Razali, M. Commercial Local Pharmacotherapeutics and Adjunctive Agents for Nonsurgical Treatment of Periodontitis: A Contemporary Review of Clinical Efficacies and Challenges. Antibiotics 2019, 9, 11.
- Wang, C.Y.; Yang, Y.H.; Li, H.; Lin, P.Y.; Su, Y.T.; Kuo, M.Y.; Tu, Y.K. Adjunctive local treatments for patients with residual pockets during supportive periodontal care: A systematic review and network meta-analysis. J. Clin. Periodontol. 2020, 47, 1496–1510.
- Matesanz, P.; Herrera, D.; Echeverria, A.; O’Connor, A.; Gonzalez, I.; Sanz, M. A randomized clinical trial on the clinical and microbiological efficacy of a xanthan gel with chlorhexidine for subgingival use. Clin. Oral Investig. 2013, 17, 55–66.
- Wang, Z.; Ma, Q.; Dong, X.; Li, D.; Xi, X.; Yu, W.; Wang, J.; Liu, G. Assembly of 1D nanofibers into a 2D bi-layered composite nanofibrous film with different functionalities at the two layers via layer-by-layer electrospinning. Phys. Chem. Chem. Phys. 2016, 19, 118–126.
- Li, D.; Qiu, Y.; Zhang, S.; Zhang, M.; Chen, Z.; Chen, J. A Multifunctional Antibacterial and Osteogenic Nanomedicine: QAS-Modified Core-Shell Mesoporous Silica Containing Ag Nanoparticles. Biomed. Res. Int. 2020, 2020, 4567049.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
873
Revisions:
2 times
(View History)
Update Date:
14 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




