Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shabnam Nisha | + 2197 word(s) | 2197 | 2021-12-02 03:37:22 | | | |
| 2 | Rita Xu | Meta information modification | 2197 | 2021-12-10 10:28:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nisha, S. Microalgae in Global CO2 Sequestration. Encyclopedia. Available online: https://encyclopedia.pub/entry/16977 (accessed on 08 March 2026).
Nisha S. Microalgae in Global CO2 Sequestration. Encyclopedia. Available at: https://encyclopedia.pub/entry/16977. Accessed March 08, 2026.
Nisha, Shabnam. "Microalgae in Global CO2 Sequestration" Encyclopedia, https://encyclopedia.pub/entry/16977 (accessed March 08, 2026).
Nisha, S. (2021, December 10). Microalgae in Global CO2 Sequestration. In Encyclopedia. https://encyclopedia.pub/entry/16977
Nisha, Shabnam. "Microalgae in Global CO2 Sequestration." Encyclopedia. Web. 10 December, 2021.
Copy Citation
The rising concentration of global atmospheric carbon dioxide (CO2) has severely affected our planet’s homeostasis. Efforts are being made worldwide to curb carbon dioxide emissions, but there is still no strategy or technology available to date that is widely accepted.
microalgae
pyrenoid
carbon sequestration
carbon emissions
1. Introduction
Climate change is a major threat that severely hampers the survival of various plant and animal species as well as humans. The continuous increase in the emissions of several greenhouse gasses (GHGs), including carbon dioxide (CO2), water vapor, methane (CH4), nitrous oxide (N2O), and fluorinated gases, has aggravated climate change [1][2]. The rise in GHGs emissions is mostly associated with anthropogenic actions, with the use of fossil fuels being the largest contributor [3]. The world’s atmospheric CO2 has increased from ~313 ppm (in 1960) to ~411 ppm at present [4]. A high level of CO2 in the atmosphere raises the acidity of ocean water and affects the marine ecosystem to a significant extent [5][6]. Hence, it is highly imperative at this moment to develop an appropriate strategy to reduce or stabilize the CO2 content in the atmosphere. Various countries have signed many international protocols to curb GHGs emissions, e.g., COP26, Kyoto Protocol (1997), and the Paris agreement (2015).
Two basic approaches for reducing CO2 emissions include (i) the decreased use of fossil fuels complemented with the increased use of renewable energy sources; (ii) and carbon capture and storage via various biological, chemical, or physical methods [7][8]. The physical methods for carbon emission reduction have been extensively explored. Still, there are several technological and economic limitations with the existing technologies. Therefore, it is crucial to upgrade the existing technologies as well as develop suitable alternatives. Among various others, biological CO2 fixation seems to be a relatively cost-effective and eco-friendly approach in comparison to the physical and chemical methods. Photosynthetic organisms assimilate CO2 via the dark phase of photosynthesis and play a key role in maintaining the balance of CO2 levels in the atmosphere. Compared to other photosynthetic organisms, phytoplankton had higher CO2 fixation efficiency and biomass productivity [6]. Marine phytoplankton accounts for half of the total global primary productivity by fixing ~ 50 gigatons of CO2 annually [6]. In this context, research on CO2 sequestration by microalgae has attracted attention across the globe [9][10][11][12][13][14]. Microalgae can assimilate CO2 10–50 times more effectively, compared to vascular plants without competing or providing food to humans/animals [15][16][17]. Microalgae have a special mechanism to assimilate carbon dioxide known as the carbon concentration mechanism (CCM). In this mechanism, a specialized organelle i.e., pyrenoid increases the concentration of CO2 around the thylakoid membranes [18]. The increased concentration of carbon dioxide around the thylakoid membrane enhances the efficiency of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), an important photosynthetic enzyme for carbon assimilation or sequestration. Rubisco has a low affinity for carbon dioxide as it has been evolved in high CO2 and low O2 environments, so the pyrenoid constantly provides an environment for enhanced CO2 fixation [18][19]. It is evident from Table 1 that microalgae grown in various cultivation conditions for carbon sequestration showed higher tolerance for increased CO2 concentration. Some of the investigations also reported that microalgae can also be used for flue gas (NOx and SOx) sequestration [20]. Microalgae have high biomass yield and tolerance for adverse environmental conditions. Therefore, microalgae are considered a potential feedstock for CO2 sequestration and bioenergy production [21][22].
Microalgae are also used for wastewater treatment and biomass production, which can further be exploited for various applications, including biogas, bioplastics, and fertilizer production [23][24]. The low nutrition conditions and high photosynthetic efficiency have made it easy to cultivate algae for their exploitation for various applications. The previously published reviews extensively majorly explored the carbon sequestration of microalgae and their physiological mechanism, however detailed information on the role of pyrenoids in the sequestration of CO2 is missing, which are key aspects with specific reference to the global CO2 mitigation using algal technologies [25][26]. This review comprehensively discusses the physiological mechanism of carbon sequestration, the role of pyrenoids, and the impact of environmental factors in the carbon concentration mechanism. Besides this, the review also provides the current global CO2 emission status and scenarios.
1.1. Global CO2 Emission Status
Worldwide progress in the economy and the population boom have led to a continuously rapid rise in the emissions of carbon dioxide in the last few decades [27]. The rising level of CO2 in the atmosphere leads to an increased global average surface temperature, which directly and indirectly influences the global weather and climatic phenomenon (e.g., excessive rainstorms, drought) [27][28]. In order to combat the increasing earth’s surface temperature, the Paris agreement came into force, which was ratified by 196 countries to limit global warming below 1.5 °C compared to the pre-industrial era. This can be achieved through reducing greenhouse emissions by Nationally Determined Contributions (NDCs). The global carbon dioxide emissions have increased by 0.9% in 2019 compared to 2018. The largest emitters were China, USA, India, EU27 + UK, Russia, and Japan as per the Emission Database for Global Atmospheric Research (EDGAR) [29][30]. Demographically, these countries comprise 51% of the global population but contribute to ~67% of CO2 emissions. A detailed CO2 emission scenarios of major contributing countries from 1990 to 2020 is published elsewhere [29][30]. Surprisingly, compared to that in 2018, the level of CO2 emission in 2019 increased in China and India but decreased in EU28, the USA, and Russia (Figure 1). Global carbon emissions showed a 5% drop in the first quarter of 2020 compared to the first quarter of 2019, due to the decline in the demand for coal (8%), oil (4.5%), and natural gases (2.3%). In another report, the daily, weekly, and seasonal dynamics of CO2 emissions were presented and estimated a ~8.8% decrease in the CO2 emissions in the first half of 2020 [29][30]. The decline in global CO2 emissions in 2020 was due to the COVID-19 pandemic, which recorded the most significant decline since the end of World War II [28]. EDGAR estimated that 2020 showed a decline, with global anthropogenic fossil CO2 emissions 5.1% lower than in 2019, at 36.0 Gt CO2, just below the 36.2 Gt CO2 emission level registered in 2013 [29][30].
In 2019, global carbon emissions (fossil fuels) per unit of Gross Domestic Products (GDP) showed a declining trend reaching an average value of 0.298 tCO2/k USD/yrs., while per capita carbon emissions remained stable at 4.93TCO2/capita/yrs., confirming a 15.9% surge from 1990 [29][30] as published by [29. The COVID-19 pandemic has had a significant impact on social activities worldwide, and thus, on energy use and carbon emissions as well. According to the International Energy Agency (IEA), the energy demand in 2020 declined by 6%, which is seven times higher than the financial crisis energy demand of 2008 [31].
1.2. Carbon Sequestration Technologies
There are various physical, chemical, and biological methods in operation for reducing atmospheric CO2 emissions [7][8][32]. The carbon sequestration or fixation strategies are popularly known as carbon capture and storage/utilization (CCS/U). Carbon emission reductions in CCS is carried out in various stages such as CO2 capture, separation, transportation, utilization, and storage. A detailed discussion of all these steps is demonstrated elsewhere [7][8][32]. A major system frequently used for carbon capture comprises (i) pre-combustion, wherein CO2 is removed before combustion and the fuel is broken down to yield synthesis gas, a mixture of CO2 and H2; subsequently, CO2 is separated into various processes, and H2 is used as a clean fuel; (ii) post-combustion, where CO2 is captured after the combustion of fuels using chemical absorption; (iii) oxy-fuel where the fuel is combusted in the presence of pure oxygen to produce high levels of CO2; and (iv) chemical looping combustion, where oxygen carrier (solid metal oxides) particles are continuously circulated to supply oxygen to react with fuel, wherein the combustion of metal oxide and fuel produce metal, CO2, and H2O [33]. The separation of CO2 from flue gas also plays a vital role in carbon capture and storage technologies. Many separation techniques in operation include absorption/adsorption, membrane separation, and cryogenic distillation [34]. After capture at the source, CO2 needs to be transported to the sink, which requires further various methodologies described elsewhere [35].
2. Physiological Mechanism of Carbon Sequestration in Algae
Aquatic photosynthetic organisms, mainly phytoplankton, are responsible for 50% of the global carbon assimilation [36][37][38]. It has been stated in the literature that 1.0 kg of cultivated microalgae may assimilate 1.83 kg of CO2 [39][40]. There are three different processes i.e., photoautotrophic, heterotrophic, and mixotrophic metabolisms, involved in algae that help CO2 assimilation [41][42][43]. Microalgae take up inorganic carbon in three different ways: (i) The transformation of bicarbonates into CO2 by extracellular carbonic anhydrase that readily diffuses inside the cells without any hindrance; (ii) straight absorption of CO2 via the plasma membrane; and (iii) direct intake of bicarbonates by resolute carriers in the membrane, also known as dissolved inorganic carbon (DIC) pumps (Figure 1A) [44].
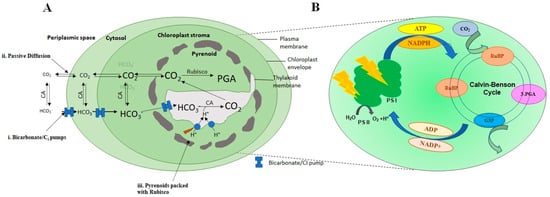
Figure 1. Typical figures of carbon concentration mechanism in microalgae Chlamydomonas reinhardtii (A) showing (i) Bicarbonate/Ci pumps for Ci transportation; (ii) passive diffusion of CO2 through membrane pores; (iii) pyrenoid packed with Rubisco. (B) Magnified view of chloroplast where photosynthetic CO2 reduction takes place through Calvin–Benson cycle.
2.1. Photoautotrophic Metabolism
The majority of the microalgae are photoautotrophic, requiring inorganic carbon and light to transform (inorganic) CO2 into carbohydrates by photosynthesis. The algae fix CO2 through the Calvin–Benson cycle (Figure 1A), where the enzyme Rubisco plays a key role in converting CO2 into organic compounds [41][45]. In microalgae, the photosynthetic reaction can be classified as a light-dependent reaction and a light-independent or dark reaction (Figure 1B). The first phase of photosynthesis is light-driven, and here light transforms NADP+ and ADP into energy-storing NADPH and ATP molecules [46]. The second phase, i.e., the dark phase, consists of CO2 fixation and assimilation via the Calvin–Benson cycle in order to create organic compounds (glucose) with the aid of NADPH and ATP, produced in the first phase [47]. Here, Ribulose bisphosphate carboxylase/oxygenase (Rubisco) plays a significant role in the sequestration of CO2 [48][49]. Rubisco catalyzes the conversion of CO2 to 3-phosphoglycerate. However, due to the oxygenase character, Rubisco binds very weakly binds with CO2, which makes it a poor CO2 fixer [48][49]. These phosphoglycerates are then involved in yielding carbohydrates. Furthermore, these phosphoglycerates are mostly used to regenerate RuBP, which is then employed to continue the carbon-fixing cycle. The oxygen ion of Rubisco produces phosphoglycolate, which in turn hinders the carboxylase function of Rubisco. The phosphoglycolate is further transformed into phosphoglycerate (3-PGA) by exploiting ATP and releasing CO2. This reaction is known as photorespiration, in which O2 is utilized and CO2 is released [50]. Therefore, photorespiration leads to the wastage of carbon and energy, eventually decreasing the yield of photosynthesis [51]. Nonetheless, atmospheric O2 concentration usually remains higher compared to atmospheric CO2, thus further favoring the oxygenase functionality of Rubisco and thereby promoting photorespiration. To counter this situation, microalgae have developed CO2 concentrating mechanisms (CCMs) to enhance the concentrations of CO2 within close range of Rubisco [52][53].
2.2. Heterotrophic Metabolism
Heterotrophic metabolism occurs with or without solar energy, and it requires organic carbon. Although the majority of microalgae are photoautotrophic, there are cases where several microalgae can grow via heterotrophic metabolism under dark conditions or under low-light conditions, which is insufficient for autotrophic metabolism. These particular algae heterotrophically metabolize a wide range of organic carbon sources in these light-deprived environments [54][55][56][57]. This metabolism follows the pentose phosphate pathway (PPP), which involves the usage of organic carbons derived from acetate, glucose, lactate, and glycerol, and different enzymes involved in transportation, phosphorylation, anabolic and catabolic metabolism, and yielding energy via the substrate or respiration [43]. However, in a few algal strains, heterotrophy can also occur in the presence of light, and such processes are termed photoheterotrophy [58]. The characteristics of the heterotrophic microalgae cultivation are (i) comparably higher capacity to assimilate and grow under light-impoverished conditions; (ii) a fast growth rate; and (iii) the capability to metabolize various types of resources of organic carbon sources [56]. Numerous microalgal strains have been examined in heterotrophic conditions for the production of biomass, and various important metabolites using glucose as a carbon source [59][60].
2.3. Mixotrophic Metabolism
Mixotrophic metabolism obeys both autotrophic photosynthesis and heterotrophic assimilation. This metabolism can be considered a derivative of the heterotrophic metabolism as both CO2 and organic carbon are used together. Mixotrophic metabolism is accompanied by respiration and photosynthesis, resulting in maximum glucose usage [61]. Thus, mixotrophic metabolism can employ both organic and inorganic carbon, thereby leading to the high production of biomass [62][63]. The organic carbon is captured via aerobic respiration, whereas inorganic carbon is absorbed through photosynthesis [64]. Mixotrophic microalgae cultivation delivers higher cell yields per unit of energy input compared to autotrophic or heterotrophic cultivations [65]. Furthermore, mixotrophic metabolism manifests a lower energy-conversion efficiency compared to heterotrophic metabolism [65]. However, both these mechanisms preserve the important pigments and photosynthetic carotenoids under solar irradiation [66][67]. There are certain aspects where mixotrophic cultivation offers extra benefits over photoautotrophic cultivation, such as an increased growth rate, decreased growth cycles, insignificant decrement of biomass in the dark, and overall higher biomass yields [68][69]. However, mixotrophic metabolisms have their own disadvantages, i.e., comparably costly due to the high requirement of organic carbon resources and are vulnerable to intrusive heterotrophic bacteria in bare pond arrangements Moreover, balancing two kinds of metabolisms is another challenge for the mixotrophic mechanism. However, mixotrophic metabolisms have their own disadvantages, as they are costly due to their necessity of organic carbon resources and are vulnerable to intrusive heterotrophic bacteria in bare pond arrangements [70]. Moreover, balancing two kinds of metabolisms is another challenge for the mixotrophic mechanism.
References
- Hansen, J.; Sato, M.; Ruedy, R.; Lacis, A.; Oinas, V. Global warming in the twenty-first century: An alternative scenario. Proc. Natl. Acad. Sci. USA 2000, 97, 9875–9880.
- IPCC. Special Report on Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014; ISBN 92-9169-1135.
- Available online: https://www.epa.gov/ghgemissions (accessed on 20 November 2021).
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide; National Oceanic and Atmospheric Administration: Copenhagen, Denmark, 2020.
- Joint, I.; Doney, S.C.; Karl, D.M. Will ocean acidification affect marine microbes? ISME J. 2011, 5, 1–7.
- Yang, X.; Liu, L.; Yin, Z.; Wang, X.; Wang, S.; Ye, Z. Quantifying photosynthetic performance of phytoplankton based on photosynthesis—Irradiance response models. Environ. Sci. Eur. 2020, 32, 24.
- Shreyash, N.; Sonker, M.; Bajpai, S.; Tiwary, S.K.; Khan, M.A. The Review of Carbon Capture-Storage Technologies and Developing Fuel Cells for Enhancing Utilization. Energies 2021, 14, 4978.
- Osman, A.I.; Hefny, M.; Maksoud, M.I.A.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849.
- Chew, K.W.; Yap, Y.J.; Show Loke, P.; Suan, H.N.; Ching, J.J.; Chuan, T. Microalgae biorefinery: High-value products perspectives. Bioresour. Technol. 2017, 229, 53–62.
- Faried, M.; Samer, M.; Abdelsalam, E.; Yousef, R.S.; Attia, Y.A.; Ali, A.S. Biodiesel production from microalgae: Processes, technologies and recent advancements. Renew. Sustain. Energy Rev. 2017, 79, 893–913.
- Klinthong, W.; Yang, Y.; Huang, C.; Tan, C. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742.
- Camerini, F.; de Morais, M.G.; da Silva Vaz, B.; de Morais, E.G.; Costa, J.A.V. Biofixation of CO2 on a pilot scale: Scaling of the process for industrial application. Afr. J. Microbiol. Res. 2016, 10, 768–774.
- Thomas, D.M.; Mechery, J.; Paulose, S.V. Carbon dioxide capture strategies from flue gas using microalgae: A review. Environ. Sci. Pollut. Res. 2016, 16926–16940.
- Zhu, X.; Rong, J.; Chen, H.; He, C.; Hu, W.; Wang, Q. An informatics-based analysis of developments to date and prospects for the application of microalgae in the biological sequestration of industrial flue gas. Appl. Microbiol. Biotechnol. 2016, 100, 2073–2082.
- Cuellar-bermudez, S.P.; Garcia-perez, J.S.; Rittmann, B.E.; Parra-saldivar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third-generation biofuels. J. Clean. Prod. 2020, 98, 53–65.
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control 2012, 10, 456–469.
- Khan, S.A.; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372.
- Barrett, J.; Girr, P.; Mackinder, L.C.M. Pyrenoids: CO2-fixing phase separated liquid organelles one phase two phase. BBA-Mol. Cell Res. 2021, 1868, 118949.
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015, 82, 429–448.
- Yen, H.; Ho, S.; Chen, C.; Chang, J. CO2, NOx and SOx removal from flue gas via microalgae cultivation: A critical review. Biotechnol. J. 2015, 10, 829–839.
- Larkum, A.W.D.; Ross, I.L.; Kruse, O.; Hankamer, B. Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol. 2012, 30, 198–205.
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M. A review on sustainable microalgae-based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59.
- Quiroz, C.E.; Peebles, C.; Bradley, T.H. Scalability of combining microalgae-based biofuels with wastewater facilities: A review. Algal Res. 2015, 9, 160–169.
- Kothari, R.; Prasad, R.; Kumar, V.; Singh, D.P. Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresour. Technol. 2013, 144, 499–503.
- Bhola, V.; Swalaha, F.; Kumar, R.R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 2103–2118.
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Pohrmen, C.B.; Kumar, V. Photosynthetic microalgae—Based carbon sequestration and generation of biomass in biorefinery approach for renewable biofuels for a cleaner environment. Biomass Conser. Biorefin. 2021, 11, 1–19.
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2014; p. 151.
- Liu, Z. Near-real-time monitoring of global CO2 emissions reveals the effects of the COVID-19 pandemic. Nat. Commun. 2020, 11, 1–11.
- Crippa, M.; Guizzardi, D.; Solazzo, E.; Muntean, M.; Schaaf, E.; Monforti-Ferrario, F.; Banja, M.; Olivier, J.G.J.; Grassi, G.; Rossi, S.; et al. GHG Emissions of All World Countries—2021 Report; EUR 30831 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-41546-6.
- Crippa, M.; Solazzo, E.; Huang, G.; Guizzardi, D.; Koffi, E.; Muntean, M.; Schieberle, C.; Friedrich, R.; Janssens-Maenhout, G. High-resolution temporal profiles in the Emissions Database for Global Atmospheric Research. Sci. Data 2020, 7, 121.
- IEA. World Energy Outlook; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/world-energy-outlook-2020 (accessed on 20 November 2021).
- Leung, D.Y.C.; Caramanna, G.; Maroto-valer, M.M. An overview of current status of carbon dioxide captures and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Jerndal, E.; Mattisson, T.; Lyngfelt, A.; Combustion, C.; After, O. Thermal analysis of chemical-looping combustion. Chem. Eng. Res. Des. 2006, 84, 795–806.
- Pires, J.C.M.; Martins, F.G.; Simões, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renew. Sustain. Energy Rev. 2012, 16, 3043–3053.
- Suzuki, T.; Toriumi, M.; Sakemi, T.; Masui, N. Conceptual Design of CO2 Transportation System for CCS. Energy Procedia 2013, 37, 2989–2996.
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–241.
- Moroney, J.V.; Ynalvez, R.A. Proposed Carbon Dioxide Concentrating Mechanism in Chlamydomonas reinhardtii. Eukaryot. Cell 2007, 6, 1251–1259.
- Prasad, R.; Shabnam, N.; Pardha-Saradhi, P. Immobilization on cotton cloth pieces is ideal for storage and conservation of microalgae. Algal Res. 2016, 20, 172–179.
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21.
- Available online: http://capitalenergy.biz/?p=16989 (accessed on 13 October 2021).
- Aci, G.; Matito-martos, I.; Sepúlveda, C.; Cintia, G.; Perez-carbajo, J.; Ania, C. Potential of CO2 capture from flue gases by physicochemical and biological methods: A comparative study. Chem. Eng. J. 2021, 417, 1–10.
- Vuppaladadiyam, A.K.; Yao, J.G.; Florin, N.; George, A.; Wang, X.; Labeeuw, L.; Jiang, Y.; Davis, R.W.; Abbas, A.; Ralph, P.; et al. Impact of Flue Gas Compounds on Microalgae and Mechanisms for Carbon Assimilation and Utilization. ChemSusChem 2018, 11, 334–355.
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 Concentrating Mechanisms in Algae: Mechanisms, Environmental Modulation, and Evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131.
- Spalding, M.H.; Biology, C. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J. Exp. Bot. 2008, 59, 1463–1473.
- Min, M.; Hu, B.; Zhou, W.; Li, Y.; Chen, P.; Ruan, R. Mutual influence of light and CO2 on carbon sequestration via cultivating mixotrophic alga Auxenochlorella protothecoides UMN280 in an organic carbon-rich wastewater. J. Appl. Phycol. 2012, 24, 1099–1105.
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288.
- Bassaham, J.A.; Benson, A.A.; Kay, L.D.; Harris, A.Z.; Wilsson, A.T.C.M. The Path of Carbon in Photosynthesis. XXI. The Cyclic Regeneration of Carbon. J. Am. Chem. Soc. 1953, 76, 1760–1770.
- Schloss, J.V. Oxygen toxicity from plants to people. Planta 2002, 216, 38–43.
- Williams, P.J.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590.
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Phosphoclycol4te Production Catalyzed By Ribulose Diphosphate Carboxylase. Biochem. Biophys. Res. Commun. 1971, 45, 716–722.
- Koning, R.E. Photorespiration. Plant Physiol. 1994. Available online: http://plantphys.info/plant_physiology/photoresp.shtml (accessed on 20 November 2021).
- Sayre, R. Microalgae: The Potential for Carbon Capture. Bioscience 2010, 60, 722–727.
- Moroney, J.V.; Jungnick, N.; Dimario, R.J.; Longstreth, D.J. Photorespiration and carbon concentrating mechanisms: Two adaptations to high O2, low CO2 conditions. Photosynth. Res. 2013, 117, 121–131.
- Tuchman, N.C.; Schollett, M.A.; Rier, S.T.; Geddes, P. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 2006, 561, 167–177.
- Lowrey, J.; Armenta, R.E.; Brooks, M.S. Nutrient and media recycling in heterotrophic microalgae cultures. Appl. Microbiol. Biotechnol. 2016, 100, 1061–1075.
- Morales-Sanchez, D.; Kyndt, J.; Martinez, A. Heterotrophic growth of microalgae: Metabolic aspects. World J. Microbiol. Biotechnol. 2015, 31, 1–9.
- Liu, J.; Sun, Z.; Chen, F. Biofuels from Algae, 1st ed.; Elsevier: San Diego, CA, USA, 2014; pp. 111–142.
- Ingram, L.O.; Baalen, C.; Van Calder, J.A. Role of Reduced Exogenous Organic Compounds in the Physiology of the Blue-Green Bacteria (Algae): Photoheterotrophic Growth of an “Autotrophic” Blue-Green Bacterium. J. Bacteriol. 1973, 114, 701–705.
- Yan, D.; Lu, Y.; Chen, Y.; Wu, Q. Waste molasses alone displaces glucose-based medium for microalgal fermentation towards cost-saving biodiesel production. Bioresour. Technol. 2011, 102, 6487–6493.
- Pleissner, D.; Chi, W.; Sun, Z.; Sze, C.; Lin, K. Biore source Technology Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 2013, 137, 139–146.
- Lee, Y.K. Algal nutrition. Heterotrophic carbon nutrition. In Handbook of Microalgal Culture. Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing: Oxford, UK, 2004; p. 116.
- Wang, J.; Yang, H.; Wang, F. Mixotrophic Cultivation of Microalgae for Biodiesel Production: Status and Prospects. Appl. Biochem. Biotechnol. 2014, 172, 3307–3329.
- Mohan, S.V.; Devi, M.P. Bioresource Technology Salinity stress induced lipid synthesis to harness biodiesel during dual mode cultivation of mixotrophic microalgae. Bioresour. Technol. 2014, 165, 288–294.
- Hu, B.; Min, M.; Zhou, W.; Li, Y.; Mohr, M.; Cheng, Y.; Lei, H.; Liu, Y.; Lin, X.; Chen, P.; et al. Influence of Exogenous CO2 on Biomass and Lipid Accumulation of Microalgae Auxenochlorella protothecoides Cultivated in Concentrated Municipal Wastewater. Appl. Biochem. Biotechnol. 2012, 166, 1661–1673.
- Yang, C.; Hua, Q.; Shimizu, K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 2000, 6, 87–102.
- Del Campo, J.A.; García-gonzález, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174.
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431.
- Park, K.C.; Whitney, C.; Mcnichol, J.C.; Dickinson, K.E.; Macquarrie, S.; Skrupski, B.P.; Zou, J.; Wilson, K.E.; O’Leary, S.J.; McGinn, P.J. Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: Potential applications for wastewater remediation for biofuel production. J. Appl. Phycol. 2012, 24, 339–348.
- Kong, W.; Song, H.; Hua, S.; Yang, H.; Yang, Q.; Xia, C. Enhancement of biomass and hydrocarbon productivities of Botryococcus braunii by mixotrophic cultivation and its application in brewery wastewater treatment. Afr. J. Microbiol. Res. 2012, 6, 1489–1496.
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 1–14.
More
Information
Subjects:
Area Studies
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.7K
Revisions:
2 times
(View History)
Update Date:
10 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





