
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lin Hu | + 2787 word(s) | 2787 | 2021-12-02 03:48:16 | | | |
| 2 | Peter Tang | Meta information modification | 2787 | 2021-12-10 04:16:44 | | |
Video Upload Options
Solution-processed polymer solar cells (PSCs) have advantages of low cost, solution processability, light weight, and excellent flexibility. Recent progress in materials synthesis and devices engineering has boosted the power conversion efficiency (PCE) of single-junction PSCs over 17%. Poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT:PSS) is one of the most promising candidates for electrodes due to its high conductivity (>4000 S/cm), excellent transmittance (>90%), intrinsically high work function (WF > 5.0 eV), and aqueous solution processability. To date, a great number of single-junction PSCs based on PEDOT:PSS electrodes have realized a PCE over 12%.
1. Introduction
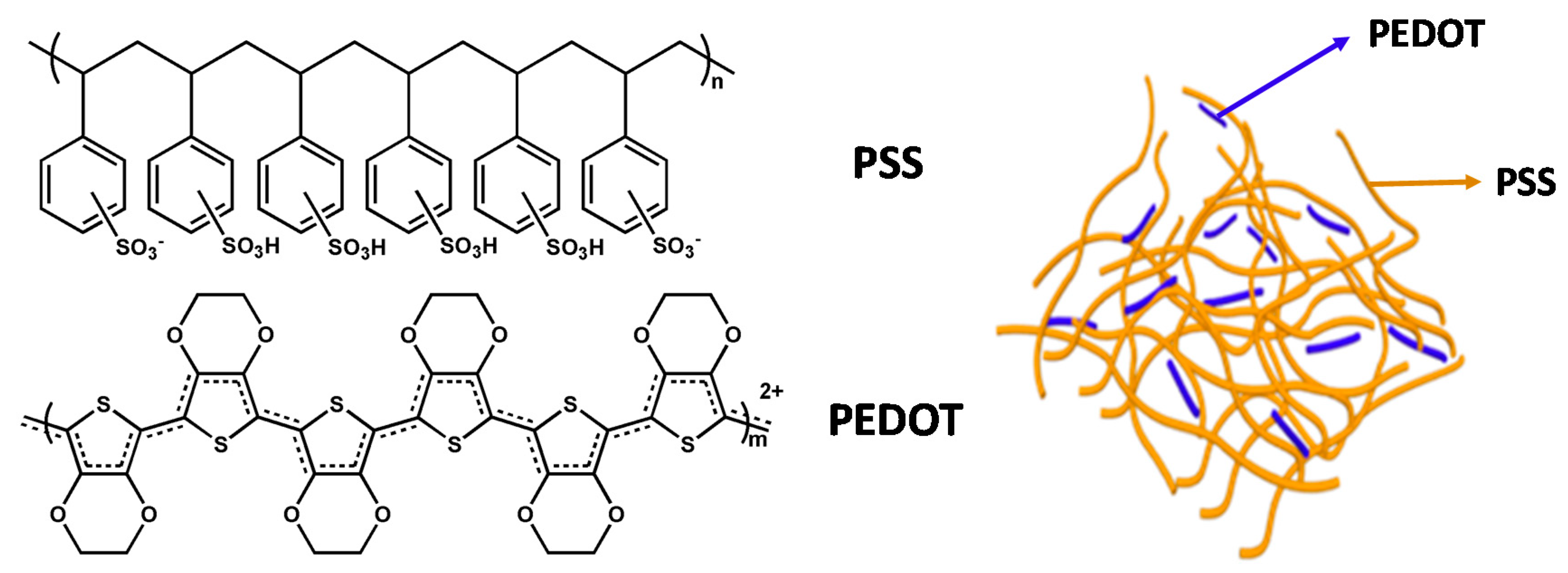
2. PEDOT:PSS Electrodes
2.1. Synthesis and Commercialization of PEDOT:PSS Complex
|
Trade Name |
Solids Content in Water (wt.%) |
PEDOT:PSS Ratio (w/w) |
Viscosity at 20 °C (mPa) |
Particle Size d50 (nm) |
Conductivity (S/cm) |
|---|---|---|---|---|---|
|
Clevios P |
1.3 |
1:2.5 |
80 |
80 |
<10 |
|
Clevios PH |
1.3 |
1:2.5 |
20 |
30 |
<10 |
|
Clevios PVP AI 4083 |
1.5 |
1:6 |
10 |
40 |
10−3 |
|
Clevios PVP CH800 |
2.8 |
1:20 |
15 |
25 |
10−5 |
|
Clevios PH500 |
1.1 |
1:2.5 |
25 |
30 |
500 a |
|
Clevios PH750 |
1.1 |
1:2.5 |
25 |
30 |
750 a |
|
Clevios PH1000 |
1.1 |
1:2.5 |
30 |
30 |
1000 a |
a Conductivities of Clevios PH500, PH750, and PH1000 are measured for dispersions containing 5% dimethyl sulfoxide.
2.2. Properties of PEDOT:PSS
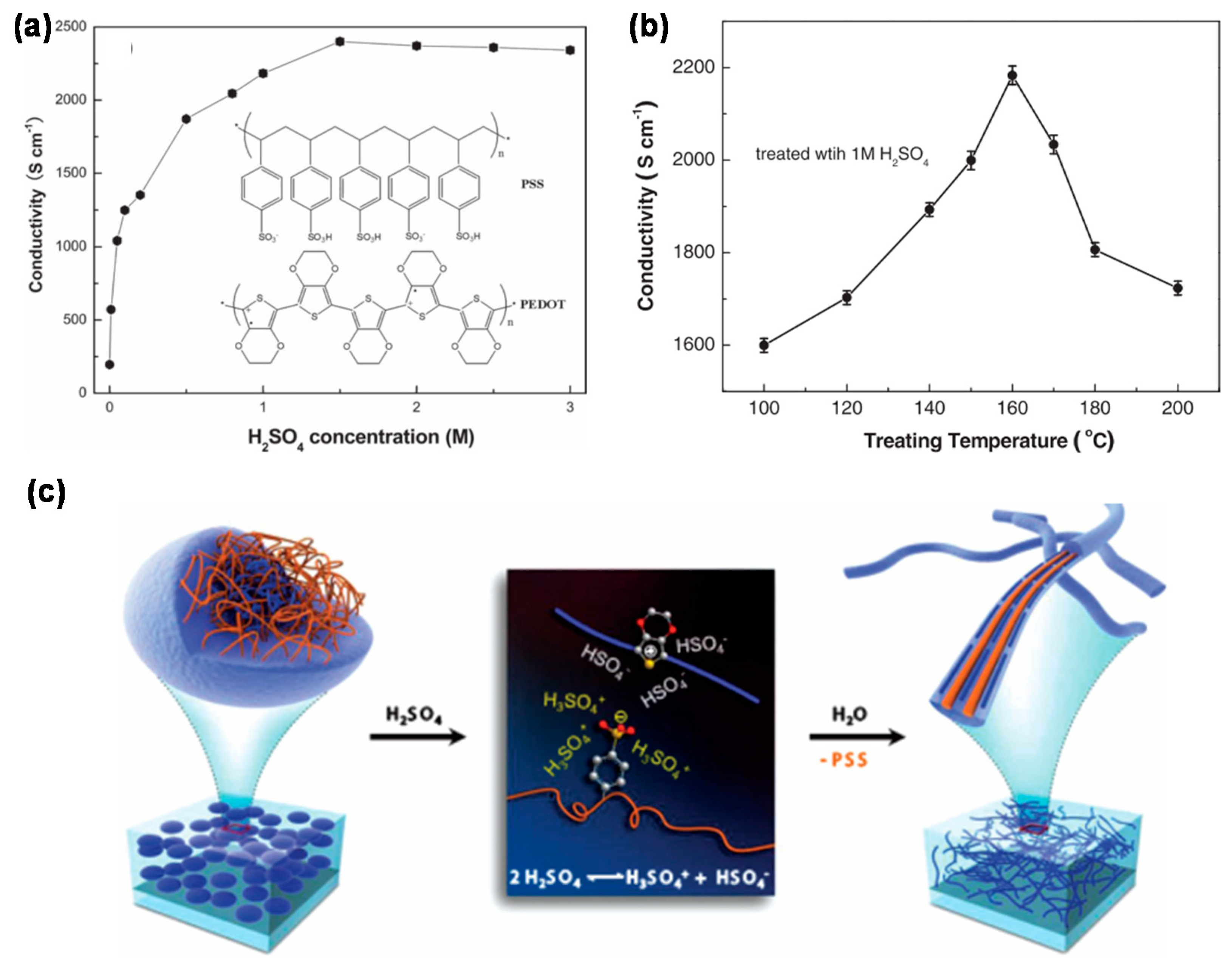
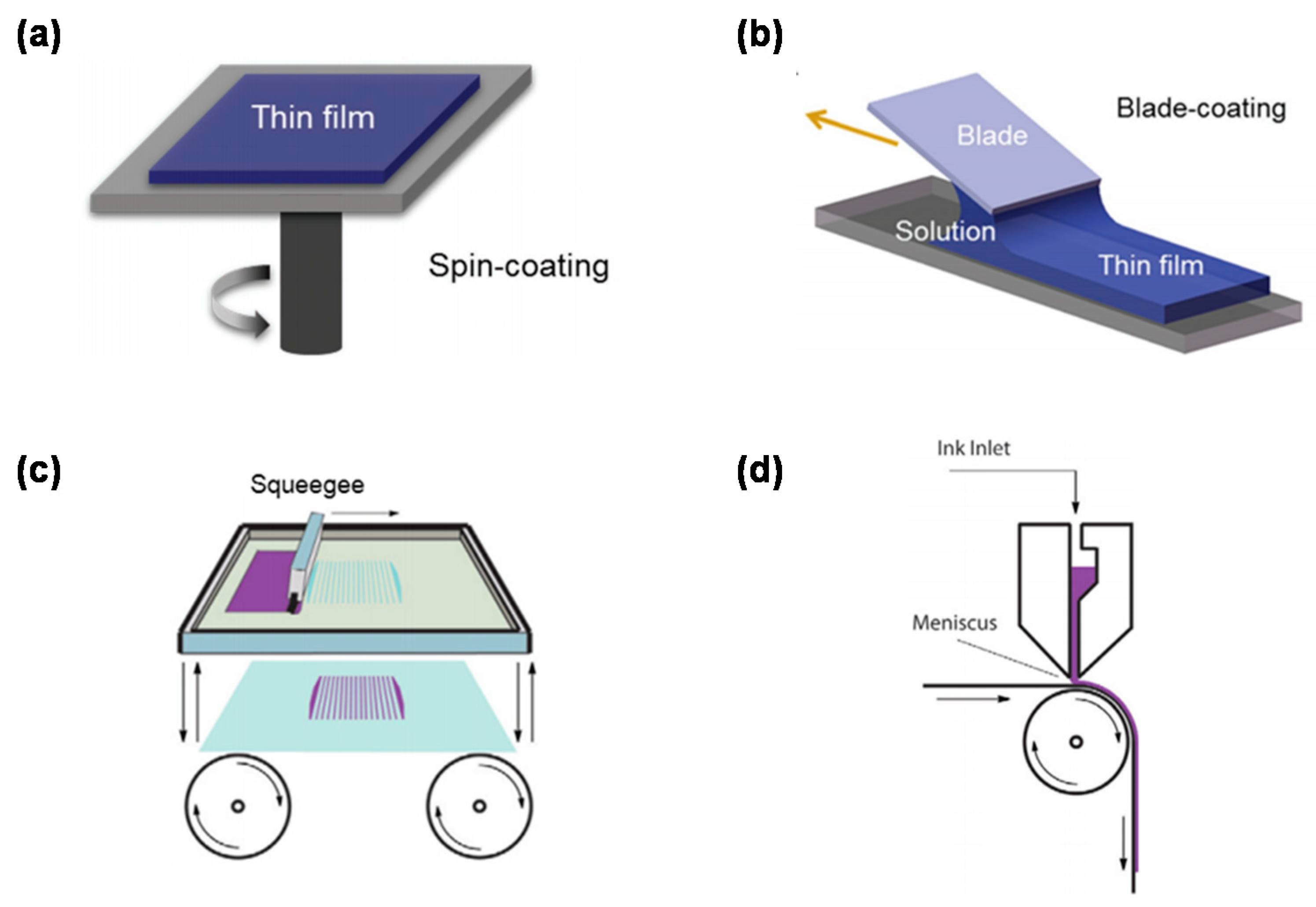
2.3. Fabrication Technologies of PEDOT:PSS Electrodes
3. Polymer Solar Cells Based on PEDOT:PSS
|
Device |
Thickness (nm) |
R (Ω/sq) |
T (%) |
JSC (mA/cm2) |
VOC (V) |
FF |
PCE (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
Glass/PEDOT:PSS:S/MEH-PPV/PCBM/Al |
150 |
~103 |
80 |
1.6 |
0.75 |
0.30 |
0.36 |
[3] |
|
Glass/EG-PEDOT:PSS/MEH-PPV:PCBM/Ca/Al |
250 |
250 |
- |
5.1 |
0.74 |
0.39 |
1.5 |
[28] |
|
Glass/PH500:5%DMSO/P3HT:PC61BM/Ca/Al |
100 |
213 |
90 |
9.73 |
0.63 |
0.54 |
3.27 |
[29] |
|
PET/PH500:5%DMSO/P3HT:PC61BM/Ca/Al |
100 |
213 |
90 |
9.16 |
0.61 |
0.50 |
2.8 |
[29] |
|
Glass/Methanol treated PH1000/P3HT:PC61BM/Ca/Al |
~50 |
25 |
85 |
9.51 |
0.58 |
0.67 |
3.71 |
[30] |
|
Glass/H2SO4 treated PH1000/PEDOT:PSS(4083)/P3HT:PC61BM/Ca/Al |
70 |
67 |
87 |
9.29 |
0.59 |
0.65 |
3.56 |
[20] |
|
Glass/PEDOT:PSS:CNTs/PEIE/ZnO/PBDBTTT-C-T:PC71BM/V2O5-RGO/Ag |
- |
40.51 |
80 |
15.76 |
0.77 |
0.62 |
7.47 |
[31] |
|
Glass/Ag grid/PH500 /ZnO/C60SAM/P3HT:PC61BM/PEDOT:PSS(4083)/Ag |
- |
9.1 |
79 |
9.39 |
0.60 |
0.57 |
3.21 |
[32] |
|
Glass/CH4SO3 treated PH1000/PEDOT:PSS(4083)/PBDB-T:IT-M/PDINO/Al |
80 |
40 |
- |
16.01 |
0.925 |
0.72 |
10.60 |
[33] |
|
PET/ CH4SO3 treated PH1000/PEDOT:PSS(4083)/PBDB-T:IT-M/PDINO/Al |
80 |
40 |
90 |
15.49 |
0.93 |
0.70 |
10.12 |
[34] |
|
Glass/ITO/ZnO/P3HT:PC61BM/CPP:PEDOT:PH1000 |
- |
420 |
- |
7.2 |
0.55 |
0.58 |
2.4 |
[35] |
|
Glass/ITO/PEI/P3HT:ICBA/PH1000:PEG-TmDD |
- |
526 |
- |
8.70 |
0.78 |
0.60 |
4.1 |
[14] |
|
Glass/ITO/ZnO/PBDB-T:ITIC/MC-PH1000:EG:PEG-TmDD |
- |
- |
- |
13.0 |
0.86 |
0.66 |
7.38 |
[36] |
|
Glass/metal/ZnO/P3HT:PCBM/PH1000T/Ag-busbar |
190 |
- |
- |
6.96 |
0.58 |
0.65 |
3.08 |
[37] |
|
Glass/ITO/PEI/P3HT:ICBA/PH1000/PEI/P3HT:ICBA/PH1000:EG:PEG-TmDD T |
- |
- |
- |
3.10 |
1.62 |
0.68 |
3.60 |
[38] |
|
Glass/ITO/PEI/P3HT:ICBA/PEDOT:PSS(4083)/HCT-PEDOT:PSS T |
2780 |
2.60 |
- |
8.65 |
0.81 |
0.66 |
4.6 |
[39] |
|
Glass/PH500:5%DMSO/ZnO-NPs/C60-SAM/P3HT:PCBM/PEDOT:PSS(4083)/PH500:5%DMSO T |
130 |
370 |
- |
5.49 |
0.31 |
0.28 |
0.47 |
[40] |
|
PES/PH1000 5% DMSO/PEI/P3HT:ICBA/PH1000:CPP-PEDOT T |
130 (bottom) 160 (top) |
- |
- |
7.1 |
0.80 |
0.52 |
3.0 |
[41] |
|
PES/PH1000:5%DMSO/PEI/P3HT:ICBAT/PH1000:5%DMSO T |
120 (bottom) 150 (top) |
- |
- |
5.6 |
0.80 |
0.55 |
2.4 |
[42] |
|
Glass/LWF-PEDOT:PSS/P3HT:ICBA/HWF-PH1000:EG:PEG-TmDD T |
124 (bottom) 150 (top) |
- |
- |
8.10 |
0.81 |
0.61 |
4.0 |
[43] |
|
PES/H3PO4-PEDOT:PSS/PEI/P3HT:ICBA/EG-PEDOT:PSS |
85 (bottom) 150 (top) |
120 (bottom) |
- |
6.6 |
0.84 |
0.60 |
3.3 |
[44] |
|
PES/hc-PEDOT:PSS/PEI/P3HT:ICBA/PEDOT:PSS/PEI/…P3HT:ICBA/hc-PEDOT:PSST |
- |
- |
- |
0.40 |
5.40 |
0.40 |
0.85 |
[45] |
Recent representative research progress on PEDOT:PSS-based solar cells including served as bottom, top and both electrodes are included in this table. The parameters, such as thickness, sheet resistance (R) and transmittance are referring to the PEDOT:PSS-based bottom or top electrodes in the PSCs device. The superscript T of the electrode in the device structure refers to the PEDOT:PSS layer deposited via film transfer lamination technique. CNTs: Carbon nanotubes. JSC: Short-circuit current density. VOC: open-circuit voltage. FF: Fill factor. PCE: Power conversion efficiency.
References
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M. 17% Efficient organic solar cells based on liquid exfoliated WS2 as a replacement for PEDOT: PSS. Adv. Mater. 2019, 1902965.
- Machui, F.; Hösel, M.; Li, N.; Spyropoulos, G.D.; Ameri, T.; Søndergaard, R.R.; Jørgensen, M.; Scheel, A.; Gaiser, D.; Kreul, K.; et al. Cost analysis of roll-to-roll fabricated ITO free single and tandem organic solar modules based on data from manufacture. Energy Environ. Sci. 2014, 7, 2792–2802.
- Zhang, F.; Johansson, M.; Andersson, M.R.; Hummelen, J.C.; Inganäs, O. Polymer photovoltaic cells with conducting polymer anodes. Adv. Mater. 2002, 14, 662–665.
- Lei, T.; Peng, R.; Huang, L.; Song, W.; Yan, T.; Zhu, L.; Ge, Z. 13.5% flexible organic solar cells achieved by robust composite ITO/PEDOT:PSS electrodes. Mater. Today Energy 2019, 14, 100334.
- Zeng, G.; Zhang, J.; Chen, X.; Gu, H.; Li, Y.; Li, Y. Breaking 12% efficiency in flexible organic solar cells by using a composite electrode. Sci. China. Chem. 2019, 62, 851–858.
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088.
- Ghosh, S.; Inganäs, O. Self-assembly of a conducting polymer nanostructure by physical crosslinking: Applications to conducting blends and modified electrodes. Synth. Met. 1999, 101, 413–416.
- Elschner, A.; Lövenich, W. Solution-deposited PEDOT for transparent conductive applications. MRS Bull. 2011, 36, 794–798.
- Lövenich, W. PEDOT-properties and applications. Polym. Sci. Ser. C 2014, 56, 135–143.
- Li, Z.; Qin, F.; Liu, T.; Ge, R.; Meng, W.; Tong, J.; Xiong, S.; Zhou, Y. Optical properties and conductivity of PEDOT:PSS films treated by polyethylenimine solution for organic solar cells. Org. Electron. 2015, 21, 144–148.
- Kim, J.; Jung, J.; Lee, D.; Joo, J. Enhancement of electrical conductivity of poly (3, 4-ethylenedioxythiophene)/poly (4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316.
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High-conductivity poly (3, 4-ethylenedioxythiophene): Poly (styrene sulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater. 2005, 15, 203–208.
- Fan, B.; Mei, X.; Ouyang, J. Significant conductivity enhancement of conductive poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) films by adding anionic surfactants into polymer solution. Macromolecules 2008, 41, 5971–5973.
- Li, Z.; Meng, W.; Tong, J.; Zhao, C.; Qin, F.; Jiang, F.; Xiong, S.; Zeng, S.; Xu, L.; Hu, B.; et al. A nonionic surfactant simultaneously enhancing wetting property and electrical conductivity of PEDOT:PSS for vacuum-free organic solar cells. Sol. Energy Mater. Sol. Cells 2015, 137, 311–318.
- Döbbelin, M.; Marcilla, R.; Salsamendi, M.; Pozo-Gonzalo, C.; Carrasco, P.M.; Pomposo, J.A.; Mecerreyes, D. Influence of ionic liquids on the electrical conductivity and morphology of PEDOT:PSS films. Chem. Mater. 2007, 19, 2147–2149.
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT: PSS nanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272.
- Ely, F.; Matsumoto, A.; Zoetebier, B.; Peressinotto, V.S.; Hirata, M.K.; de Sousa, D.A.; Maciel, R. Handheld and automated ultrasonic spray deposition of conductive PEDOT:PSS films and their application in AC EL devices. Org. Electron. 2014, 15, 1062–1070.
- Luo, J.; Billep, D.; Waechtler, T.; Otto, T.; Toader, M.; Gordan, O.; Sheremet, E.; Martin, J.; Hietschold, M.; Zahn, D.R. Enhancement of the thermoelectric properties of PEDOT: PSS thin films by post-treatment. J. Mater. Chem. A 2013, 1, 7576–7583.
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671.
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440.
- Bae, E.J.; Kang, Y.H.; Jang, K.-S.; Cho, S.Y. Enhancement of thermoelectric properties of PEDOT: PSS and tellurium-PEDOT: PSS hybrid composites by simple chemical treatment. Sci. Rep. 2016, 6, 18805.
- Zhi, C.; Dai, L. Flexible Energy Conversion and Storage Devices; John Wiley & Sons: Hoboken, NJ, USA, 2018.
- Savva, A.; Neophytou, M.; Koutsides, C.; Kalli, K.; Choulis, S.A. Synergistic effects of buffer layer processing additives for enhanced hole carrier selectivity in inverted organic photovoltaics. Org. Electron. 2013, 14, 3123–3130.
- Shin, D.; Kang, D.; Lee, J.B.; Ahn, J.H.; Cho, I.W.; Ryu, M.Y.; Cho, S.W.; Jung, N.E.; Lee, H.; Yi, Y. Electronic structure of nonionic surfactant-modified PEDOT: PSS and its application in perovskite solar cells with reduced interface recombination. ACS Appl. Mater. Interfaces 2019, 11, 17028–17034.
- Savva, A.; Georgiou, E.; Papazoglou, G.; Chrusou, A.Z.; Kapnisis, K.; Choulis, S.A. Photovoltaic analysis of the effects of PEDOT:PSS-additives hole selective contacts on the efficiency and lifetime performance of inverted organic solar cells. Sol. Energy Mater. Sol. Cells 2015, 132, 507–514.
- Rühle, S.; Greenshtein, M.; Chen, S.-G.; Merson, A.; Pizem, H.; Sukenik, C.S.; Cahen, D.; Zaban, A. Molecular adjustment of the electronic properties of nanoporous electrodes in dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 18907–18913.
- Jang, Y.; Jo, J.; Kim, D.S. Control of doctor-blade coated poly (3, 4-ethylenedioxythiophene)/poly (styrenesulfonate) electrodes shape on prepatterned substrates via microflow control in a drying droplet. J. Polym. Sc. Part B Polym. Phys. 2011, 49, 1590–1596.
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. Polymer Optoelectronic Devices with High-Conductivity Poly(3,4-Ethylenedioxythiophene) Anodes. J. Macromol. Sci. Part A 2004, 41, 1497–1511.
- Na, S.-I.; Kim, S.-S.; Jo, J.; Kim, D.-Y. Efficient and flexible ito-free organic solar cells using highly conductive polymer anodes. Adv. Mater. 2008, 20, 4061–4067.
- Ahlswede, E.; Mühleisen, W.; bin Moh Wahi, M.W.; Hanisch, J.; Powalla, M. Highly efficient organic solar cells with printable low-cost transparent contacts. Appl. Phys. Lett. 2008, 92, 143307.
- Hu, X.; Chen, L.; Zhang, Y.; Hu, Q.; Yang, J.; Chen, Y. Large-scale flexible and highly conductive carbon transparent electrodes via roll-to-roll process and its high performance lab-scale indium tin oxide-free polymer solar cells. Chem. Mater. 2014, 26, 6293–6302.
- Zou, J.; Yip, H.-L.; Hau, S.K.; Jen, A.K.Y. Metal grid/conducting polymer hybrid transparent electrode for inverted polymer solar cells. Appl. Phys. Lett. 2010, 96, 203301.
- Song, W.; Fan, X.; Xu, B.; Yan, F.; Cui, H.; Wei, Q.; Peng, R.; Hong, L.; Huang, J.; Ge, Z. All-solution-processed metal-oxide-free flexible organic solar cells with over 10% efficiency. Adv. Mater. 2018, 30, e1800075.
- Hu, X.; Chen, L.; Tan, L.; Ji, T.; Zhang, Y.; Zhang, L.; Zhang, D.; Chen, Y. In situ polymerization of ethylenedioxythiophene from sulfonated carbon nanotube templates: Toward high efficiency ITO-free solar cells. J. Mater. Chem. A 2016, 4, 6645–6652.
- Zhou, Y.; Cheun, H.; Choi, S.; Fuentes-Hernandez, C.; Kippelen, B. Optimization of a polymer top electrode for inverted semitransparent organic solar cells. Org. Electron. 2011, 12, 827–831.
- Mao, L.; Luo, B.; Sun, L.; Xiong, S.; Fan, J.; Qin, F.; Hu, L.; Jiang, Y.; Li, Z.; Zhou, Y. Writable, patternable organic solar cells and modules inspired from old Chinese calligraphy tradition. Mater. Horiz. 2018, 5, 123–130.
- Gupta, D.; Wienk, M.M.; Janssen, R.A.J. Efficient polymer solar cells on opaque substrates with a laminated PEDOT:PSS top electrode. Adv. Energy Mater. 2013, 3, 782–787.
- Tong, J.; Xiong, S.; Li, Z.; Jiang, F.; Mao, L.; Meng, W.; Zhou, Y. Vacuum-free and metal electrode-free organic tandem solar cells. Appl. Phys. Lett. 2015, 106, 053306.
- Li, Z.; Ma, G.; Ge, R.; Qin, F.; Dong, X.; Meng, W.; Liu, T.; Tong, J.; Jiang, F.; Zhou, Y.; et al. Free-standing conducting polymer films for high-performance energy devices. Angew. Chem. Int. Ed. 2016, 55, 979–982.
- Hau, S.K.; Yip, H.-L.; Zou, J.; Jen, A.K.Y. Indium tin oxide-free semi-transparent inverted polymer solar cells using conducting polymer as both bottom and top electrodes. Org. Electron. 2009, 10, 1401–1407.
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J. A universal method to produce low–work function electrodes for organic electronics. Science 2012, 336, 327–332.
- Jin, Y.; Li, Z.; Qin, L.; Liu, X.; Mao, L.; Wang, Y.; Qin, F.; Liu, Y.; Zhou, Y.; Zhang, F. Laminated free standing PEDOT:PSS electrode for solution processed integrated photocapacitors via hydrogen-bond interaction. Adv. Mater. Interfaces 2017, 4, 1700704.
- Zhou, Y.; Khan, T.M.; Shim, J.W.; Dindar, A.; Fuentes-Hernandez, C.; Kippelen, B. All-plastic solar cells with a high photovoltaic dynamic range. J. Mater. Chem. A 2014, 2, 3492–3497.
- Li, Z.; Liang, Y.; Zhong, Z.; Qian, J.; Liang, G.; Zhao, K.; Shi, H.; Zhong, S.; Yin, Y.; Tian, W. A low-work-function, high-conductivity PEDOT:PSS electrode for organic solar cells with a simple structure. Synth. Met. 2015, 210, 363–366.
- Meng, W.; Ge, R.; Li, Z.; Tong, J.; Liu, T.; Zhao, Q.; Xiong, S.; Jiang, F.; Mao, L.; Zhou, Y. Conductivity enhancement of PEDOT:PSS films via phosphoric acid treatment for flexible all-plastic solar cells. ACS Appl. Mater. Interfaces 2015, 7, 14089–14094.




