
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rima Hajjo | + 1428 word(s) | 1428 | 2021-04-29 08:13:48 | | | |
| 2 | Dean Liu | -10 word(s) | 1418 | 2021-12-10 01:48:58 | | |
Video Upload Options
Machine learning (ML) and artificial intelligence (AI) have enabled the identification of highly predictive, disease-specific biomarkers.
1. Introduction
Imaging is routinely used for cancer diagnosis and staging, for monitoring treatment efficacy, for detecting disease recurrence, or generally for cancer surveillance [1][2][3][4]. Understanding the anatomical and physiological aspects of medical images allows experts to distinguish aberrant from normal appearance [5]. Advances in analytical methods and the application of machine learning methods enabled the use of medical images as biomarkers that can potentially optimize cancer care and improve clinical outcome [5]. The imaging biomarkers that are currently, and successfully, used for clinical diagnosis have attracted many researchers’ attention as described in multiple publications [1][5][6][7][8][9][10][11][12][13][14][15][16][17][18].
Magnetic resonance imaging (MRI) is a diagnostic imaging technique that applies strong magnetic and radio waves to generate high quality MRI scans of body organs facilitating the diagnosis of tumors and other conditions such as brain and spinal cord diseases. Currently, MRI is one of the of the big data producers in biomedicine, and is being exploited as important generator of cancer biomarkers. In essence, a biomarker is a characteristic that is measured as an indicator of a biological condition of interest (i.e., normal biological processes, pathogenic processes, or responses to a therapeutic intervention) [19][20]. The process of biomarker prioritization starts with a theory and ends with biomarker validation in an experimental setting. However, the current dogmas in biomedicine may hinder the process of unbiased hypothesis generation due to the complexity of cancer phenotypes and patient attributes, which makes it harder for human experts and physicians to comprehend all the details in MRI scans [21]. This led to the rise MRI biomarkers, identified by ML, that could capture disease characteristics with high accuracy, efficiency, reproducibility and interpretability [5][22].
2. Imaging Biomarkers
Biomarker stands for biological marker and it is defined by the U.S. Food and Drug Administration (FDA) as “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions” [23]. Biomarkers can measure anatomical, histological, physiological, molecular, and radiographic characteristics. Imaging biomarkers are convenient and reliable [5]. In oncology, they represent comprehensive cancer features such as apoptosis, angiogenesis, growth, metabolism, invasion, metastasis, and selective target interaction [24]. Cancer imaging biomarkers are widely used for cancer identification, for the prediction of disease outcome, and for monitoring treatment responses [5]. Examples of imaging biomarkers include Tumor, Node, Metastasis (TNM) reflecting a staging system (i.e., a prognostic biomarker) and Response Evaluation Criteria in Solid Tumors (RECIST) which can be applied as a response biomarker [1]. Confirmed imaging biomarkers are used to support decision-making in clinical practice. The necessity for quantitative evaluation in diagnosis must be validated [5]. Quantitative approach is profound and exhaustive due to technology and apparatus differences as well as quantitative development that influences the extracted data [5]. The well-established QA and QC protocols are perquisite to validate and approve the reliability of medical assessment along with endeavor made by research, radiological, and medical institution [5]. In addition, significant factors should be considered such as isolating normal healthy from ailment tissues to achieve better diagnosis [5]. Table 1 provides a summary of the various types of imaging biomarkers used in cancer besides MRI.
Table 1. Imaging biomarkers for disease detection with examples.
| Disease | Biomarker | Quantitative (Q)/Semi-Quantitative (SQ)/Non-Quantitative (NQ) | Biomarkers Uses |
|---|---|---|---|
| Malignant disease | Lung RADS, pancreatic cancer action network (PanCan), national comprehensive cancer network (NCCN) criteria [25][26] |
SQ | AUC for malignancy 0.81–0.87 [27] |
| CT blood flow, perfusion, permeability measurements |
Q | Sensitivity 0.73, specificity 0.70 [28] AUC 0.75, sensitivity 0.79, specificity 0.75 [29] |
|
| Breast imaging (BI)-RADS [30] Prostate imaging (PI)-RADS [29] Liver imaging (LI)-RADS [31] |
SQ | positive predictive value (PPV) BI-RADS 0 14.1%, BI-RADS 4 39.1%, BI-RADS 5 92.9% PI-RADS 2 pooled sensitivity 0.85, pooled specificity 0.71 pooled sensitivity for malignancy 0.93 |
|
| Apparent diffusion coefficient (ADC) | Q | Liver AUC 0.82–0.95 Prostate AUC 0.84 |
|
| RECIST/morphological volume |
Q | Ongoing guidelines for treatment evaluation [32] | |
| Positron emission response criteria in solid tumors (PERCIST) /metabolic Volume [33] |
Q | Ongoing guidelines for treatment evaluation [32] | |
| Liver cancer Recurrent glioblastoma |
Dynamic contrast enhanced (DCE) metrics (perfusion parameters Ktrans, Kep, blood flow, Ve) |
Q | Hepatocellular cancer AUC 0.85, sensitivity 0.85, specificity 0.81 [29] Brain- Ktrans accuracy 86% [34] |
| Cancer Sarcoma [35] Lung cancer [36] |
18FDG- standardized uptake value (SUV) |
Q | Sarcoma-sensitivity 0.91, specificity 0.85, accuracy 0.88 Lung-sensitivity 0.68–0.95 |
| Cancer | Targeted radionuclides [37] In-octreotide [38][39] 68Gallium (Ga)-DOTA-TOC [39] and 68Ga DOTA-TATE [39][40][41] 68Ga prostate-specific membrane antigen (PSMA) [42] |
NQ | Sensitivity 97%, specificity 92% for octreotide [43] Sensitivity 100%, specificity 100% for PSMA [44] |
| Brain cancer | Dynamic susceptibility contrast (DSC)-MRI | SQ | AUC = 0.77 for classifying glioma grades II and III [45] |
| Glioma | Adjuvant paclitaxel and trastuzumab (APT) trial | Q | APT accords with cancer grade and Ki67 index [46] |
| Rectal cancer Lung cancer |
DCE-CT parameters Blood flow, permeability |
Q | Blood flow 75% accuracy for detecting rectal cancers with lymph node metastases [47] CT permeability anticipated survival regardless of treatment in lung cancer [48] |
| Cervix cancer Endometrial cancer Rectal cancer Breast cancer |
DCE-MRI parameters |
Q | Cancer volume with increasing metrics is considered a significant independent factor for disease-free survival (DFS) and overall survival (OS) in cervical cancer [49] Low cancer blood flow and low rate constant for contrast agent intravasation (kep) correlated with high risk of endometrial cancer [50] Ktrans, Kep and Ve are higher in rectal cancers accompanied with metastasis [51] Ktrans, iAUC qualitative and ADC anticiptate low-risk breast cancers (AUC of combined parameters 0.78) |
| Diverse cancer types [52][53] | Radiomic signature [54] DCE-MR parameters |
Q | Data endpoints, feature detection protocols, and classifiers are important factors in lung cancer prediction [55] Radiomic signature is significantly associated with lymph node (LN) status in colorectal cancer [56] Evaluating therapeutic effect subsequent to antiangiogenic agents [57] |
| Lymphoma | Deauville or response evaluation criteria in lymphoma (RECIL) score on 18F-FDG-PET |
SQ | Assessment of lymphoma treatment in clinical trials employs the summation of longest diameters of three target lesions [58] |
| Breast cancer [59] Prostate cancer [60] |
Receptor tyrosine-protein kinase erbB-2, CD340, and HER2 prostate-specific membrane antigen (PSMA) |
SQ | Selective cancer receptor; investigation of cancer treatment on receptor expression. Assessing therapy response to antiangiogenic agents [57] |
| Oesophageal cancer |
CT perfusion/blood flow | Q | Multivariate analysis detects blood flow as a predictor of response [61] |
| Gastrointestinal stromal cancers |
CT density HU | Q | Decrease in cancer density of > 15% on CT associated with a sensitivity of 97% and a specificity of 100% in identifying PET responders compared to 52% and 100% by RECIST [61] |
3. MRI Biomarkers
MRI can be exploited to extract numerous variables according to diverse inherent tissue properties such as proton density, diffusion, and T1-and T2 relaxation times [1]. In addition, MRI can probe the alterations in parameters due to the association of macromolecules and contrast agents [5]. For example, the apparent diffusion coefficient (ADC) is an extensively used criterion in cancer identification [16][62], diagnosis, and treatment assessment [63][64]. However, post-processing tools to derive absolute quantitation are widely disputed [65][66][67], although the protocol itself is versatile and reliable for cancer detection [68]. Quantification of T1 relaxation has an impact on cardiovascular MRI rather than depending on image contrast [69]. T1 values are significant in differentiating cardiac inflammation [70], multiple sclerosis [71][72], liver fat and iron concentration [73][74], and endocrine glands [75].
Quantitative chemical exchange saturation transfer (CEST) imaging is promising in evaluating brain ischemic disease [76], osteoarthritis [77], lymphedema [78], cancer pH and metabolomics [79]. Furthermore, MRI offers beneficial effects such as optimum images distinction, superior resolution, providing many contrasts per each testing; probing histological features (oxygenation, perfusion, and angiogenesis) [1].
Distinctive MRI biomarkers have been assigned in cancer diagnosis [1] including Breast Imaging Reporting and Data System (BI-RADS) [2], Liver Imaging Reporting and Data System (LI-RADS) [80][81], Prostate Imaging Reporting and Data System (PI-RADS) [4], TNM, and RECIST [1]. Quantitative biomarkers have been employed in clinical research studies such as initial area under the gadolinium curve (iAUGC) or transfer constant (Ktrans) from dynamic gadolinium enhanced (DGE) imaging and apparent diffusion coefficient (ADC) [1]. Morphological-based cancer biomarkers use many contrasts and moderate to high spatial resolution of MRI [1][82][83][84]. T1-weighted and T2-weighted imaging are utilized in cancer profiling [1].
4. MRI Data Preprocessing
Applying machine learning directly on raw MRI scans often yields poor results due to noise and information redundancy. Furthermore, machines read and store images in the form of number matrices. Raw MRI data are transformed into numerical features that can be processed by machines while preserving the information in the original data set.
5. Machine Learning for MRI Data
Machine learning (ML) algorithms are becoming useful components of computer-aided disease diagnosis and decision support systems. Computers seem to be able to recognize patterns that humans cannot perceive. Hence, ML provides a tool to analyze and utilize a massive amount of data more efficiently than the conventional analysis carried by human. This realization has led to heightened interest in ML and AI applications to medical images. Recently, employing ML in analyzing big data resulting from medical images, including MRI data, have been useful in obtaining significant clinical information that can aid physicians in making important decisions regarding clinical diagnosis, clinical prognosis, or treatment outcome [55][85][86]. ML can be used also to prioritize MRI biomarkers. The workflow for prioritizing MRI biomarkers using ML is summarized in Figure 1.
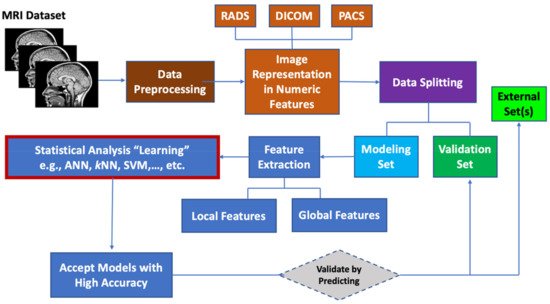
Figure 1. Workflow for prioritizing ML MRI biomarkers.
References
- Dregely, I.; Prezzi, D.; Kelly-Morland, C.; Roccia, E.; Neji, R.; Goh, V. Imaging Biomarkers in Oncology: Basics and Application to MRI. J. Magn. Reson. Imaging 2018, 48, 13–26.
- Mercado, C.L. BI-RADS Update. Radiol. Clin. N. Am. 2014, 52, 481–487.
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354.
- Barentsz, J.O.; Weinreb, J.C.; Verma, S.; Thoeny, H.C.; Tempany, C.M.; Shtern, F.; Padhani, A.R.; Margolis, D.; Macura, K.J.; Haider, M.A.; et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur. Urol. 2016, 69, 41–49.
- DeSouza, N.M.; Achten, E.; Alberich-Bayarri, A.; Bamberg, F.; Boellaard, R.; Clément, O.; Fournier, L.; Gallagher, F.; Golay, X.; Heussel, C.P.; et al. Validated Imaging Biomarkers as Decision-Making Tools in Clinical Trials and Routine Practice: Current Status and Recommendations from the EIBALL* Subcommittee of the European Society of Radiology (ESR). Insights Imaging 2019, 10, 1–6.
- Leithner, D.; Helbich, T.H.; Bernard-Davila, B.; Marino, M.A.; Avendano, D.; Martinez, D.F.; Jochelson, M.S.; Kapetas, P.; Baltzer, P.A.T.; Haug, A.; et al. Multiparametric 18F-FDG PET/MRI of the Breast: Are There Differences in Imaging Biomarkers of Contralateral Healthy Tissue between Patients with and without Breast Cancer? J. Nucl. Med. 2020, 61, 20–25.
- Jalali, S.; Chung, C.; Foltz, W.; Burrell, K.; Singh, S.; Hill, R.; Zadeh, G. MRI Biomarkers Identify the Differential Response of Glioblastoma Multiforme to Anti-Angiogenic Therapy. Neuro-Oncol. 2014, 16, 868–879.
- Moffa, G.; Galati, F.; Collalunga, E.; Rizzo, V.; Kripa, E.; D’Amati, G.; Pediconi, F. Can MRI Biomarkers Predict Triple-Negative Breast Cancer? Diagnostics 2020, 10, 1090.
- Grand, D.; Navrazhina, K.; Frew, J.W. A Scoping Review of Non-Invasive Imaging Modalities in Dermatological Disease: Potential Novel Biomarkers in Hidradenitis Suppurativa. Front. Med. 2019, 6.
- Just, N. Improving Tumour Heterogeneity MRI Assessment with Histograms. Br. J. Cancer 2014, 111, 2205–2213.
- Padhani, A.R.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; van Cauteren, M.; Collins, D.; et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. In Neoplasia; Elsevier B.V.: Amsterdam, The Netherlands, 2009; Volume 11, pp. 102–125.
- Qiao, J.; Xue, S.; Pu, F.; White, N.; Jiang, J.; Liu, Z.R.; Yang, J.J. Molecular Imaging of EGFR/HER2 Cancer Biomarkers by Protein MRI Contrast Agents Topical Issue on Metal-Based MRI Contrast Agents. J. Biol. Inorg. Chem. 2014, 19, 259–270.
- Watson, M.J.; George, A.K.; Maruf, M.; Frye, T.P.; Muthigi, A.; Kongnyuy, M.; Valayil, S.G.; Pinto, P.A. Risk Stratification of Prostate Cancer: Integrating Multiparametric MRI, Nomograms and Biomarkers. Future Oncol. 2016, 12, 2417–2430.
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16.
- O’Flynn, E.A.M.; Nandita, M.D. Functional Magnetic Resonance: Biomarkers of Response in Breast Cancer. Breast Cancer Res. 2011, 13, 204.
- Lopci, E.; Franzese, C.; Grimaldi, M.; Zucali, P.A.; Navarria, P.; Simonelli, M.; Bello, L.; Scorsetti, M.; Chiti, A. Imaging Biomarkers in Primary Brain Tumours. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 597–612.
- Weaver, O.; Leung, J.W.T. Biomarkers and Imaging of Breast Cancer. Am. J. Roentgenol. 2018, 210, 271–278.
- O’Connor, J.P.B.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.W.L.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging Biomarker Roadmap for Cancer Studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186.
- Booth, T.C.; Williams, M.; Luis, A.; Cardoso, J.; Ashkan, K.; Shuaib, H. Machine Learning and Glioma Imaging Biomarkers. Clin. Radiol. 2020, 75, 20–32.
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools). Updated Sept. 25 2017, 55. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 1 March 2021).
- Waldstein, S.M.; Seeböck, P.; Donner, R.; Sadeghipour, A.; Bogunović, H.; Osborne, A.; Schmidt-Erfurth, U. Unbiased Identification of Novel Subclinical Imaging Biomarkers Using Unsupervised Deep Learning. Sci. Rep. 2020, 10, 12954.
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500–510.
- About Biomarkers and Qualification|FDA. Available online: https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification (accessed on 16 February 2021).
- European Society of Radiology. White Paper on Imaging Biomarkers. Insights Imaging 2010, 1, 42–45.
- Zhang, D.; Xu, A. Application of Dual-Source CT Perfusion Imaging and MRI for the Diagnosis of Primary Liver Cancer. Oncol. Lett. 2017, 14, 5753–5758.
- Heuvelmans, M.A.; Walter, J.E.; Vliegenthart, R.; van Ooijen, P.M.A.; de Bock, G.H.; de Koning, H.J.; Oudkerk, M. Disagreement of Diameter and Volume Measurements for Pulmonary Nodule Size Estimation in CT Lung Cancer Screening. Thorax 2018, 73, 779–781.
- Van Riel, S.J.; Ciompi, F.; Jacobs, C.; Winkler Wille, M.M.; Scholten, E.T.; Naqibullah, M.; Lam, S.; Prokop, M.; Schaefer-Prokop, C.; van Ginneken, B. Malignancy Risk Estimation of Screen-Detected Nodules at Baseline CT: Comparison of the PanCan Model, Lung-RADS and NCCN Guidelines. Eur. Radiol. 2017, 27, 4019–4029.
- Matoba, M.; Tsuji, H.; Shimode, Y.; Nagata, H.; Tonami, H. Diagnostic Performance of Adaptive 4D Volume Perfusion CT for Detecting Metastatic Cervical Lymph Nodes in Head and Neck Squamous Cell Carcinoma. Am. J. Roentgenol. 2018, 211, 1106–1111.
- Zhang, L.; Tang, M.; Chen, S.; Lei, X.; Zhang, X.; Huan, Y. A Meta-Analysis of Use of Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) with Multiparametric MR Imaging for the Detection of Prostate Cancer. Eur. Radiol. 2017, 27, 5204–5214.
- Timmers, J.M.H.; van Doorne-Nagtegaal, H.J.; Zonderland, H.M.; van Tinteren, H.; Visser, O.; Verbeek, A.L.M.; den Heeten, G.J.; Broeders, M.J.M. The Breast Imaging Reporting and Data System (Bi-Rads) in the Dutch Breast Cancer Screening Programme: Its Role as an Assessment and Stratification Tool. Eur. Radiol. 2012, 22, 1717–1723.
- Van der Pol, C.B.; Lim, C.S.; Sirlin, C.B.; McGrath, T.A.; Salameh, J.P.; Bashir, M.R.; Tang, A.; Singal, A.G.; Costa, A.F.; Fowler, K.; et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy—A Systematic Review. Gastroenterology 2019, 156, 976–986.
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and Disease-Specific Adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145.
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nuc. Med. 2009, 50, 122S–150S.
- Nael, K.; Bauer, A.H.; Hormigo, A.; Lemole, M.; Germano, I.M.; Puig, J.; Stea, B. Multiparametric MRI for Differentiation of Radiation Necrosis from Recurrent Tumor in Patients with Treated Glioblastoma. Am. J. Roentgenol. 2018, 210, 18–23.
- Bastiaannet, E.; Groen, B.; Jager, P.L.; Cobben, D.C.P.; van der Graaf, W.T.A.; Vaalburg, W.; Hoekstra, H.J. The Value of FDG-PET in the Detection, Grading and Response to Therapy of Soft Tissue and Bone Sarcomas; a Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2004, 30, 83–101.
- Chang, C.Y.; Chang, S.J.; Chang, S.C.; Yuan, M.K. The Value of Positron Emission Tomography in Early Detection of Lung Cancer in High-Risk Population: A Systematic Review. Clin. Respir. J. 2013, 7, 1–6.
- Parekh, V.S.; Macura, K.J.; Harvey, S.; Kamel, I.; EI-Khouli, R.; Bluemke, D.A.; Jacobs, M.A. Multiparametric Deep Learning Tissue Signatures for a Radiological Biomarker of Breast Cancer: Preliminary Results. Med. Phys. 2018, 47, 75–88.
- Lu, S.J.; Gnanasegaran, G.; Buscombe, J.; Navalkissoor, S. Single Photon Emission Computed Tomography/Computed Tomography in the Evaluation of Neuroendocrine Tumours: A Review of the Literature. Nucl. Med. Commun. 2013, 34, 98–107.
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients with Stable Chest Pain: Insights from the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332.
- Ambrosini, V.; Campana, D.; Tomassetti, P.; Fanti, S. 68Ga-Labelled Peptides for Diagnosis of Gastroenteropancreatic NET. Eur. J. Nuc. Med. Mol. Imaging 2012, 39, 52–60.
- Maxwell, J.E.; Howe, J.R. Imaging in Neuroendocrine Tumors: An Update for the Clinician. Int. J. Endocr. Oncol. 2015, 2, 159–168.
- Zacho, H.D.; Nielsen, J.B.; Afshar-Oromieh, A.; Haberkorn, U.; deSouza, N.; de Paepe, K.; Dettmann, K.; Langkilde, N.C.; Haarmark, C.; Fisker, R.V.; et al. Prospective Comparison of 68Ga-PSMA PET/CT, 18F-Sodium Fluoride PET/CT and Diffusion Weighted-MRI at for the Detection of Bone Metastases in Biochemically Recurrent Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1884–1897.
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-Octreotide PET in Neuroendocrine Tumors: Comparison with Somatostatin Receptor Scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518.
- Park, S.Y.; Zacharias, C.; Harrison, C.; Fan, R.E.; Kunder, C.; Hatami, N.; Giesel, F.; Ghanouni, P.; Daniel, B.; Loening, A.M.; et al. Gallium 68 PSMA-11 PET/MR Imaging in Patients with Intermediate- or High-Risk Prostate Cancer. Radiology 2018, 288, 495–505.
- Delgado, A.F.; Delgado, A.F. Discrimination between Glioma Grades II and III Using Dynamic Susceptibility Perfusion MRI: A Meta-Analysis. Am. J. Neuroradiol. 2017, 38, 1348–1355.
- Su, C.; Liu, C.; Zhao, L.; Jiang, J.; Zhang, J.; Li, S.; Zhu, W.; Wang, J. Amide Proton Transfer Imaging Allows Detection of Glioma Grades and Tumor Proliferation: Comparison with Ki-67 Expression and Proton MR Spectroscopy Imaging. Am. J. Neuroradiol. 2017, 38, 1702–1709.
- Hayano, K.; Shuto, K.; Koda, K.; Yanagawa, N.; Okazumi, S.; Matsubara, H. Quantitative Measurement of Blood Flow Using Perfusion CT for Assessing Clinicopathologic Features and Prognosis in Patients with Rectal Cancer. Dis. Colon Rectum 2009, 52, 1624–1629.
- Win, T.; Miles, K.A.; Janes, S.M.; Ganeshan, B.; Shastry, M.; Endozo, R.; Meagher, M.; Shortman, R.I.; Wan, S.; Kayani, I.; et al. Tumor Heterogeneity and Permeability as Measured on the CT Component of PET/CT Predict Survival in Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2013, 19, 3591–3599.
- Lund, K.V.; Simonsen, T.G.; Kristensen, G.B.; Rofstad, E.K. Pretreatment Late-Phase DCE-MRI Predicts Outcome in Locally Advanced Cervix Cancer. Acta Oncol. 2017, 56, 675–681.
- Fasmer, K.E.; Bjørnerud, A.; Ytre-Hauge, S.; Grüner, R.; Tangen, I.L.; Werner, H.M.J.; Bjørge, L.; Salvesen, Ø.O.; Trovik, J.; Krakstad, C.; et al. Preoperative Quantitative Dynamic Contrast-Enhanced MRI and Diffusion-Weighted Imaging Predict Aggressive Disease in Endometrial Cancer. Acta Radiol. 2018, 59, 1010–1017.
- Yu, J.; Xu, Q.; Huang, D.Y.; Song, J.C.; Li, Y.; Xu, L.L.; Shi, H.B. Prognostic Aspects of Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Synchronous Distant Metastatic Rectal Cancer. Eur. Radiol. 2017, 27, 1840–1847.
- Lee, J.W.; Lee, S.M. Radiomics in Oncological PET/CT: Clinical Applications. Nucl. Med. Mol. Imaging 2018, 52, 170–189.
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The Process and the Challenges. Magn. Reson. Imaging 2012, 30, 1234–1248.
- Wilson, R.; Devaraj, A. Radiomics of Pulmonary Nodules and Lung Cancer. Transl. Lung Cancer Res. 2017, 6, 86–91.
- Zhang, Y.; Oikonomou, A.; Wong, A.; Haider, M.A.; Khalvati, F. Radiomics-Based Prognosis Analysis for Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7.
- Huang, Y.Q.; Liang, C.H.; He, L.; Tian, J.; Liang, C.S.; Chen, X.; Ma, Z.L.; Liu, Z.Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164.
- O’Connor, J.P.B.; Jackson, A.; Parker, G.J.M.; Roberts, C.; Jayson, G.C. Dynamic Contrast-Enhanced MRI in Clinical Trials of Antivascular Therapies. Nat. Rev. Clin. Oncol. 2012, 9, 167–177.
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group Consensus Response Evaluation Criteria in Lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447.
- Dalm, S.U.; Verzijlbergen, J.F.; de Jong, M. Review: Receptor Targeted Nuclear Imaging of Breast Cancer. Int. J. Mol. Sci. 2017, 18, 260.
- Bakht, M.K.; Oh, S.W.; Youn, H.; Cheon, G.J.; Kwak, C.; Kang, K.W. Influence of Androgen Deprivation Therapy on the Uptake of PSMA-Targeted Agents: Emerging Opportunities and Challenges. Nucl. Med. Mol. Imaging 2017, 51, 202–211.
- Hayano, K.; Okazumi, S.; Shuto, K.; Matsubara, H.; Shimada, H.; Nabeya, Y.; Kazama, T.; Yanagawa, N.; Ochiai, T. Perfusion CT Can Predict the Response to Chemoradiation Therapy and Survival in Esophageal Squamous Cell Carcinoma: Initial Clinical Results. Oncol. Rep. 2007, 18, 901–908.
- Bittencourt, L.K.; de Hollanda, E.S.; de Oliveira, R.V. Multiparametric MR Imaging for Detection and Locoregional Staging of Prostate Cancer. Top. Magn. Reson. Imaging 2016, 25, 109–117.
- Galbán, C.J.; Hoff, B.A.; Chenevert, T.L.; Ross, B.D. Diffusion MRI in Early Cancer Therapeutic Response Assessment. NMR Biomed. 2017, 30, e3458.
- Shukla-Dave, A.; Obuchowski, N.A.; Chenevert, T.L.; Jambawalikar, S.; Schwartz, L.H.; Malyarenko, D.; Huang, W.; Noworolski, S.M.; Young, R.J.; Shiroishi, M.S.; et al. Quantitative Imaging Biomarkers Alliance (QIBA) Recommendations for Improved Precision of DWI and DCE-MRI Derived Biomarkers in Multicenter Oncology Trials. J. Magnet. Reson. Imaging 2019, 49, e101–e121.
- Zeng, Q.; Shi, F.; Zhang, J.; Ling, C.; Dong, F.; Jiang, B. A Modified Tri-Exponential Model for Multi-b-Value Diffusion-Weighted Imaging: A Method to Detect the Strictly Diffusion-Limited Compartment in Brain. Front. Neurosci. 2018, 12, 102.
- Langkilde, F.; Kobus, T.; Fedorov, A.; Dunne, R.; Tempany, C.; Mulkern, R.V.; Maier, S.E. Evaluation of Fitting Models for Prostate Tissue Characterization Using Extended-Range b-Factor Diffusion-Weighted Imaging. Magn. Reson. Med. 2018, 79, 2346–2358.
- Keene, J.D.; Jacobson, S.; Kechris, K.; Kinney, G.L.; Foreman, M.G.; Doerschuk, C.M.; Make, B.J.; Curtis, J.L.; Rennard, S.I.; Barr, R.G.; et al. Biomarkers Predictive of Exacerbations in the SPIROMICS and COPDGene Cohorts. Am. J. Respir. Crit. Care Med. 2017, 195, 473–481.
- Winfield, J.M.; Tunariu, N.; Rata, M.; Miyazaki, K.; Jerome, N.P.; Germuska, M.; Blackledge, M.D.; Collins, D.J.; de Bono, J.S.; Yap, T.A.; et al. Extracranial Soft-Tissue Tumors: Repeatability of Apparent Diffusion Coefficient Estimates from Diffusion-Weighted MR Imaging. Radiology 2017, 284, 88–99.
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81.
- Toussaint, M.; Gilles, R.J.; Azzabou, N.; Marty, B.; Vignaud, A.; Greiser, A.; Carlier, P.G. Characterization of Benign Myocarditis Using Quantitative Delayed-Enhancement Imaging Based on MOLLI T1 Mapping. Medicine 2015, 94.
- Jurcoane, A.; Wagner, M.; Schmidt, C.; Mayer, C.; Gracien, R.M.; Hirschmann, M.; Deichmann, R.; Volz, S.; Ziemann, U.; Hattingen, E. Within-Lesion Differences in Quantitative MRI Parameters Predict Contrast Enhancement in Multiple Sclerosis. J. Magn. Reson. Imaging 2013, 38, 1454–1461.
- Salisbury, M.L.; Lynch, D.A.; van Beek, E.J.R.; Kazerooni, E.A.; Guo, J.; Xia, M.; Murray, S.; Anstrom, K.J.; Yow, E.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis: The Association between the Adaptive Multiple Features Method and Fibrosis Outcomes. Am. J. Respir. Crit. Care Med. 2017, 195, 921–929.
- Katsube, T.; Okada, M.; Kumano, S.; Hori, M.; Imaoka, I.; Ishii, K.; Kudo, M.; Kitagaki, H.; Murakami, T. Estimation of Liver Function Using T1 Mapping on Gd-EOB-DTPA-Enhanced Magnetic Resonance Imaging. Investig. Radiol. 2011, 46, 277–283.
- Mozes, F.E.; Tunnicliffe, E.M.; Moolla, A.; Marjot, T.; Levick, C.K.; Pavlides, M.; Robson, M.D. Mapping Tissue Water T1 in the Liver Using the MOLLI T1 Method in the Presence of Fat, Iron and B0 Inhomogeneity. NMR Biomed. 2019, 32.
- Adam, S.Z.; Nikolaidis, P.; Horowitz, J.M.; Gabriel, H.; Hammond, N.A.; Patel, T.; Yaghmai, V.; Miller, F.H. Chemical Shift MR Imaging of the Adrenal Gland: Principles, Pitfalls, and Applications. Radiographics 2016, 36, 414–432.
- Tietze, A.; Blicher, J.; Mikkelsen, I.K.; Østergaard, L.; Strother, M.K.; Smith, S.A.; Donahue, M.J. Assessment of Ischemic Penumbra in Patients with Hyperacute Stroke Using Amide Proton Transfer (APT) Chemical Exchange Saturation Transfer (CEST) MRI. NMR Biomed. 2014, 27, 163–174.
- Krishnamoorthy, G.; Nanga, R.P.R.; Bagga, P.; Hariharan, H.; Reddy, R. High Quality Three-Dimensional GagCEST Imaging of in Vivo Human Knee Cartilage at 7 Tesla. Magn. Reson. Med. 2017, 77, 1866–1873.
- Donahue, M.J.; Donahue, P.C.M.; Rane, S.; Thompson, C.R.; Strother, M.K.; Scott, A.O.; Smith, S.A. Assessment of Lymphatic Impairment and Interstitial Protein Accumulation in Patients with Breast Cancer Treatment-Related Lymphedema Using CEST MRI. Magn. Reson. Med. 2016, 75, 345–355.
- Lindeman, L.R.; Randtke, E.A.; High, R.A.; Jones, K.M.; Howison, C.M.; Pagel, M.D. A Comparison of Exogenous and Endogenous CEST MRI Methods for Evaluating in Vivo PH. Magn. Reson. Med. 2018, 79, 2766–2772.
- Tang, A.; Bashir, M.R.; Corwin, M.T.; Cruite, I.; Dietrich, C.F.; Do, R.K.G.; Ehman, E.C.; Fowler, K.J.; Hussain, H.K.; Jha, R.C.; et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-Based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018, 286, 29–48.
- Mitchell, D.G.; Bruix, J.; Sherman, M.; Sirlin, C.B. LI-RADS (Liver Imaging Reporting and Data System): Summary, Discussion, and Consensus of the LI-RADS Management Working Group and Future Directions. Hepatology 2015, 61, 1056–1065.
- Degani, H.; Gusis, V.; Weinstein, D.; Fields, S.; Strano, S. Mapping Pathophysiological Features of Breast Tumors by MRI at High Spatial Resolution. Nat. Med. 1997, 3, 780–782.
- Uecker, M.; Zhang, S.; Voit, D.; Karaus, A.; Merboldt, K.D.; Frahm, J. Real-Time MRI at a Resolution of 20 Ms. NMR Biomed. 2010, 23, 986–994.
- Van Wijk, D.F.; Strang, A.C.; Duivenvoorden, R.; Enklaar, D.-J.F.; Zwinderman, A.H.; van der Geest, R.J.; Kastelein, J.J.P.; de Groot, E.; Stroes, E.S.G.; Nederveen, A.J. Increasing the Spatial Resolution of 3T Carotid MRI Has No Beneficial Effect for Plaque Component Measurement Reproducibility. PLoS ONE 2015, 10, e0130878.
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat. Commun. 2014, 5, 1–9.
- Trebeschi, S.; Drago, S.G.; Birkbak, N.J.; Kurilova, I.; Cǎlin, A.M.; Delli Pizzi, A.; Lalezari, F.; Lambregts, D.M.J.; Rohaan, M.W.; Parmar, C.; et al. Predicting Response to Cancer Immunotherapy Using Noninvasive Radiomic Biomarkers. Ann. Oncol. 2019, 30, 998–1004.




