Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aurore DANIGO | + 1008 word(s) | 1008 | 2021-11-22 09:09:40 | | | |
| 2 | Nora Tang | Meta information modification | 1008 | 2021-12-13 01:55:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Danigo, A. CCK2R Structure and Functions. Encyclopedia. Available online: https://encyclopedia.pub/entry/16929 (accessed on 08 February 2026).
Danigo A. CCK2R Structure and Functions. Encyclopedia. Available at: https://encyclopedia.pub/entry/16929. Accessed February 08, 2026.
Danigo, Aurore. "CCK2R Structure and Functions" Encyclopedia, https://encyclopedia.pub/entry/16929 (accessed February 08, 2026).
Danigo, A. (2021, December 09). CCK2R Structure and Functions. In Encyclopedia. https://encyclopedia.pub/entry/16929
Danigo, Aurore. "CCK2R Structure and Functions." Encyclopedia. Web. 09 December, 2021.
Copy Citation
Postoperative pain is defined as acute pain experienced by the patient after a surgical procedure. Good management of postoperative pain is important in reducing complications and facilitating rehabilitation. Postoperative pain relief is mainly based on opioids, and so is associated with side effects such as constipation, respiratory distress and the development of tolerance to administered opioids.
cholecystokinin type 2 receptor
pain management
antagonist
rodent model
clinical trial
1. Gene Localization and Related Diseases
Human CCK2R was cloned for the first time in 1993 [1]. Cck2r, the gene coding for CCK2R, which is located on the terminal short arm of chromosomes 11 in humans (11p15.4), is 12 kbp in length and is composed of 5 exons. According to the Ensembl database, three transcripts have been identified in humans. CCK2R splice variants and mutations have been shown to be involved in cell proliferation and cancer pathogenesis [2]. Mutations, as well as overexpression of CCK2R, are associated with carcinogenesis via the modulation of processes including cell proliferation and cell adhesion.
2. Structural Features of the CCK2R
CCK1R and CCK2R belong to the family of G-protein-coupled receptors (GPCRs). The two genes share only 50% sequence homology, mostly in domains characteristic of GPCRs and in sequence signatures that are required for receptor activation, which might explain their differences in affinity for ligands [3]. Though there remain some controversies, CCK2R can be generally associated with Gq protein, and to a lesser extent with Gi, contrary to CCK1R which is associated with Gs protein [4]. The activated Gq pathway passes through phospholipase C beta (PLCβ), which hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to inositol-trisphosphate (IP3), leading to the release of intracellular Ca2+ stores into the cytoplasm, and to diacylglycerol (DAG), leading to the activation of protein kinase C (PKC). Ca2+ is a major effector of neuronal function, involved in neurotransmitter release and membrane excitability. It has been shown that PKC can play a crucial role in the regulation of GPCRs [5]. GPCRs are comprised of seven α-helical transmembrane domains, an extracellular N terminus with three extracellular loops, and an intracellular C terminus with three intracellular loops. GPCRs are a major class of proteins that are potentially involved in pain transmission and so, represent a preferred therapeutic target for novels analgesics [6]. For example, serotonin, apelin, dopamine, GABAB, opioid receptors and the recently clinically-implicated angiotensin II type 2 receptor (AT2R), all use signaling that passes through the GPCR family proteins and have roles in pain modulation [6][7].
3. Cellular Localization and Tissue Distribution in the Nervous System
CCK2R is localized in the plasma membrane and can be internalized for desensitization, an essential mechanism for maintaining physiologically appropriate cellular responses to extracellular stimuli. The internalization of the receptor can be decreased by removal of the C terminus, without affecting G protein coupling [8].
Only a few studies have investigated the expression of CCKRs in the human brain. Most of these explored the binding of radiolabeled ligands, which did not allow the discrimination between CCK1R and CCK2R [9][10]. However, a study using integrative data from several sources (transcriptomics, single-cell genomics, in situ hybridization and antibody-based protein profiling) has shown the localization and expression level of the receptors in human, pig and mouse brain [11]. This database detected CCK2R mRNA in most parts of the human brain, except the thalamus. Some of the structures that express CCK2R are directly implicated in the modulation of pain, such as the hypothalamus, the basal ganglia and the amygdala.
The hypothalamus has a role in modulating the affective dimension of pain and is part of the spinothalamic tract, the principal pain tract [12] (Figure 1). The basal ganglia (mainly including the putamen and the caudate nucleus, and the nucleus accumbens (NAc)) have been principally studied for their motor functions, but are also involved in different aspects of pain including the sensory-discriminative, affective and cognitive dimension of pain and the modulation of nociceptive stimulus intensity information [13]. The amygdala plays an important role in emotional responses and affective states, in disorders such as learned fear, anxiety and depression, and also in pain modulation [14].
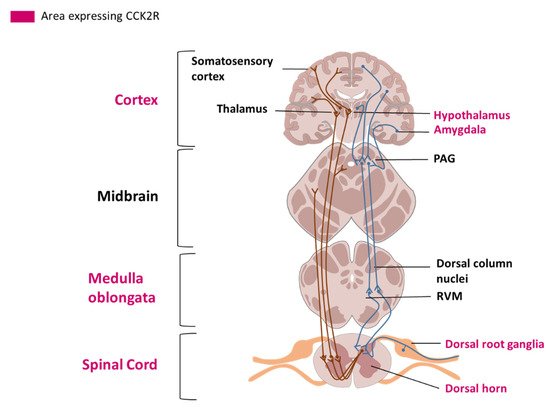
Figure 1. Representation of the spinothalamic tract and descending pain modulation pathway. Areas where CCK2R has been detected are labelled in pink. A: Ascending pain pathways. Nociceptors (C and Aδ fibers) send pain information from peripheral tissues, their cell bodies are localized in the dorsal root ganglia (DRG). Fibers transmit nociceptive signals to a second order neuron in the dorsal horn of the spinal cord. The second order neuron then crosses over to the opposite side, where it forms the ascending spinothalamic tract. This tract takes signals to nuclei in the medulla oblongata and midbrain on the way up to the thalamus. The thalamus relays the information to the somatosensory and insular cortex, as well as cortical regions mediating different aspects of the pain experience such as affective responses in the cingulate cortex. B: Descending pain modulation pathways: Information from the environment and some motivational states can activate this top-down pathway. Several areas in the limbic forebrain including the anterior cingulate and insular cortex, nuclei in the amygdala and the hypothalamus, project to the midbrain periaqueductal grey (PAG), which then modulates ascending pain transmission from the afferent pain system indirectly through the rostral ventromedial medulla (RVM) in the brainstem.
Low level expression of CCK2R has been detected in the spinal cord [11]. Interestingly, the expression of CCK2R in the human peripheral nervous system has not been explored. In rats, CCK2R mRNA has been detected in dorsal root ganglia (DRGs) using in situ hybridization. Only 3% to 4% of all DRG neurons in physiological conditions expressed CCK2R mRNA [15]. Another study using [125I]-CCK-8S labelled with Bolton-Hunter reagent to localize its binding site in DRG, led to the same conclusion [16]. Moreover, in a model of unilateral peripheral transection of the sciatic nerve in the rat [15], in situ hybridization highlighted an increase in CCK2R mRNA in the L4 and L5 DRGs after axotomy. Localization of CCK2R in nociception-linked areas and upregulation of CCK2R mRNA after traumatic lesions underlined the interest of evaluating the roles of CCK2R in pain modulation.
References
- Noble, F.; Roques, B.P. CCK-B Receptor: Chemistry, Molecular Biology, Biochemistry and Pharmacology. Prog. Neurobiol. 1999, 58, 349–379.
- Willard, M.D.; Lajiness, M.E.; Wulur, I.H.; Feng, B.; Swearingen, M.L.; Uhlik, M.T.; Kinzler, K.W.; Velculescu, V.E.; Sjöblom, T.; Markowitz, S.D.; et al. Somatic Mutations in CCK2R Alter Receptor Activity That Promote Oncogenic Phenotypes. Mol. Cancer Res. 2012, 10, 739–749.
- Miller, L.J.; Gao, F. Structural Basis of Cholecystokinin Receptor Binding and Regulation. Pharm. Ther. 2008, 119, 83–95.
- Piiper, A.; Stryjek-Kaminska, D.; Klengel, R.; Zeuzem, S. CCK, Carbachol, and Bombesin Activate Distinct PLC-Beta Isoenzymes via Gq/11 in Rat Pancreatic Acinar Membranes. Am. J. Physiol. 1997, 272, G135–G140.
- Zhang, J.-G.; Cong, B.; Li, Q.-X.; Chen, H.-Y.; Qin, J.; Fu, L.-H. Cholecystokinin Octapeptide Regulates Lipopolysaccharide-Activated B Cells Co-Stimulatory Molecule Expression and Cytokines Production In Vitro. Immunopharmacol. Immunotoxicol. 2011, 33, 157–163.
- Stone, L.S.; Molliver, D.C. In Search of Analgesia: Emerging Poles of GPCRs in Pain. Mol. Interv. 2009, 9, 234–251.
- Danigo, A.; Rovini, A.; Bessaguet, F.; Bouchenaki, H.; Bernard, A.; Sturtz, F.; Bourthoumieu, S.; Desmoulière, A.; Magy, L.; Demiot, C. The Angiotensin II Type 2 Receptor, a Target for Protection and Regeneration of the Peripheral Nervous System? Pharmaceuticals 2021, 14, 175.
- Pohl, M.; Silvente-Poirot, S.; Pisegna, J.R.; Tarasova, N.I.; Wank, S.A. Ligand-Induced Internalization of Cholecystokinin Receptors. J. Biol. Chem. 1997, 272, 18179–18184.
- Dietl, M.M.; Probst, A.; Palacios, J.M. On the Distribution of Cholecystokinin Receptor Binding Sites in the Human Brain: An Autoradiographic Study. Synapse 1987, 1, 169–183.
- Gaudreau, P.; St-Pierre, S.; Pert, C.B.; Quirion, R. Cholecystokinin Receptors in Mammalian Brain: A Comparative Characterization and Visualization. Ann. N. Y. Acad. Sci. 1985, 448, 198–219.
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, eaay5947.
- Dafny, N.; Dong, W.Q.; Prieto-Gomez, C.; Reyes-Vazquez, C.; Stanford, J.; Qiao, J.T. Lateral Hypothalamus: Site Involved in Pain Modulation. Neuroscience 1996, 70, 449–460.
- Chudler, E.H.; Dong, W.K. The Role of the Basal Ganglia in Nociception and Pain. Pain 1995, 60, 3–38.
- Neugebauer, V. 15. Amygdala Pain Mechanisms. Handb Exp. Pharm. 2015, 227, 261–284.
- Zhang, X.; Dagerlind, A.; Elde, R.P.; Castel, M.N.; Broberger, C.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Marked Increase in Cholecystokinin B Receptor Messenger RNA Levels in Rat Dorsal Root Ganglia after Peripheral Axotomy. Neuroscience 1993, 57, 227–233.
- Ghilardi, J.R.; Allen, C.J.; Vigna, S.R.; McVey, D.C.; Mantyh, P.W. Trigeminal and Dorsal Root Ganglion Neurons Express CCK Receptor Binding Sites in the Rat, Rabbit, and Monkey: Possible Site of Opiate-CCK Analgesic Interactions. J. Neurosci. 1992, 12, 4854–4866.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
753
Revisions:
2 times
(View History)
Update Date:
13 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




