Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Passera Alessandro | + 1591 word(s) | 1591 | 2021-11-27 06:49:11 | | | |
| 2 | Yvaine Wei | Meta information modification | 1591 | 2021-12-08 04:41:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alessandro, P. Bacterial Communities in the Embryo of Maize Landraces. Encyclopedia. Available online: https://encyclopedia.pub/entry/16827 (accessed on 07 February 2026).
Alessandro P. Bacterial Communities in the Embryo of Maize Landraces. Encyclopedia. Available at: https://encyclopedia.pub/entry/16827. Accessed February 07, 2026.
Alessandro, Passera. "Bacterial Communities in the Embryo of Maize Landraces" Encyclopedia, https://encyclopedia.pub/entry/16827 (accessed February 07, 2026).
Alessandro, P. (2021, December 07). Bacterial Communities in the Embryo of Maize Landraces. In Encyclopedia. https://encyclopedia.pub/entry/16827
Alessandro, Passera. "Bacterial Communities in the Embryo of Maize Landraces." Encyclopedia. Web. 07 December, 2021.
Copy Citation
Locally adapted maize accessions (landraces) represent an untapped resource of nutritional and resistance traits for breeding, including the shaping of distinct microbiota.
Fusarium
Maize
Landraces
microbiota
1. Introduction
Maize (Zea mays L.) is one of the most widely grown crops worldwide. It is not only a staple food for people in several countries, but also a very important crop for animal feed and finds use as industrial material for production of fuels, among other technological uses. In addition to all these practical applications, maize has also been used as a model organism in scientific research, as it benefits from a high level of phenotypic and genetic variability, and quick life cycle.
While these characteristics allowed for a great increase in yield in the past century [1], the predicted demands for food in the next few decades [2] suggest that substantial changes in breeding techniques and agronomic processes will be required to reach the needed level of crop improvement and yield [3].
The history of maize as a crop is very long: its domestication is traced back to approximately 8700 BC in central America and the Spanish brought it to Europe at the end of the 16th century, where favorable environmental and social conditions allowed it to be employed with success [4]. In particular, the first written reports on the use of corn in Italy date back to 1600, proving that maize had well adapted to the climatic zone of cultivation and to the local tradition of the people living in the North-Eastern part of the peninsula. The cultivation in Italy gave rise to several landraces which were later abandoned for the more productive dent hybrids when mechanized farming practices became more common in the second half of the twentieth century [4].
The hybrids, which were selected mostly for their massive yield traits in field, have lost several useful features which were present in ancient maize varieties that gave the landraces a particular biochemical composition, including relevant nutritional components such as antioxidants and carotenoids [5], and pathogen resistance. It has been recently reported that certain Italian maize germplasm can be advantageous in resisting mycotoxin accumulation in its kernels [6][7]. This last trait is of particular importance not only for the ability of the maize plants to produce an abundant quantity of kernels, but also for their quality: several phytopathogenic fungal species belonging to the genera Aspergillus, Fusarium, and Penicillium that frequently colonize maize plants in field are known to produce mycotoxins as part of their secondary metabolism [8]. These fungi may not always produce visible damage to kernels but may still contaminate them with mycotoxins. These toxins, which are highly stable and extremely dangerous even at low concentrations, are currently one of the main concerns for human and animal nutrition all over the world [9]. The use of endophytes with biocontrol abilities against these toxigenic fungi can be of great interest for several reasons: biocontrol endophytes may prove effective against these fungi that develop at least for a portion of their life cycle inside the tissues of the host, and some endophytes are known to chelate and detoxify mycotoxins [10][11]; furthermore, the use of synthetic fungicides has given inconsistent results on this particular issue, sometimes resulting in a greater rate of mycotoxin production by the pathogens [12], making the use of biological control agents a more sustainable and potentially effective option [13].
While it has been held for a long time that healthy plant tissues were sterile, the presence of complex communities of microorganisms inside every plant tissue has been proven [14][15]. These microorganisms, called endophytes, can have beneficial, neutral, or harmful effects on the host. Since beneficial endophytes can influence the growth of the host plant, as well as its metabolic processes and resistance to both biotic and abiotic stresses [16], their presence can greatly affect the phenotype of their host. By increasing the uptake of nutrients and granting higher resistance to pathogens and pests, as well as other stresses, a positive relation between microorganisms, the plant, and the environment can greatly contribute to integrity, proper functionality, and sustainability of agro-ecosystems [17].
Moreover, seed-borne endophytes have been shown to be an important source of bacteria within other tissues. The identification of a set of endophytic microbes among Zea spp. that are conserved across evolutionary and ecological boundaries [14] suggests microbes with beneficial properties to the host plant are selected from the environment by the plants themselves. In addition to environmental origin there is also evidence that, in some plant species, bacterial endophytes can be inherited from one generation to the next through seed [18][19].
2. Accessions Characterized by Similar Embryo Microbiota Show Similar Susceptibility to Fusarium Ear Rot
The susceptibility to the disease associated with F. verticillioides, fusarium ear rot, was assayed in field, using both non-inoculated maize ears (natural infection) and performing an experimental inoculum of the silk channels with a suspension of conidia from F. verticillioides strains. The severity of the disease was assessed visually, as percentage of the ear that was infected with Fusarium (Figure 1). All the maize accessions gave similar infection rates under natural infection: while accession N and W had lower infection than the other 4, even though this difference was not statistically significant (Figure 1A). Conversely, in the F. verticillioides inoculated ears, statistically significant differences were reported: accessions N and W had infection values similar to the naturally infected ears, while the remaining four accessions had way higher infection values. This can also be seen by the pictures of representative ears of maize of the different accessions (Figure 1B–G). This suggests that these two accessions may have an adaptive advantage under conditions conducive to the establishment of Fusarium infections. An analysis of the embryo microbiota, carried out through sequencing of an hypervariable region of the gene encoding for 16S rRNA, also revealed that the two accessions N and W had a different structure of their microbiota than the other four accessions, in particular characterized by a high abundance of Firmicutes. These findings suggest that there is a connection between susceptibility to fusarium ear rot and the microbiota.
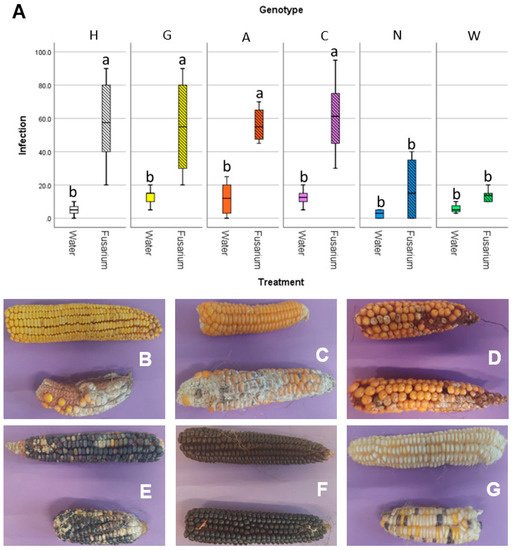
Figure 1. Results of the in field inoculation with FVm on maize ears. (A) graph reporting on the X-axis the different accessions, either mock-inoculated with water or inoculated with FVm, and on the Y-axis the infection severity, expressed as a percentage of the ear surface showing symptoms. Different letters (a, b) indicate statistically significant differences among results, according to a one-way ANOVA analysis followed by Tukey’s post-hoc test (p = 0.012). Panel showing representative maize ears either mock inoculated (top of each picture) or FVm-inoculated (bottom of each picture) for (B) accession H, (C) accession G, (D) accession A, (E) accession C, (F) accession N, and (G) accession W.
3. The Relationship Between Maize Landraces and Susceptibility to Fusarium Ear Rot
Finding new and effective solutions to the problem of mycotoxins in maize has been a primary challenge for sustainable agriculture since decades [8], as their presence in the kernels affects not only the direct consumption of corn as food but also animal feed, with effects on the meat and dairy production lines. For this reason, development of fungi-resistant maize genotypes could be a revolutionary advance in agriculture. Sadly, the genetic bases behind resistance to Fusarium spp. is not well-characterized, making breeding programs aimed in this direction very hard. Recent evidence suggests that other phenotypes which are not easily explained by simple genetic traits, such as the phenomenon of heterosis, might be related to a contribution of the microbiota [20]. A great involvement of the microbiota is expected in processes based in plant-microbe interactions such as defense mechanisms to pathogens, in which the recruitment of symbionts that can protect against pathogens is already reported [21]. This involvement is even more relevant while the plants are at the seed stage, during which they are exposed to several biotic stresses and are at their most vulnerable [22].
The situation regarding infection by F. verticillioides is further complicated by the different mechanisms by which it can infect maize. Infection can occur both by interaction with the silk channels [23], and by entering through holes and wounds present on the surface of the plant, such as those caused by insects, in particular Ostrinia nubilalis. Also, F. verticillioides can survive as an endophyte [10], causing no symptoms to the host plant, for long periods of time and therefore give rise to latent infections that only show symptoms at later times. It is also known that different fungal pathogens, such as different species of Fusarium or Fusarium with Ustilago species, can interact between themselves to promote or hinder the development of disease [23].
With these considerations in mind, both regarding the complexity of the pathosystem and the involvement of microorganisms in the infection process, the hypothesis behind the current study was that resistance to the pathogens responsible for fusarium ear rot may see contributions from the microbiota present in the kernels, not being directly/strictly associated to genes of the maize plant alone. The presence of encouraging studies in the literature showing the value of exploring the microbiota of less standardized genotypes to look for bacteria that can protect maize from pathogens [24] further reinforced the hypothesis that this approach could yield interesting results.
The characterization of the embryo-associated bacterial microbiota gave different results depending on the technique employed: cultivation-dependent and -independent approaches did not agree on the composition of the bacterial community.
The major point on which both approaches converge is that they identified a low number of bacteria from the investigated tissue. This is not surprising as the embryo is a tissue that was considered to be completely sterile for a long time even after the discovery of endophytic communities in other organs of plants and, being subject to very strict regulation and protection by several physical and biochemical barriers, it is very hard to colonize for microbes [25].
References
- Haley, C. A cornucopia of maize genes. Nat. Genet. 2011, 43, 87–88.
- Yan, J.; Warburton, M.; Crouch, J. Association Mapping for Enhancing Maize (Zea mays L.) Genetic Improvement. Crop. Sci. 2011, 51, 433–449.
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822.
- Brandolini, A.; Brandolini, A. Maize introduction, evolution and diffusion in Italy. Maydica 2009, 54, 233–242.
- Tafuri, A.; Alfieri, M.; Redaelli, R. Determination of soluble phenolics content in Italian maize varieties and lines. Tec. Mol. Int. 2014, 65, 60–69.
- Pilu, R.; Cassani, E.; Sirizzotti, A.; Petroni, K.; Tonelli, C. Effect of flavonoid pigments on the accumulation of fumonisin B1 in the maize kernel. J. Appl. Genet. 2010, 52, 145–152.
- Balconi, C.; Berardo, N.; Locatelli, S.; Lanzanova, C.; Torri, A.; Redaelli, R. Evaluation of ear rot (Fusarium verticillioides) resistance and fumonisin accumulation in Italian maize inbred lines. Phytopathol. Mediterr. 2014, 53, 14–26.
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516.
- Venturini, G.; Babazadeh, L.; Casati, P.; Pilu, R.; Salomoni, D.; Toffolatti, S.L. Assessing pigmented pericarp of maize kernels as possible source of resistance to fusarium ear rot, Fusarium spp. infection and fumonisin accumulation. Int. J. Food Microbiol. 2016, 227, 56–62.
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium Verticillioides: Managing the Endophytic Association with Maize for Reduced Fumonisins Accumulation. Toxin Rev. 2008, 27, 411–446.
- Quattrini, M.; Bernardi, C.; Stuknytė, M.; Masotti, F.; Passera, A.; Ricci, G.; Vallone, L.; De Noni, I.; Brasca, M.; Fortina, M.G. Functional characterization of Lactobacillus plantarum ITEM 17215: A potential biocontrol agent of fungi with plant growth promoting traits, able to enhance the nutritional value of cereal products. Food Res. Int. 2018, 106, 936–944.
- Edwards, S.G.; Godley, N. Reduction of Fusariumhead blight and deoxynivalenol in wheat with early fungicide applications of prothioconazole. Food Addit. Contam. Part A 2010, 27, 629–635.
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Martínez-Álvarez, J.C.; López-Meyer, M.; Lizárraga-Sánchez, G.J.; Félix-Gastélum, R.; Castro-Martínez, C.; Maldonado-Mendoza, I.E. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. SpringerPlus 2016, 5, 330.
- Links, M.; Demeke, T.; Gräfenhan, T.; Hill, J.; Hemmingsen, S.M.; Dumonceaux, T.J. Simultaneous profiling of seed-associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on T riticum and B rassica seeds. New Phytol. 2014, 202, 542–553.
- Johnston-Monje, D.; Gutiérrez, J.P.; Lopez-Lavalle, L.A.B. Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes. Front. Microbiol. 2021, 12, 2945.
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome Profiling of the Endophyte Burkholderia phytofirmans PsJN Indicates Sensing of the Plant Environment and Drought Stress. mBio 2015, 6, e00621-15.
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, L.; Colpaert, J.V.; Vangronsveld, J.; Van Der Lelie, D. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588.
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 2007, 53, 802–808.
- Johnston-Monje, D.; Mousa, W.K.; Lazarovits, G.; Raizada, M.N. Impact of swapping soils on the endophytic bacterial com-munities of pre-domesticated, ancient and modern maize. BMC Plant. Biol. 2014, 14, 233.
- Wagner, M.R.; Tang, C.; Salvato, F.; Clouse, K.M.; Bartlett, A.; Vintila, S.; Phillips, L.; Sermons, S.; Hoffmann, M.; Balint-Kurti, P.J.; et al. Microbe-dependent heterosis in maize. Proc. Natl. Acad. Sci. USA 2021, 118.
- Mousa, W.K.; Shearer, C.; Limay-Rios, V.; Ettinger, C.L.; Eisen, J.A.; Raizada, M.N. Root-hair endophyte stacking in finger millet creates a physicochemical barrier to trap the fungal pathogen Fusarium graminearum. Nat. Microbiol. 2016, 1, 16167.
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72.
- Thompson, M.; Raizada, M.N. Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road. Pathogens 2018, 7, 81.
- Mousa, W.K.; Shearer, C.R.; Limay-Rios, V.; Zhou, T.; Raizada, M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant. Sci. 2015, 6, 805.
- Kuźniar, A.; Włodarczyk, K.; Grządziel, J.; Goraj, W.; Gałązka, A.; Wolińska, A. Culture-independent analysis of an endophytic core microbiome in two species of wheat: Triticum aestivum L. (cv. ‘Hondia’) and the first report of microbiota in Triticum spelta L. (cv. ‘Rokosz’). Syst. Appl. Microbiol. 2019, 43, 126025.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
614
Revisions:
2 times
(View History)
Update Date:
08 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




