Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raul Avila Sosa | + 951 word(s) | 951 | 2021-11-22 09:00:53 | | | |
| 2 | Conner Chen | Meta information modification | 951 | 2021-12-07 02:04:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Avila Sosa, R. Saffron as an Aromatic Spice. Encyclopedia. Available online: https://encyclopedia.pub/entry/16790 (accessed on 07 February 2026).
Avila Sosa R. Saffron as an Aromatic Spice. Encyclopedia. Available at: https://encyclopedia.pub/entry/16790. Accessed February 07, 2026.
Avila Sosa, Raul. "Saffron as an Aromatic Spice" Encyclopedia, https://encyclopedia.pub/entry/16790 (accessed February 07, 2026).
Avila Sosa, R. (2021, December 06). Saffron as an Aromatic Spice. In Encyclopedia. https://encyclopedia.pub/entry/16790
Avila Sosa, Raul. "Saffron as an Aromatic Spice." Encyclopedia. Web. 06 December, 2021.
Copy Citation
Plant extracts can be sources of valuable chemical compounds, with particular biological properties, such as color or aroma. Tinctures or essential oils derived from plants are usually a complex mixture of bioactive components, where their combination usually present synergistic effects. Due to their aromatic nature and the biological activity of their components, spices have been valued for centuries, and among them, saffron is one of the most precious.
Saffron spice is made up of the dried stigmas of the Crocus sativus L. flower. Its primary use is in food, where it is valued for its coloring, flavoring, and aromatizing of some traditional dishes.
saffron
1. Saffron as an Aromatic Spice
Saffron is derived from the stigmas of the flower Crocus sativus L. The stigmas are dried and used as a natural dye or as flavoring in cooking [1][2]. In addition to providing color to food, it acts as an antioxidant [3] and has pharmacological properties [4]. The name saffron is derived from the Arabic za’-faran (“yellow”) or from the Persian term Za’afarn, which means golden flowers. The Greeks knew it as krokos [5][6], since mythology described how the god Hermes (Mercury) wounded his friend Krokos in the head; when he fell dead, his blood spilled onto a flower, creating three blood-colored filaments [7]. Historically, the cultivation and use of saffron has spanned more than 3500 years across multiple cultures, continents, and civilizations [8]. The origin of the practice is believed to be in the eastern Mediterranean basin, from which farming of the plant spread to other parts the “Old World”. Many species of the Crocus are obtained from Crete and islands in the Aegean sea, which can be considered as the "birthplace" of saffron [4]. Saffron is among the most traditional spices, and even appeared in a fresco from the Palace of Minos in Crete. Today, the spice it is known as “red gold” or “soft gold” [9][10][11].
2. Botanical Characterisctics
C. sativus L. is a triploid plant, geophyte of the Iridaceae family that blooms in autumn and grows in arid and semi-arid climates [12][13]. It consists of a corm, dark green leaves, and lilac flowers (Figure 1). Corms produce symmetrical white roots covered with several tunics of varying texture and color [14][15][16]. The plant can bloom with one or several flowers whose peduncle and ovary are underground. The flower has a perigonium consisting of six purple tepals (2–4.7 cm long and 1.1 and 2.3 cm wide), three stamens, and stigma. The stigma is arranged by three red filaments linked by their base to the style, with an enlarged apex in the form of a trumpet. In the anthesis, these elements remain upright, but as the flower opens, the tepals tilt downward from longer filaments than the anthers [17][18][15][19]. Sub-histerant flowers (appearing before or after the leaves) are sterile, so the plant propagates through underground corms from which flowers emerge in autumn [17][14][16][20]. The life cycle of C. sativus is composed of two stages: vegetative (to propagate corms) and reproductive (formation of floral organs) [20]. The flower number, size, and stigma are conditioned by corm characteristics [19]; however, stigma length and color are related to the development and plant growth. Some authors [21][22] have described the stigma color as changing from scarlet-yellow in the following phases: yellow stigma, closed bud inside the perianth tube (0.3 cm in length); orange stigma, closed bud inside the perianth tube (0.4 cm in length); red stigma, closed bud inside the perianth tube (0.8 cm in length); three days before anthesis (complete flower opening), dark red stigmas in closed bud outside the perianth tube (3 cm); day of anthesis, dark red stigmas form in the closed bud outside of the perianth tube (3 cm); on the day of anthesis, the dark red stigmas are 3 cm in length, and they last until two days after anthesis. Stigma coloration changes are associated with the increase of carotenoids and biosynthesis, and the increase and accumulation of apocarotenoids and zeaxanthin derivatives (crocin, crocetin and picrocrocin) [23].
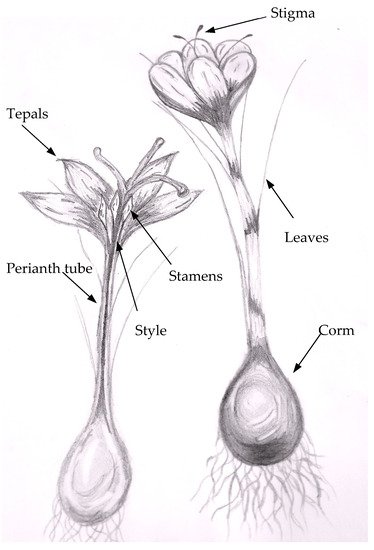
Figure 1. Crocus sativus L. plant parts.
The plant is distributed geographically around the world, but production is concentrated in Iran. However, significant producers include India, Greece, Afghanistan, Morocco, and Spain. The world production of saffron is approximately 430 tons per year [13][24], with the principal importing countries being Spain (23%), Hong Kong (8.7%), the United States (7.6%), India (7.0%), and Italy (7.0%) [17].
3. Harvest
C. sativus farmers have observed certain advantages of planting, such as high market prices, low water requirements, and a long-term exploitation opportunity with only a single crop [25]. To produce 1 kg of saffron, approximately 150,000 flowers must be collected within 370–470 h (i.e., the flowers must be picked, their stigmas removed, and the drying process conducted) [14][16]. C. sativus occupies a very special place, since it is the only plant species that naturally produces crocins, safranal, and picrocrocins in significant quantities [26]. Safranal is the main component of saffron essential oil and is responsible for its unique odor and neuropsychological effects. Crocin mainly contributes to the coloring of saffron and is widely used as a food dye [27]. Saffron production consists of several phases: harvest, collection, transport, drying, packaging, and storage [28].
Flower harvesting and stigma separation are time-consuming; for instance, collecting 1000 flowers takes approximately 45–55 min; another 100–130 min are required to remove the stigmas for drying. [5]. The flowering period lasts from 15 to 25 days, and harvesting begins before dawn, as the flower life is extremely short (from 20–24 h, bud to anthesis) [29]. The collection is completed carefully by cutting the bottom of the corolla. At that point, flowers are transferred to baskets or bags to the processing areas, avoiding excess pressure and deformation of the floral stigmas. Cutting of the flowers is done before the tepals open to prevent them from wilting in the sun (losing their color and taste) [17][4][16][30][31][32]. Traditional harvesting contaminates the product with microorganisms from soil, dust, and sewage. Ethylene oxide decontamination is a conventional method for spice sterilization. However, the process is not reliable due to possible toxic waste [33].
References
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech. Histochem. 2014, 89, 401–411.
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Feizi, J. Development of an environmentally-friendly solvent-free extraction of saffron bioactives using subcritical water. LWT Food Sci. Technol. 2019, 114, 108428.
- Kosar, M.; Demirci, B.; Goger, F.; Kara, I.; Baser, K.H.C. Volatile composition, antioxidant activity, and antioxidant components in saffron cultivated in Turkey. Int. J. Food Prop. 2017, 20, S746–S754.
- Ordoudi, S.A.; Tsimidou, M. Saffron quality: Effect of agricultural practices, processing and storage. In Production Practices and Quality Assessment of Food Crops; Dris, R., Jain, S.M., Eds.; Kluwer Academic: Amsterdam, The Netherlands, 2004; Volume 1, pp. 209–260.
- Winterhalter, P.; Straubinger, M. Saffron Renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59.
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends Food Sci. Technol. 2016, 58, 69–78.
- Karabagias, I.K.; Koutsoumpou, M.; Liakou, V.; Kontakos, S.; Kontominas, M.G. Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. Eur. Food Res. Technol. 2017, 243, 1577–1591.
- Zhao, M.; Wang, B.; Xiang, L.; Xiong, C.; Shi, Y.; Wu, L.; Meng, X.; Dong, G.; Xie, Y.; Sun, W. A novel onsite and visual molecular technique to authenticate saffron (Crocus sativus) and its adulterants based on recombinase polymerase amplification. Food Control 2019, 100, 117–121.
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron. Food Chem. 2018, 253, 284–292.
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics 2019, 6, 63.
- Rocchi, R.; Mascini, M.; Sergi, M.; Compagnone, D.; Mastrocola, D.; Pittia, P. Crocins pattern in saffron detected by UHPLC-MS/MS as marker of quality, process and traceability. Food Chem. 2018, 264, 241–249.
- Haghighi, R.; Sayed Tabatabaei, B.E.; Maibody, S.A.M.M.; Talebi, M.; Molina, R.V.; Nebauer, S.G.; Renau-Morata, B. A flowering inhibitor of the temperature-dependent pathway in Crocus sativus L. Mol. Biol. Rep. 2020, 47, 2171–2179.
- Koocheki, A.; Rezvani Moghaddam, P.; Seyyedi, S.M. Depending on mother corm size, the removal of extra lateral buds regulates sprouting mechanism and improves phosphorus acquisition efficiency in saffron (Crocus sativus L.). Ind. Crops Prod. 2019, 141, 111779.
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive volatiles in Sicilian (South Italy) saffron: Safranal and its related compounds. J. Essent. Oil Res. 2017, 29, 221–227.
- Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. The genus Crocus L.: A review of ethnobotanical uses, phytochemistry and pharmacology. Ind. Crops Prod. 2021, 171, 113923.
- Razak, S.I.A.; Anwar Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A Review on Medicinal Properties of Saffron toward Major Diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116.
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560.
- Gómez-Gómez, L.; Trapero-Mozos, A.; Gómez, M.D.; Rubio-Moraga, A.; Ahrazem, O. Identification and possible role of a MYB transcription factor from saffron (Crocus sativus). J. Plant Physiol. 2012, 169, 509–515.
- Moratalla-López, N.; Sánchez, A.M.; Lorenzo, C.; López-Córcoles, H.; Alonso, G.L. Quality determination of Crocus sativus L. flower by high-performance liquid chromatography. J. Food Compos. Anal. 2020, 93, 103613.
- Zhou, G.; Li, L.; Lu, J.; Li, J.; Yao, C.; Sun, P.; Liu, K.; Dong, Y.; Qin, L.; Qian, X. Flower cultivation regimes affect apocarotenoid accumulation and gene expression during the development of saffron stigma. Hortic. Environ. Biotechnol. 2020, 61, 473–484.
- Sánchez, A.M.; Winterhalter, P. Carotenoid cleavage products in saffron (Crocus sativus L). ACS Symp. Ser. 2013, 1134, 45–63.
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gómez, M.D.; Orzaez, D.; Granell, A.; Gómez-Gómez, L. Cytosolic and Plastoglobule-targeted Carotenoid Dioxygenases from Crocus sativus Are Both Involved in b-Ionone Release. J. Biol. Chem. 2008, 238, 24816–24825.
- Baba, S.A.; Ashraf, N. Apocarotenoid Biosynthesis in Crocus sativus L. In Apocarotenoids of Crocus sativus L: From Biosynthesis to Pharmacology; Baba, S.A., Ashraf, N., Eds.; Springer: Singapore, 2016; pp. 1–21.
- Razavi, S.E.; Jafari, S.M. Effect of corm age on the antioxidant, bactericidal and fungicidal activities of saffron (Crocus sativus L.) stigmas. Food Control 2021, 130, 108358.
- Taheri-Dehkordi, A.; Naderi, R.; Martinelli, F.; Salami, S.A. A robust workflow for indirect somatic embryogenesis and cormlet production in saffron (Crocus sativus L.) and its wild allies; C. caspius and C. speciosus. Heliyon 2020, 6, e05841.
- Yue, J.; Wang, R.; Ma, X.; Liu, J.; Lu, X.; Balaso-Thakar, S.; An, N.; Liu, J.; Xia, E.; Liu, Y. Full-length transcriptome sequencing provides insights into the evolution of apocarotenoid biosynthesis in Crocus sativus. Comput. Struct. Biotechnol. J. 2020, 18, 774–783.
- Fang, Q.; Li, Y.; Liu, B.; Meng, X.; Yang, Z.; Yang, S.; Bao, T.; Kimani, S.; Gao, X.; Wang, L. Cloning and functional characterization of a carotenoid cleavage dioxygenase 2 gene in safranal and crocin biosynthesis from Freesia hybrida. Plant Physiol. Biochem. 2020, 154, 439–450.
- Fancello, F.; Petretto, G.; Sanna, M.L.; Pintore, G.; Lage, M.; Zara, S. Isolation and characterization of microorganisms and volatiles associated with Moroccan saffron during different processing treatments. Int. J. Food Microbiol. 2018, 273, 43–49.
- Thakur, S.; Dutt, H.C. Floral Phenology and Adequate Collection Time of Flowers of Crocus sativus L.: An Expensive Spice. Natl. Acad. Sci. Lett. 2021, 44, 275–280.
- Kafi, M.; Kamili, A.N.; Husaini, A.M.; Ozturk, M.; Altay, V. An Expensive Spice Saffron (Crocus sativus L.): A Case Study from Kashmir, Iran, and Turkey. In Global Perspectives on Underutilized Crops; Springer International Publishing: Cham, Switzerland, 2018; pp. 109–149.
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. State of art of saffron (Crocus sativus L.) agronomy: A comprehensive review. Food Rev. Int. 2009, 25, 44–85.
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209.
- Amini, M.; Ghoranneviss, M.; Abdijadid, S. Effect of cold plasma on crocin esters and volatile compounds of saffron. Food Chem. 2017, 235, 290–293.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
07 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




