
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Riccardo Di Corato | + 1678 word(s) | 1678 | 2021-12-01 04:24:41 | | | |
| 2 | Catherine Yang | Meta information modification | 1678 | 2021-12-07 02:02:55 | | |
Video Upload Options
A suitable amount of an addition of zinc, manganese, cobalt or nickel to the ferrite nucleus of the Nanoparticles (NPs) results in a higher magnetic resonance imaging contrast and an increase of saturation magnetization (Ms), which can lead to more efficient magnetic targeting. In particular, the introduction of zinc and manganese into the crystalline structure of the magnetic particle generates some changes in the material properties, enhancing their potential use in theranostic applications.
1. Synthetic Strategies of Mn-Zn Ferrites
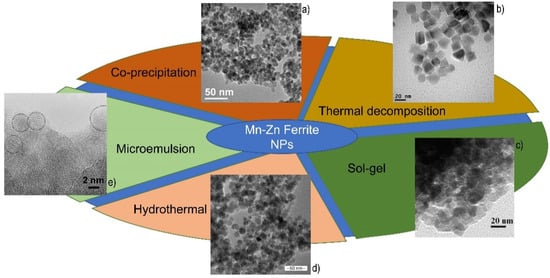
There are different preparation techniques for the synthesis of ferrite nanoparticles that exhibit novel properties which are dependent on their structural characteristics, like particle size and shape. Various methods, described below, such as co-precipitation, thermal decomposition, hydrothermal methods and sol-gel, are used to prepare spinel manganese-zinc ferrite nanocrystals.
The co-precipitation method is one of the most utilized synthesis methods for preparing magnetic nanoparticles [1]. The formation of magnetic nanoparticles is due to the nucleation followed by the crystal growth and there are two steps to follow this precipitation process [2]. The first step is to take account of the solubility for each type of solute and the second step is the addition of an excess of solute, which could result in the precipitation and formation of nanocrystals. For example, for the formation of nanoparticles, the solution has to be in a high state of saturation to start the nucleation. In this case, two kinds of nucleation processes could occur: the heterogeneous nucleation (when nuclei collide and interact with a solid surface) and homogenous nucleation (which takes place in the absence of a solid interface by combining solute molecules to produce nuclei) [3]. Furthermore, for having a uniformity of particle size, it is necessary for distribution to follow a short nucleation period in such a way so as to obtain the uniformity of the nanoparticles without further nucleation. As such, the burst nucleation (induction of a single event) is obtained by increasing the concentration of metal precursors to the critical saturation point. Their magnetization depends on different parameters, like the pH of the suspension, the reaction temperature, and the initial molar concentration [4][5]. The major drawback of the co-precipitation method is the insufficient size control distribution and the uncontrolled shape, but, nonetheless, this process is a favored route to produce magnetic nanoparticles due to it requiring a low reaction temperature, the high reaction yield and the shorter reaction time than other methods, such as thermal decomposition or hydrothermal synthesis.
Scheme 1 summarizes all the reported synthetic methods, with some representative TEM images of obtained nanoparticles.
2. In Vitro Applications of Mn-Zn Ferrites
In the selection of relevant contributions, some aspects were considered, such as the effective enhancement of the magnetic properties (e.g., magnetic saturation or r 2 relaxivity or SAR value), that justified the use of a doped ferrite in alternative to bare iron oxide nanoparticles, and the application of these nanoparticles in an in vitro assay, with particular consideration of the biocompatibility of the nanomaterial.
Qu et al. presented a study on the synthesis of MnxZn1−xFe2O4 ferrite nanoparticles with a size of around 8 nm. For these nanoparticles, obtained by thermal decomposition, the ratio between Zn and Mn was inversely varied, finding Mn0.6Zn0.4Fe2O4 as the best composition in terms of Ms (76.7 emu/g). These nanoparticles were then transferred in the aqueous phase through the use of a synthetic amphiphilic block copolymer obtained from mPEG and poly(caprolactone) (PEG-PLC), obtaining ultra-stable clusters of about 100–150 nm. The size of these clusters and their magnetization were modulated by varying the mass ratios between the particles and polymer. In terms of magnetic hyperthermia, however, it was noted that the ferrites obtained with Mn only show a higher SAR (1618 W/g) than Mn0.6Zn0.4Fe2O4 (1100 W/g). From the experiments conducted on MCF-7 and drug-resistive MCF-7/ADR cancer cells in vitro, it can been seen that the application of magnetic hyperthermia generated high levels of cellular depletion of up to 90% within 15 min, obtained mainly by induction of apoptotic and non-necrotic phenomena [11].
In addition, in the work of Guo et al. the Mn-Zn ferrite nanoparticles were synthesized by thermal decomposition, obtaining monodisperse nanoparticles of 10 nm, with a quasi-cubic morphology. In addition to a good r 2 relaxivity and an excellent biocompatibility (with cytotoxicity of 10% at the highest tested concentration and a hemolysis rate of 1.12%), these ferrites showed a very high magnetic saturation, equal to 98 emu/g. These nanoparticles were then investigated as a potential actuator for magnetic hyperthermia, obtaining an increase in temperature of up to 42–44 °C within 20 min, at 235 kHz, with a particularly low concentration of 60 μg/mL. In addition, the nanoparticles were evaluated as carriers for gene therapy, assessing the ability to transfect CD44-shRNA-EGFP plasmids into the ovarian cancer SK-OV-3 cells, obtaining a transfection efficiency of 58%, which is comparable to the standard liposome method [12].
Applying a different synthetic method, Sobhani et al. reported the preparation of manganese-zinc ferrite nanoparticles that were obtained by a hydrothermal route, obtaining particles with a TEM diameter of approximately 14 nm and a hydrodynamic diameter of approximately 400 nm. These particles, stabilized with a PEG outer layer, showed a higher relaxivity r 2, 85 mM −1 s −1 compared to the commercial equivalent (Sinerem). Taking into account a low-mild cytotoxicity, two different cell lineages, 4T1 and L929 cells, were incubated and then analyzed by MRI to evaluate the T 2 signal decrease, obtaining a decrease of signal intensity in T 2-weighted images, of 25% at an iron concentration of 0.4 mg/mL and 45% at a concentration of 0.8 mg/mL [13].
3. In Vivo Applications of Mn-Zn Ferrites
For the medical validation of this quite novel nanomaterial, the administration in a living system is very important, because many results concerning magnetic nanoparticles, fully demonstrated in solution or by in vitro experiments, were not reproduced in vivo. As in the previous section, the analysis is focused on those papers that provided real advancement in the medical usage of Mn-Zn ferrite nanoparticles. It should be noted that all these studies have been published in the last six years, reflecting the very recent development of the medical application of these ferrites.
In 2015, Leng et al. reported the preparation of hydrophobic spherical Mn0.6Zn0.4Fe2O4 nanocrystals, with an average diameter of 8 nm, and a star-shaped copolymer 4sPCL- b -P(MEO 2MA- co -OEGMA), starting from the in vitro study reported in [11]. The magnetic nanoparticles, obtained by a standard thermal decomposition method, were then resuspended in THF with the polymer and quite small nanoclusters were obtained, with an average size of 122 nm. These composites showed low toxicity, by in vitro assay, and a relevant r 2 of 138 mM −1 s −1 at 3.0 T. For assessing the MRI potential of the system, the magnetic micelles were intravenously injected in mice that were bearing a xenograft tumor at a low dose of 0.007mmol Fe/kg. By MRI, a significant darkening of the tumor region was observed after 1 h in T 2-weighted spin-echo analyses, with a maximum signal recorded at 4 h. At 8h after injection, the signal in the tumor region recovered the contrast level of pre-injection imaging [11][14].
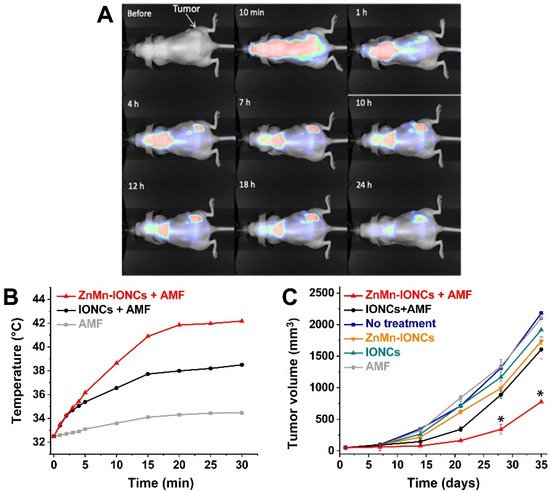
Albarqi et al. recently proposed the preparation of small nanoclusters for the therapy of prostatic cancer. Iron oxide nanoparticles doped with zinc and manganese, with a size of 13 nm, were obtained by the thermal decomposition of Fe(acac)3, ZnCl2 and MnCl2. These hydrophobic nanoparticles were subsequently transferred in water using the solvent evaporation method, adding PEG-PCL as template for the preparation of very small clusters of 100 nm. As expected, the doped nanoparticles were characterized by a higher saturation magnetization value (60.33 Am 2/kg) than iron oxide nanoparticles (57 Am2/kg). Moreover, the SAR values (measured in THF at 420 kHz, 26.9 kA/m) were improved for doped nanoparticles (1615.1 W/g) if compared to bare iron oxide nanoparticles (586.2 W/g). When the nanoparticles were clustered and transferred in water, a slight decrease of SAR values was observed, but, regardless, the Mn-Zn magnetic cluster showed a higher performance (1010.0 W/g) than the iron oxide nanoclusters (493.8 W/g). The clusters were intravenously administrated to some nude mice bearing subcutaneous xenografts of DU145 prostate cancer cells. The particles started to accumulate in the tumor site (via passive targeting) after 1 h and reached a maximum concentration after 7 h, with a minor accumulation in other organs ( Figure 1 A). The application of AFM to the animal induced an intertumoral temperature of 42 °C and an evident inhibition of the tumor growth, also in comparison to the bare iron oxide clusters ( Figure 1 B) [15].
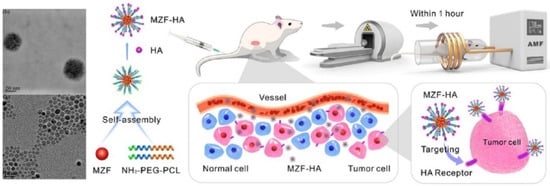
Wang et al. started from hydrophobic Mn0.6Zn0.4Fe2O4 nanoparticles for the preparation of magnetic PEG- b -PCL block copolymer micelles. The surface of the magnetic cluster was then engineered with hyaluronic acid (HA) for the active targeting of tumor cells, such as A549 (human lung adenocarcinoma cell line), over-expressing the receptor (CD44) for the HA. The purpose of this approach was to combine a magnetic hyperthermia therapeutic approach with radiotherapy for the treatment of non-small-cell lung cancer ( Figure 2 ). By the in vitro test, a synergistic effect of the dual therapy was observed, with a total apoptosis rate of 32.7%, almost four times that of the results of hyperthermia and five times for radiotherapy. The synergistic effect was obtained also by in vivo treatment of xenograft tumor in mice. The nanocomposite was injected by tail vein in the animal and the therapeutic scheme was applied on days 1 and 4. The growth of the tumor appeared to be significantly inhibited with the relative tumor volume ratio shrinking to 49.6% by day 13 in the dual therapy group [16].
References
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248.
- Sun, T.; Borrasso, A.; Liu, B.; Dravid, V. Synthesis and Characterization of nanocrystalline zinc manganese ferrite. J. Am. Ceram. Soc. 2011, 94, 1490–1495.
- Sugimoto, M. The past, present, and future of ferrites. J. Am. Ceram. Soc. 2004, 82, 269–280.
- Lu, A.-H.; Salabas, E.L.; Schueth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244.
- Babes, L.; Denizot, B.; Tanguy, G.; Le Jeune, J.J.; Jallet, P. Synthesis of iron oxide nanoparticles used as MRI contrast agents: A parametric study. J. Colloid Interface Sci. 1999, 212, 474–482.
- De Mello, L.B.; Varanda, L.C.; Sigoli, F.A.; Mazali, I.O. Co-precipitation synthesis of (Zn-Mn)-co-doped magnetite nanoparticles and their application in magnetic hyperthermia. J. Alloys Compd. 2018, 779, 698–705.
- Orsini, N.J.; Milic, M.M.; Torres, T.E. Zn- and (Mn, Zn)-substituted versus unsubstituted magnetite nanoparticles: Structural, magnetic and hyperthermic properties. Nanotechnology 2020, 31, 225707.
- Thota, S.; Kashyap, S.C.; Sharma, S.K.; Reddy, V.R. Micro Raman, Mossbauer and magnetic studies of manganese substituted zinc ferrite nanoparticles: Role of Mn. J. Phys. Chem. Solids 2016, 91, 136–144.
- Kaewmanee, T.; Wannapop, S.; Phuruangrat, A.; Thongtem, T.; Wiranwetchayan, O.; Promnopas, W.; Sansongsiri, S.; Thongtem, S. Effect of oleic acid content on manganese-zinc ferrite properties. Inorg. Chem. Commun. 2019, 103, 87–92.
- Makovec, D.; Kodre, A.; Arčon, I.; Drofenik, M. Structure of manganese zinc ferrite spinel nanoparticles prepared with co-precipitation in reversed microemulsions. J. Nanoparticle Res. 2008, 11, 1145–1158.
- Qu, Y.; Li, J.; Ren, J.; Leng, J.; Lin, C.; Shi, D. Enhanced magnetic fluid hyperthermia by micellar magnetic nanoclusters composed of MnxZn1−xFe2O4 nanoparticles for induced tumor cell apoptosis. ACS Appl. Mater. Interfaces 2014, 6, 16867–16879.
- Guo, T.; Dou, F.; Lin, M.; Huang, J.; Zhou, C.; Zhang, J.; Yu, H.; Jiang, X.; Ye, J.; Shi, Y.; et al. Biological characteristics and carrier functions of pegylated manganese zinc ferrite nanoparticles. J. Nanomater. 2019, 2019, 1–10.
- Sobhani, T.; Shahbazi-Gahrouei, D.; Rostami, M.; Zahraei, M.; Farzadniya, A. Assessment of manganese-zinc ferrite nanoparticles as a novel magnetic resonance imaging contrast agent for the detection of 4T1 breast cancer cells. Med. Signals Sens. 2019, 9, 245–251.
- Leng, J.; Li, J.; Ren, J.; Deng, L.; Lin, C. Star–block copolymer micellar nanocomposites with Mn, Zn-doped nano-ferrite as superparamagnetic MRI contrast agent for tumor imaging. Mater. Lett. 2015, 152, 185–188.
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020.
- Wang, Y.; Zou, L.; Qiang, Z.; Jiang, J.; Zhu, Z.; Ren, J. Enhancing targeted cancer treatment by combining hyperthermia and radiotherapy using Mn-Zn ferrite magnetic nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 3550–3562.




