Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Erik Maronde | + 901 word(s) | 901 | 2021-12-01 07:06:16 | | | |
| 2 | Camila Xu | Meta information modification | 901 | 2021-12-07 07:45:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maronde, E. Cyclic Nucleotide (cNMP) Analogues. Encyclopedia. Available online: https://encyclopedia.pub/entry/16777 (accessed on 07 February 2026).
Maronde E. Cyclic Nucleotide (cNMP) Analogues. Encyclopedia. Available at: https://encyclopedia.pub/entry/16777. Accessed February 07, 2026.
Maronde, Erik. "Cyclic Nucleotide (cNMP) Analogues" Encyclopedia, https://encyclopedia.pub/entry/16777 (accessed February 07, 2026).
Maronde, E. (2021, December 06). Cyclic Nucleotide (cNMP) Analogues. In Encyclopedia. https://encyclopedia.pub/entry/16777
Maronde, Erik. "Cyclic Nucleotide (cNMP) Analogues." Encyclopedia. Web. 06 December, 2021.
Copy Citation
Cyclic nucleotides are important signaling molecules involved in cellular events in pro- and eukaryotes, and analogues of this type of molecules are promising drug candidates.
cyclic nucleotide analogues
cAMP
cGMP
cGAMP
c-hexa-AMP
cCMP
1. Introduction
Since the discovery of the first so-called second messenger molecules by Earl Sutherland and coworkers [1][2], namely, “3′,5′-cyclic adenosine monophosphate” (cAMP; Figure 1, compound 1) and “3′,5′-cyclic guanosine monophosphate” (cGMP; Figure 1, compound 2), vast knowledge to understand their chemistry, mechanisms of action and their physiological functions has been accumulated.
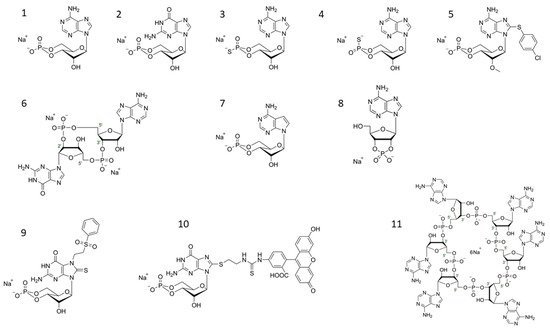
Figure 1. Selected structures of cyclic nucleotides (1) cAMP (Adenosine-3′,5′-cyclic monophosphate syn-conformation), (2) cGMP (Guanosine-3′,5′-cyclic monophosphate), (3) Rp-cAMPS (Adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer; PKA antagonist), (4) Sp-cAMPS (Adenosine-3′,5′-cyclic monophosphorothioate, Sp-isomer; PKA agonist), (5) 8-pCPT-2′-O-Me-cAMP (8-(4-Chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate; EPAC activator), (6) cGAMP(2′-5′)/2′3′-cGAMP/2′,5′-3′,5′-cGAMP (Cyclic (guanosine-(2′ − 5′)-monophosphate-adenosine-3′ − 5′)- monophosphate)STING ligand), (7) 7-CH-cAMP/cTuMP (7-Deazaadenosine-3′,5′-cyclic monophosphate; HCN ligand), (8) 2′,3′-cAMP (Adenosine-2′,3′-cyclic monophosphate), (9) 7-PS(O)2E-8-T-cGMP (7-[2-(Phenylsulfonyl)ethyl]-8-thioguanosine-3′,5′-cyclic monophosphate; selective CNGolf/ret ligand), (10) 8-[Fluo]-cGMP/8-[Fluo]-AET-cGMP (8-(2-[Fluoresceinyl]aminoethylthio)guanosine-3′,5′-cyclic monophosphate PKG ligand, general cGMP-like binding fluorescence tool), (11) c-hexa-AMP; cyclic hexaadenylate (Cyclic hexa-adenosine monophosphate; CRISPR-CAS III ligand).
For the finding that cAMP and cGMP bind to and activate certain protein kinases (which are nowadays called cAMP- or cGMP-dependent protein kinases, PKA and PKG), Edwin Krebs and Edwin Fisher were honored by receiving the Nobel Prize for Medicine or Physiology in 1992 [3].
Soon after their discovery, it became evident that the membrane permeability of cAMP or cGMP is too low to pass through cellular plasma membranes [4][5], one line of research became the synthesis of variants of cyclic nucleotides (cyclic nucleotide analogues/cNMPs) that maintain their principal functions while becoming more cell membrane permeable and thus applicable for use in living systems [6]. Soon cNMPs modified at various positions, which made them more (or less) potent, more target specific, or, while maintaining the principal function, less toxic in vitro or in vivo and provided higher cell membrane permeability, became available [5]. Moreover, analogues of the non-canonical cyclic nucleotides cCMP/cUMP and 2′,3′-cAMP (Figure 1, compound 8) have been made [7] and analyzed for their occurrence and potential biological role [8][9].
2. Practical Considerations for the Use of cNMP Analogues in Biological Systems
Despite vast accumulated and confirmed knowledge about cNMPs and their abilities (including advantages and disadvantages), cNMP analogues are often used inappropriately or at least not in an optimal manner for the biological system of interest. Below is a list of points worth considering before using certain cNMP analogues to investigate (or modulate) cNMP-regulated pathways.
2.1. Cell Permeability/Membrane Passage
It is well established that unmodified cNMPs do not notably enter a cell if applied extracellularly [4]. Purine ring modified analogues (like 8-Br-cAMP or N6,O2′-dibutyryl-cAMP short db-cAMP) are more lipophilic and enter cells more efficiently but are often prone to degradation processes (see below). An extended analysis of the hydrophobicity (lipophilicity) of cNMPs and sources for the chromatographic retention parameters used for the determination of lipophilicity can be found in [10].
2.2. Metabolism/Degradation of the Analog
Many 8-substituted-cAMP analogues are degraded by phosphodiesterase (PDE) activity present in animal sera like fetal bovine serum which many cell culture systems contain [11]. The analogue metabolites may have unwanted activities like binding to adenosine receptors [12] or being transformed via the salvage pathway, thereby interfering with RNA and DNA metabolism. Although, for example, db-cAMP ideally hydrolyzes after cell entry into the butyrate molecules and mono- or non-butyrylated cAMP, the resulting butyrates may interfere with other cellular pathways. Moreover, db-cAMP may hydrolyze before entering the cell and release one or two butyrates thereby posing potential other, extracellular, side effects. On the other hand, in biological systems where butyrate and cAMP act in an additive manner (for example in nerve cell- or adipocyte-differentiation) this butyrate effect may be desirable. Under such conditions, db-cAMP acts as the “trojan horse” carrying several biologically active components into the cells.
In case the abovementioned effects and side effects are not desired, PDE-dependent hydrolysis by 3′,5′-cyclic phosphodiesterases can be avoided by using the PDE-resistant cNMP-phosphorothioate analogues like Sp- and Rp-cAMPS and their derivatives. Phosphorothioate analogues are also more lipophilic and thus enter cells easier. However, in the case of the presence of high-affinity adenosine receptors (KD in the nanomolar range), it may be necessary to add the enzyme adenosine deaminase to the cell culture medium to convert the minute amounts (0.05%) of adenosine that may be present in Rp-cAMPS preparations to its inactive metabolite inosine [12].
2.3. Target Selectivity
As outlined above, cNMPs bind with high affinity to cAMP/cGMP-dependent protein kinases, 3′,5′-cyclic phosphodiesterases, EPAC/GEF proteins, ion channels (CNG; HCN), organic anion exchangers (OAG) and other target proteins. Each of the binding sites for cNMPs on these cNMP target molecules are different in terms of space, charge and flexibility so that in principle every modification that provides a gain of function on one of the target molecules may alter binding to another one thereby reducing the selectivity of the compound. An expression analysis of which cNMP target protein is present in a given biological system may help with the selection of the appropriate cNMP analog.
Taken together, the choice for the “ideal” cNMP analog for a certain experiment takes into account the nature of the investigated organism and/or the intended cell type targeted, combining good and fast permeability through the plasma membrane, stability against PDE hydrolysis and other enzymatic modifications outside and inside the cell with a high target selectivity and no or low toxicity.
References
- Sutherland, E.W.; Rall, T.W. The properties of an adenine ribonucleotide produced with cellular particles, ATP, Mg++, and epinephrine or glucagon. J. Am. Chem. Soc. 1957, 79, 3608.
- Butcher, R.W.; Sutherland, E.W. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J. Biol. Chem. 1962, 237, 1244–1250.
- Krebs, E.G.; Fischer, E.H. Phosphorylase activity of skeletal muscle extracts. J. Biol. Chem. 1955, 216, 113–120.
- Bartsch, M.; Zorn-Kruppa, M.; Kühl, N.; Genieser, H.-G.; Schwede, F.; Jastorff, B. Bioactivatable, Membrane-Permeant Analogs of Cyclic Nucleotides as Biological Tools for Growth Control of C6 Glioma Cells. Biol. Chem. 2003, 384, 1321–1326.
- Schultz, C.; Vajanaphanich, M.; Genieser, H.G.; Jastorff, B.; Barrett, K.E.; Tsien, R.Y. Membrane-permeant derivatives of cy-clic AMP optimized for high potency, prolonged activity, or rapid reversibility. Mol. Pharmacol. 1994, 46, 702–708.
- Posternak, T.; Sutherland, E.; Henion, W. Derivatives of cyclic 3′,5′-adenosine monophosphate. Biochim. Biophys. Acta 1962, 65, 558–560.
- Schwede, F.; Rentsch, A.; Genieser, H.-G. Medicinal Chemistry of the Noncanonical Cyclic Nucleotides cCMP and cUMP. Handb. Exp. Pharmacol. 2017, 238, 307–337.
- Ludwig, N.; Yerneni, S.S.; Menshikova, E.V.; Gillespie, D.G.; Jackson, E.K.; Whiteside, T.L. Simultaneous Inhibition of Glycolysis and Oxidative Phosphorylation Triggers a Multi-Fold Increase in Secretion of Exosomes: Possible Role of 2′,3′-cAMP. Sci. Rep. 2020, 10, 6948, Author Correction in 2020, 10, 14027.
- Jackson, E.K. Discovery and Roles of 2′,3′-cAMP in Biological Systems. Handb. Exp. Pharmacol. 2017, 238, 229–252.
- Braumann, T.; Jastorff, B.; Richter-Landsberg, C. Fate of Cyclic Nucleotides in PC12 Cell Cultures: Uptake, Metabolism, and Effects of Metabolites on Nerve Growth Factor-Induced Neurite Outgrowth. J. Neurochem. 2006, 47, 912–919.
- Vintermyr, O.K.; Bøe, R.; Brustugun, O.T.; Maronde, E.; Aakvaag, A.; ODøskeland, S. Cyclic adenosine monophosphate (cAMP) analogs 8-Cl- and 8-NH2-cAMP induce cell death independently of cAMP kinase-mediated inhibition of the G1/S transition in mammary carcinoma cells (MCF-7). Endocrinology 1995, 136, 2513–2520.
- Musgrave, I.F.; Genieser, H.G.; Maronde, E.; Seifert, R. Preparations of Rp-cyclic adenosine 3′,5′-phosphorothioate (Rp-cAMPS) can contain biologically active amounts of adenosine. FEBS Lett. 1993, 318, 227–230.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
829
Revisions:
2 times
(View History)
Update Date:
07 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




