Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ao, T. Vitamin D on Immune System. Encyclopedia. Available online: https://encyclopedia.pub/entry/16760 (accessed on 07 February 2026).
Ao T. Vitamin D on Immune System. Encyclopedia. Available at: https://encyclopedia.pub/entry/16760. Accessed February 07, 2026.
Ao, Tomoka. "Vitamin D on Immune System" Encyclopedia, https://encyclopedia.pub/entry/16760 (accessed February 07, 2026).
Ao, T. (2021, December 06). Vitamin D on Immune System. In Encyclopedia. https://encyclopedia.pub/entry/16760
Ao, Tomoka. "Vitamin D on Immune System." Encyclopedia. Web. 06 December, 2021.
Copy Citation
Vitamin D deficiency, which causes an imbalance in bone remodeling, is a global public health problem and its frequency is increasing.
vitamin D
immune system
COVID-19
rheumatoid arthritis (RA)
systemic lupus erythematosus (SLE)
multiple sclerosis (MS)
1. Introduction
Vitamin D deficiency, which causes an imbalance in bone remodeling, is a global public health problem and its frequency is increasing. Due to the pleiotropic effects of vitamin D, its deficiency is related to a higher risk of cardiovascular diseases [1][2][3], infectious diseases, and autoinflammatory diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS). In addition, vitamin D taken for the treatment and prevention of disease has been debated, given its immunosuppressive effect. Anti-cancer effects of vitamin D have found application in cancer treatment [4].
2. Vitamin D and the Immune Cells
Granulocytes, dendritic cells, monocytes/macrophages, and lymphocytes play an important role in the regulation of the immune system, the inflammatory response, and bone remodeling. In the 1980s, Abe et al. reported that vitamin D induces differentiation of monocytes and macrophages [5]. It has also been demonstrated that dendritic cells, monocytes/macrophages, and T and B cells express vitamin D and 1α-hydroxylase (CYP27B1), the vitamin D-activating enzyme [6]. In this section, we discuss the function of vitamin D in these immune cells, which play important roles in infectious and autoimmune diseases.
2.1. Dendritic Cells
A dendritic cell acts as an antigen-presenting cell to T cells, priming the adaptive immune response. Stimulation with the active form of vitamin D downregulates MHC class II and co-stimulatory molecules (such as CD40, CD80, and CD86), expressed on dendritic cells, resulting in T cell activation. In addition, activated vitamin D or vitamin D analogues suppress dendritic cell cytokine production, specifically, interleukin (IL)-12, which affects the differentiation of T helper cells into Th1 cells, and IL-23, which affects the differentiation of T helper cells into Th17 cells. Vitamin D also promotes expression of the anti-inflammatory cytokine IL-10 [7][8].
2.2. Monocytes/Macrophages
Monocytes/macrophages play an important role in the protection of infections by producing inflammatory cytokines. Components from bacteria, viruses, and fungi are recognized by toll-like receptors expressed on the surfaces of monocytes and macrophages, which upregulate the expression of the vitamin D receptor (VDR) and CYP27B1 [9]. After transport into the cell, 25-hydroxyvitamin D (25D) is metabolized into the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25D), by CYP27B1. Within the cell, 1,25D binds to VDR, which exists in the cytosol or nucleus, and the activated VDR forms a heterodimer with the retinoid-X receptor (RXR). The heterodimer binds to DNA and induces the production of antibiotic peptides, such as cathelicidin and β-defensin 2 [10][11]. These peptides produce antibiotic effects by destroying the cell membranes of bacteria and viruses or by activating an antibiotic signaling cascade in infected cells.
The NF-κB transcription factor is required for the expression of DEFB4, the gene encoding β-defensin 2, and 1,25D has been shown to affect NF-κB activation. 1,25D induces the expression of nucleotide-binding oligomerization domain 2 (NOD2), an intracellular pathogen-recognizing protein. NOD2 binds to muramyl dipeptide, a common peptidoglycan among gram-negative bacteria, to promote the transcription of DEFB4 via NF-κB [12]. Furthermore, 1,25D has been shown to induce autophagy, a conserved cellular degradation and recycling process in eukaryotes, in macrophages, and to promote antibiotic activity (Figure 1). Yuk et al. reported that 1,25D induces transcription of the autophagy-associated proteins Atg-5 and Beclin-1, which promote autophagy via the induction of cathelicidin and its downstream factors (p38, ERK, and C/EBPβ) [13]. 1,25D produced by monocytes and macrophages induces the expression of cathelicidin and β-defensin 2, which contribute to protection against infections [14]. It also regulates the epigenetic programming of monocytes/macrophages during immune challenges and, thereby, affects immunological memory and subtype differentiation of immune cells [15].
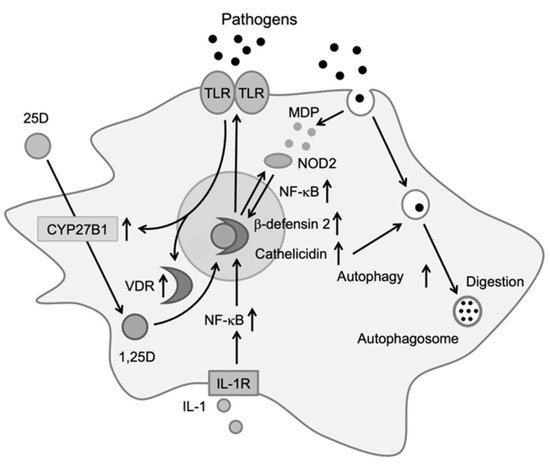
Figure 1. Effects of vitamin D on immune cells. Activation of toll-like receptors by pathogens increases the expression of vitamin D receptor (VDR) and CYP27B1. Upon entering the cell, 25D is metabolized to 1,25D by CYP27B1. 1,25D then binds to VDR, which induces cathelicidin and β-defensin 2. Cathelicidin promotes antibiotic activity via autophagy [14].
2.3. T Cells
T cells interact with antigen-presenting dendritic cells to induce an antigen-specific immune response. T cells express both the VDR and CYP27B1. Naïve T cells express a low level of the VDR, which gradually increases upon activation. Moreover, 1,25D suppresses the proliferation and differentiation of CD4-positive T cells via cytokine secretion. Specifically, 1,25D reduces Th1-type differentiation and the secretion of inflammatory cytokines (IL-2, IFNγ, and TNF-α), and promotes Th2-type differentiation and the secretion of anti-inflammatory cytokines (IL-4, IL-5, and IL-10) [16]. Additionally, 1,25D inhibits the secretion of Th17-related cytokines (IL-17, IFNγ, IL-21, and IL-22) and negatively regulates the RAR-related orphan receptor C and the aryl hydrocarbon receptor, which are master regulators of Th17-type differentiation [17][18]. Conversely, 1,25D promotes the differentiation of regulatory T cells, preventing an increased autoimmune response by inducing the anti-inflammatory cytokine IL-10 and the FoxP3 transcription factor [19].
2.4. B Cells
B cells play a key role in autoimmune disease via the production of autoantibodies; they also express the VDR and CYP27B1. Previous studies have indicated that B cell differentiation, proliferation, and antibody production are suppressed by 1,25D-treated T helper cells. However, a recent study demonstrated that 1,25D itself suppresses naïve B cell differentiation or maturation to memory B and plasma cells [20].
3. Vitamin D and Infectious Disease
In recent years, epidemiological data have shown that vitamin D deficiency is associated with morbidity in several infectious diseases. However, vitamin D supplementation as a treatment for infectious diseases remains controversial, in part due to conflicting clinical study results [21]. In this section, we will focus on respiratory infections, including the 2019 coronavirus disease (COVID-19) and the flu. We reviewed recent clinical studies on the correlation between vitamin D deficiency and morbidity, the effects of vitamin D supplementation in randomized controlled trials (RCT), and the underlying mechanisms.
3.1. Flu and Vitamin D
It has been reported that a low serum level of 25D in patients is positively correlated with morbidity in upper respiratory tract infections, including the flu. The increased morbidity of the flu in the winter may be related to decreased exposure to sunlight since synthesis of the active form of vitamin D requires sunlight. Interestingly, in cases with an increase in the serum 1,25D level by 10 nmol/L, the risk of infection decreases by 7% [22]. However, the use of vitamin D supplementation continues to be controversial. Hayashi et al. found that mice fed a diet consisting of a high dose of 25D and infected with the influenza virus exhibited decreased production of the inflammatory cytokines, IL-5 and IFN-γ [23]. In an RCT conducted by Murdoch, healthy adults were given more than 100,000 IU of vitamin D3 for 1 month; however, morbidity resulting from upper respiratory tract infections did not decrease [24]. Conversely, another RCT conducted by Camargo demonstrated that vitamin D3 administration (300 IU/day for 3 months) resulted in decreased morbidity related to upper respiratory tract infections in Mongolian children [25]. However, another RCT targeting immunodeficient patients in Sweden showed that daily administration of vitamin D3 (4000 IU/day) reduced symptoms, the amount of pathogen detected in mucus, and the duration of antibiotic treatment [26][27]. Moreover, a double-blind trial conducted by Urashima et al. indicated that children treated with vitamin D3 (1200 IU/day) had a significantly lower rate of flu type A (18.6%) compared to the placebo group (10.8%) [28]. In that trial, vitamin D supplementation was significantly effective in children with asthma.
3.2. COVID-19 and Vitamin D
COVID-19 is a serious public health threat. Its pathogen, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causes respiratory symptoms. Many observational studies have demonstrated that serum vitamin D levels are inversely correlated with the incidence and severity of COVID-19 [29]. The suggested mechanism is that vitamin D suppresses the renin-angiotensin system, increases ACE2 concentration in acute lung injury, and induces an interferon (IFN)-mediated antiviral reaction. Xu et al. suggested that 1,25D alleviates lipopolysaccharide-induced acute lung injury through renin suppression and Ang Ⅱ expression [30]. A similar mechanism may be expected in SARS-CoV-2-related acute respiratory distress syndrome (ARDS). Type Ⅰ IFNs are natural antiviral mediators, and there is evidence that their response contributes to COVID-19 severity [31]. A molecular study has described a constitutive inhibitory interaction between unbound VDR and STAT1, a transcription factor in Type Ⅰ IFN signaling. Consequently, vitamin D deficiency could reduce the effectiveness of the IFN-mediated antiviral response due to higher levels of unbound VDR [32]. T Therefore, vitamin D supplementation may contribute to the prevention of severe COVID-19.
References
- Buleu, F.N.; Luca, C.T.; Tudor, A.; Badalica-Petrescu, M.; Caraba, A.; Pah, A.; Georgescu, D.; Christodorescu, R.; Dragan, S. Correlations between Vascular Stiffness Indicators, OPG, and 25-OH Vitamin D3 Status in Heart Failure Patients. Medicina 2019, 55, 309.
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275.
- Bahrami, L.S.; Ranjbar, G.; Norouzy, A.; Arabi, S.M. Vitamin D supplementation effects on the clinical outcomes of patients with coronary artery disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12923.
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14.
- Abe, E.; Miyaura, C.; Sakagami, H.; Takeda, M.; Konno, K.; Yamazaki, T.; Yoshiki, S.; Suda, T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1981, 78, 4990–4994.
- Hart, P.H.; Gorman, S.; Finlay-Jones, J.J. Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat. Rev. Immunol. 2011, 11, 584–596.
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411.
- Berer, A.; Stöckl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp. Hematol. 2000, 28, 575–583.
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773.
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077.
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243.
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912.
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231.
- Kikuta, J.; Ishii, M. Current Topics on Vitamin D. The effects of vitamin D on the immune system. Clin. Calcium 2015, 25, 359–365.
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211.
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23.
- Ikeda, U.; Wakita, D.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Iwakura, Y.; Nishimura, T. 1α,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol. Lett. 2010, 134, 7–16.
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell Biol. 2011, 31, 3653–3669.
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabryšová, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708.
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647.
- Lee, C. Controversial Effects of Vitamin D and Related Genes on Viral Infections, Pathogenesis, and Treatment Outcomes. Nutrients 2020, 12, 962.
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440.
- Hayashi, H.; Okamatsu, M.; Ogasawara, H.; Tsugawa, N.; Isoda, N.; Matsuno, K.; Sakoda, Y. Oral Supplementation of the Vitamin D Metabolite 25(OH)D. Nutrients 2020, 12, 2000.
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: The VIDARIS randomized controlled trial. JAMA 2012, 308, 1333–1339.
- Camargo, C.A.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, e561–e567.
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Björkhem-Bergman, L.; Ekström, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. BMJ Open 2012, 2.
- Van Amerongen, B.M.; Dijkstra, C.D.; Lips, P.; Polman, C.H. Multiple sclerosis and vitamin D: An update. Eur. J. Clin. Nutr. 2004, 58, 1095–1109.
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260.
- Ben-Eltriki, M.; Hopefl, R.; Wright, J.M.; Deb, S. Association between Vitamin D Status and Risk of Developing Severe COVID-19 Infection: A Meta-Analysis of Observational Studies. J. Am. Coll. Nutr. 2021.
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438.
- Pellegrini, S.; Uzé, G. An Old Cytokine Against a New Virus? J. Interferon Cytokine Res. 2020, 40, 425–428.
- Lange, C.M.; Gouttenoire, J.; Duong, F.H.; Morikawa, K.; Heim, M.H.; Moradpour, D. Vitamin D receptor and Jak-STAT signaling crosstalk results in calcitriol-mediated increase of hepatocellular response to IFN-α. J. Immunol. 2014, 192, 6037–6044.
More
Information
Subjects:
Rheumatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
942
Revisions:
2 times
(View History)
Update Date:
06 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




