
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hanieh Montaseri | + 1924 word(s) | 1924 | 2021-12-01 07:22:24 | | | |
| 2 | Yvaine Wei | Meta information modification | 1924 | 2021-12-06 03:04:28 | | | | |
| 3 | Yvaine Wei | Meta information modification | 1924 | 2021-12-06 03:05:18 | | | | |
| 4 | Dean Liu | -3 word(s) | 1921 | 2021-12-06 03:17:04 | | | | |
| 5 | Nkune Nkune | Meta information modification | 1921 | 2021-12-08 08:37:56 | | | | |
| 6 | Nkune Nkune | Meta information modification | 1921 | 2021-12-08 08:38:38 | | |
Video Upload Options
Photodynamic therapy (PDT) is a promising non-invasive phototherapeutic approach for cancer therapy that can eliminate local tumor cells and produce systemic antitumor immune responses. Significant efforts have been made in developing strategies to further investigate the immune mechanisms triggered by PDT. The majority of in vitro experimental models still rely on the two-dimensional (2D) cell cultures that do not mimic a three-dimensional (3D) cellular environment in the human body, such as cellular heterogeneity, nutrient gradient, growth mechanisms, and the interaction between cells as well as the extracellular matrix (ECM) and therapeutic resistance to anticancer treatments. In addition, in vivo animal studies are highly expensive and time consuming, which may also show physiological discrepancies between animals and humans. In this sense, there is growing interest in the utilization of 3D tumor models, since they precisely mimic different features of solid tumors. This entry summarizes the characteristics and techniques for 3D tumor model generation. Furthermore, researchers provide an entry of innate and adaptive immune responses induced by PDT in several in vitro and in vivo tumor models. Future perspectives are highlighted for further enhancing PDT immune responses as well as ideal experimental models for antitumor immune response sudies.
1. Introduction
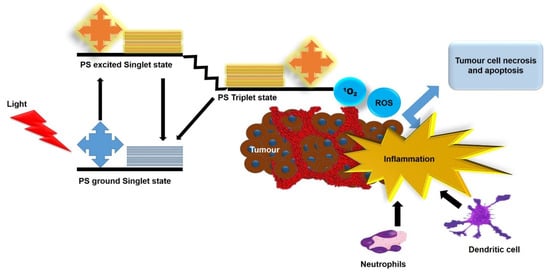
2. Methods for 3D Tumor Generation
3. PDT-Induced Antitumor Immune Responses
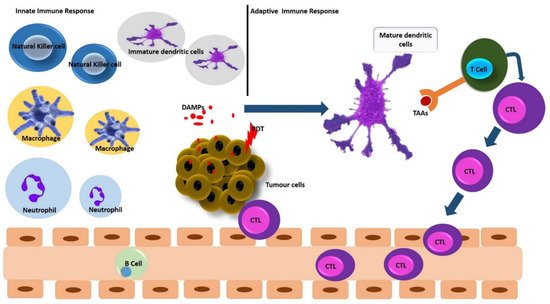
3.1. PDT and Innate Immune Response
3.2. PDT and Adaptive Immune Response
4. Enhancing PDT-Induced Antitumor Immune Responses
4.1. Intracellular Accumulation of PSs
4.2. ER-Targeted PDT
4.3. Mitochondria Targeted PDT
4.4. Application of Nanotechnology
References
- Kruger, C.A.; Abrahamse, H. Utilisation of Targeted Nanoparticle Photosensitiser Drug Delivery Systems for the Enhancement of Photodynamic Therapy. Molecules 2018, 23, 2628.
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastatis Treat. 2019, 5, 25.
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional Nanoparticles as Photosensitizer Delivery Carriers for Enhanced Photodynamic Cancer Therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111099.
- Yang, Y.; Hu, Y.; Wang, H. Targeting Antitumor Immune Response for Enhancing the Efficacy of Photodynamic Therapy of Cancer: Recent Advances and Future Perspectives. Oxid. Med. Cell. Longev. 2016, 2016, e5274084.
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune Response after Photodynamic Therapy Increases Anti-Cancer and Anti-Bacterial Effects. World J. Immunol. 2014, 4, 1–11.
- Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333.
- Chen, H.; Dai, Z. Antitumor Immune Responses Induced by Photodynamic and Sonodynamic Therapy: A Narrative Review. J. Bio-X Res. 2021, 4, 77–86.
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the Tumor Microenvironment: Removing Obstruction to Anticancer Immune Responses and Immunotherapy. Ann. Oncol. 2016, 27, 1482–1492.
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of Photodynamic Therapy (PDT) and Anti-Tumor Immunity in Cancer Therapy. J. Pharm. Investig. 2018, 48, 143–151.
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108.
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-Dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery. Oncol. Lett. 2017, 14, 6999–7010.
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an In Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083.
- Mroz, P.; Hashmi, J.T.; Huang, Y.-Y.; Lange, N.; Hamblin, M.R. Stimulation of Anti-Tumor Immunity by Photodynamic Therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91.
- Vatansever, F.; Hamblin, M.R. Photodynamic Therapy and Antitumor Immune Response. Cancer Immunol. 2015, 383–399.
- Wachowska, M.; Muchowicz, A.; Demkow, U. Immunological Aspects of Antitumor Photodynamic Therapy Outcome. Cent. Eur. J. Immunol. 2015, 40, 481–485.
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell Death in Photodynamic Therapy: From Oxidative Stress to Anti-Tumor Immunity. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1872, 188308.
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324.
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620.
- Gift, M.; Ann, K.; Mfouo Tynga, I.; Abrahamse, H. A Review of Nanoparticle Photosensitizer Drug Delivery Uptake Systems for Photodynamic Treatment of Lung Cancer. Photodiagn. Photodyn. Ther. 2018, 22, 147–154.
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107.
- Yuan, B.; Liu, J.; Guan, R.; Jin, C.; Ji, L.; Chao, H. Endoplasmic Reticulum Targeted Cyclometalated Iridium(III) Complexes as Efficient Photodynamic Therapy Photosensitizers. Dalton Trans. 2019, 48, 6408–6415.
- Zhuang, Z.; Dai, J.; Yu, M.; Li, J.; Shen, P.; Hu, R.; Lou, X.; Zhao, Z.; Tang, B.Z. Type I Photosensitizers Based on Phosphindole Oxide for Photodynamic Therapy: Apoptosis and Autophagy Induced by Endoplasmic Reticulum Stress. Chem. Sci. 2020, 11, 3405–3417.
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in Innate Immune Responses. Nat. Rev. Immunol. 2011, 11, 389–402.
- Zhang, K.; Kaufman, R.J. From Endoplasmic-Reticulum Stress to the Inflammatory Response. Nature 2008, 454, 455–462.
- Garg, A.D.; Dudek, A.M.; Ferreira, G.B.; Verfaillie, T.; Vandenabeele, P.; Krysko, D.V.; Mathieu, C.; Agostinis, P. ROS-Induced Autophagy in Cancer Cells Assists in Evasion from Determinants of Immunogenic Cell Death. Autophagy 2013, 9, 1292–1307.
- Michaud, M.; Sukkurwala, A.Q.; Di Sano, F.; Zitvogel, L.; Kepp, O.; Kroemer, G. Synthetic Induction of Immunogenic Cell Death by Genetic Stimulation of Endoplasmic Reticulum Stress. OncoImmunology 2014, 3, e28276.
- Garg, A.D.; Agostinis, P. ER Stress, Autophagy and Immunogenic Cell Death in Photodynamic Therapy-Induced Anti-Cancer Immune Responses. Photochem. Photobiol. Sci. 2014, 13, 474–487.
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281.
- Md, S.; Haque, S.; Madheswaran, T.; Zeeshan, F.; Meka, V.S.; Radhakrishnan, A.K.; Kesharwani, P. Lipid Based Nanocarriers System for Topical Delivery of Photosensitizers. Drug Discov. Today 2017, 22, 1274–1283.
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 3349.
- Noh, I.; Lee, D.; Kim, H.; Jeong, C.-U.; Lee, Y.; Ahn, J.-O.; Hyun, H.; Park, J.-H.; Kim, Y.-C. Enhanced Photodynamic Cancer Treatment by Mitochondria-Targeting and Brominated Near-Infrared Fluorophores. Adv. Sci. 2018, 5, 1700481.
- Yang, Y.; Gao, N.; Hu, Y.; Jia, C.; Chou, T.; Du, H.; Wang, H. Gold Nanoparticle-Enhanced Photodynamic Therapy: Effects of Surface Charge and Mitochondrial Targeting. Ther. Deliv. 2015, 6, 307–321.
- Xu, J.; Zeng, F.; Wu, H.; Yu, C.; Wu, S. Dual-Targeting Nanosystem for Enhancing Photodynamic Therapy Efficiency. ACS Appl. Mater. Interfaces 2015, 7, 9287–9296.
- Soler, D.C.; Ohtola, J.; Sugiyama, H.; Rodriguez, M.E.; Han, L.; Oleinick, N.L.; Lam, M.; Baron, E.D.; Cooper, K.D.; McCormick, T.S. Activated T Cells Exhibit Increased Uptake of Silicon Phthalocyanine Pc 4 and Increased Susceptibility to Pc 4-Photodynamic Therapy-Mediated Cell Death. Photochem. Photobiol. Sci. 2016, 15, 822–831.
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7.
- Tang, C.; Li, H. Application of Nanoparticles in the Early Diagnosis and Treatment of Tumors: Current Status and Progress. Tradit. Med. Res. 2020, 5, 34–43.
- Monge-Fuentes, V.; Muehlmann, L.A.; de Azevedo, R.B. Perspectives on the Application of Nanotechnology in Photodynamic Therapy for the Treatment of Melanoma. Nano Rev. 2014, 5, 24381.
- Nkune, N.; Kruger, C.; Abrahamse, H. Possible Enhancement of Photodynamic Therapy (PDT) Colorectal Cancer Treatment When Combined with Cannabidiol. Anti-Cancer Agents Med. Chem. 2020, 20, 137–148.
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795.
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307.




