Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ivan Addae-Mensah | + 1172 word(s) | 1172 | 2021-12-03 03:53:43 | | | |
| 2 | Dean Liu | Meta information modification | 1172 | 2021-12-06 01:40:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Addae-Mensah, I. Heterocyclic Anticancer Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/16738 (accessed on 07 February 2026).
Addae-Mensah I. Heterocyclic Anticancer Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/16738. Accessed February 07, 2026.
Addae-Mensah, Ivan. "Heterocyclic Anticancer Compounds" Encyclopedia, https://encyclopedia.pub/entry/16738 (accessed February 07, 2026).
Addae-Mensah, I. (2021, December 05). Heterocyclic Anticancer Compounds. In Encyclopedia. https://encyclopedia.pub/entry/16738
Addae-Mensah, Ivan. "Heterocyclic Anticancer Compounds." Encyclopedia. Web. 05 December, 2021.
Copy Citation
Heterocyclic organic compounds are designated as integral components on a wide array of structures with both pharmacological and biological importance. They constitute a large cohort of structures with immense importance in the life sciences.
homeostasis
heterocyclic compounds
multiple biological targets
1. Introduction
Cancer is a complex group of diseases the onset of which is characterized by abnormal cell division with the potential of spreading to other parts of the body over time [1][2]. The abnormal cell differentiation is normally accompanied by drastic weight loss, prolonged cough, and abnormal lump growth [3]. The prevalence of cancer is spiraling globally. Currently, cancer ranks second as the leading cause of death worldwide and in the year 2020 alone, an estimated 19.3 million new cases with 10 million deaths were recorded [4]. These statistics translate into an annual death rate that is a little over 50% of the annual new cases. Cancers of the stomach, lung, breast, prostate/cervix, and colorectum have all been reported but the most frequently diagnosed are lung and breast cancers followed by prostate which occurs predominantly among the aging male population [5]. Sex and age have become of paramount importance in cancer susceptibility and treatment, and men are said to be more prone to infections than women [6][7].
Prior to cancer becoming metastatic, its spread is initiated by pro-angiogenic factors consisting of vascular endothelial growth factor (VEGF) receptors, fibroblast growth factor (FGF), platelet derived growth factors (PDGF), epidermal growth factor (EGF), thymidine phosphorylase (TP), neuropeptide Y4 (NY4), and platelet factor 4 (PF4), etc. [8][9]. Upon initiation, the survival of these cancer cells and their proliferation are dependent on the supply of oxygen, nutrients, and clearance of waste products [10][11]. All these processes are dependent on the sprouting of vascular networks. New blood vessels form through a process called angiogenesis [12]. In the absence of the vascular extension, cancer cells become necrotic or undergo a process called apoptosis, limiting the proliferation rate [10]. The level of expression of necrotic or apoptotic factors is a reflection of the aggressiveness of a tumor [13][14]. Generally vascular development starts by basement membrane destruction which leads to the release of angiogenic factors. This activates endothelial cells to migrate which then proliferate and stabilize until a safeguard sets in [10][15][16]. Regulation of neoplastic vascularization as an important step in limiting cancer proliferation has received immense attention and extensive research [8][12]. To starve these tumor cells, cautious targeting of vessel sprouting activators with a foreknowledge of how they control this chemical signal is crucial in maintaining homeostasis [17][18].
Numerous cancer targets including VEGF, FGF, PGF, EGF, PDGF, topoisomerase I and II, histone deacetylase, tyrosine kinase, and transforming growth factor-alpha (TGF-α) have been elucidated with multiple studies focused mainly on targeting the initiator VEGF [1][19][20][21]. However, halting VEGF signaling has not been very effective as reports of disease progression after treatment have become rampant [22].
A number of heterocyclic anticancer agents both synthetic and naturally occurring are in use and more are still being sought after [23][24][25][26]. Some examples are as shown in Figure 1. Heterocyclic compounds (ring compounds containing C and any of the atoms N, O, and S) have been explored for their medicinal properties for the treatment of various diseases including cancer. Introduction of these heteroatoms improves solubility, polarity, and hydrogen bonding abilities leading to ADMET (Adsorption, Distribution, Metabolism, Excretion, and Toxicity) optimization of druggable candidates. Therapeutic heterocyclic agents such as 5- fluorouracil 1, orlistat 2, vandetanib 3, rapamycin 4, axitinib 5, sorafenib 6, epigallocatechin 7, doxorubicin 8, daunorubicin 9, and Taxol 10 (Figure 1) have demonstrated high inhibition against different types of cancer cells [27][28][29].
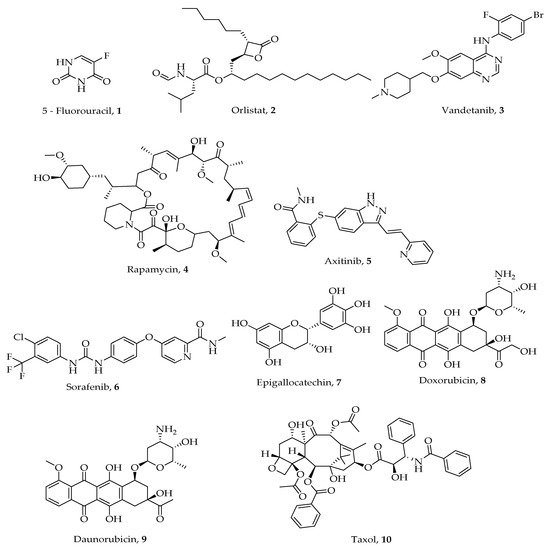
Figure 1. Chemical structures of some heterocyclic compounds used for treating various types of cancer.
Brevilin A, 11, a heterocyclic sesquiterpene lactone natural product isolated from Centipeda minima exhibits anticancer properties [30]. Studies have shown that Brevilin A attenuates the signal transducer and activator of transcription (STATS 3) and Janus kinase and tyrosine kinase activity thereby inhibiting cell growth, inducing apoptosis and reducing cell metastasis [31]. Lee, Chan et al., synthesized analogues of Brevilin A and found that 13 and 14 exerted greater anticancer properties than 11. Aldol reaction of 11 and paraformaldehyde in the presence of sodium carbonate produced 12. Acetylation of 12 with p-nitrobenzoyl chloride and methacrylic anhydride afforded 13 and 14, respectively (Scheme 1) [26].
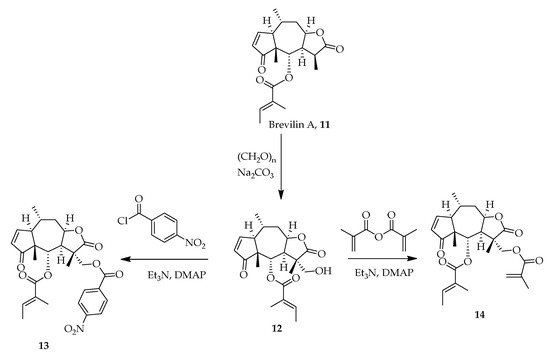
Scheme 1. Synthetic routes of Brevilin A analogues [26].
Challenges associated with cancer treatments such as drug resistance, systemic toxicity of administered drugs, and drug ineffectiveness are widespread [32][33]. Furthermore, confounding factors such as the multiple signaling nature of pathways and the propensity of most cancer cells to mutate have hindered the search for an effective therapeutic agent for combating cancers, calling for urgent search to identify new anticancer agents as drug leads [19][34]. To overcome these challenges, multi-target heterocyclic inhibitors are proposed as an option in achieving success in the fight against various forms of cancers.
Emerging heterocyclic compounds with demonstrable anticancer activities include sunitinib 15, midostaurin 16, and vorinostat 17 (Figure 2). They possess multi-regulatory activity against growth factors like vascular endothelium growth factor receptor (VEGFR), platelet-derived growth factor receptor alpha (PDGFRA’s c-Kit) and tyrosine kinase 3 (FLT-3) [35]. In the same vein, gefitinib 18, erlotinib 19, lapatinib 20, and sotagliflozin 21 (Figure 2) with magnificent inhibitory potentials against human epidermal growth factor receptor 1 (HER1) and 2 (HER2) are also in phase 3 clinical trials confirming that multi-targeted therapy has a future [36][37].
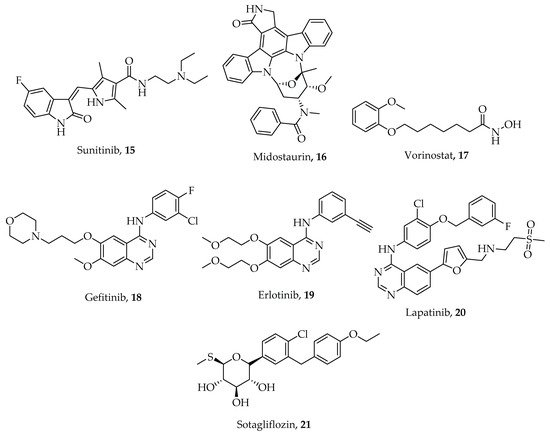
Figure 2. Chemical structures of some heterocyclic multi-target anticancer agents. Compounds 15–17 are in clinical use while 18–21 are in various stages of clinical trials.
This review therefore seeks to highlight the various types of heterocyclic multimodality anticancer agents (synthetic and natural products) and their biological targets with some emphasis on their mechanism of action. Various systematic rigorous methodological approaches were used to search literature to identify, collate, and critically appraise a body of previously published works relevant to the topic. The search comprised of putting relevant key words into Scifinder, Scopus, Google scholar, PubMed, and others and appraising them for their suitability. Original hard copy offprints of published papers in our various personal archives were also consulted for relevant information [38][39].
2. Heterocyclic Compounds
Heterocyclic organic compounds are designated as integral components on a wide array of structures with both pharmacological and biological importance. They constitute a large cohort of structures with immense importance in the life sciences. Their diverse characteristics include the display of a wide variety of intermolecular interactions, different ring sizes, their planarity if aromatic, and functional group versatility. Among the twenty amino acids, proline, histidine, and tryptophan contain heterocyclic rings. Furthermore, the basic units that contain instructions for development, growth, and reproduction are all made of a heterocyclic nucleus.
Many natural products such as alkaloids, flavonoids, terpenoids, coumarins, anthocyanins, isothiazoles, etc., contain heterocyclic rings, possess a broad spectrum of activities against numerous disease-causing organisms. In addition, recent structural assessment of heterocyclic moieties among synthetic compounds by Marson has revealed that their incorporation plays a very significant role in drug-likeness and target binding by limiting toxic metabolite production, increasing water solubility, and lowering conformational entropy [40]. Heterocyclic chemotypes like quinoline, quinazoline, quinoxaline, pyrimidine, pyrazoline, 1,2,4-triazole, imidazole, benzimidazole, isoxazoline, isoquinoline, pyrazole, and isoxazole (Figure 3) can be found in compounds used in the treatment of debilitating diseases like malaria, tuberculosis, cancer, neuro-degenerative diseases, fungal, and bacterial infections [41][42][43]. The following are heterocyclic compounds that, if present as moieties in synthetic or naturally occurring compounds, may confer anti-cancer properties.
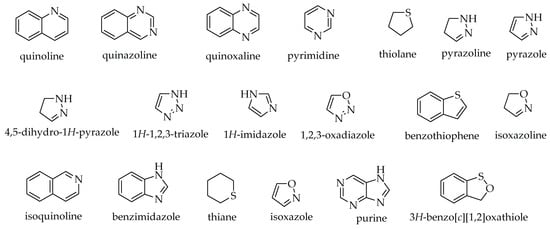
Figure 3. Examples of heterocyclic chemotypes.
References
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorg. Med. Chem. 2020, 28, 115599.
- Abbot, V.; Sharma, P.; Dhiman, S.; Noolvi, M.N.; Patel, H.M.; Bhardwaj, V. Small hybrid heteroaromatics: Resourceful biological tools in cancer research. RSC Adv. 2017, 7, 28313–28349.
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as potential anticancer agents. MedChemComm 2017, 8, 1742–1773.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Yee, E.M.H.; Pasquier, E.; Iskander, G.; Wood, K.; Black, D.S.; Kumar, N. Synthesis of novel isoflavene-propranolol hybrids as anti-tumor agents. Bioorg. Med. Chem. 2013, 21, 1652–1660.
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 1–11.
- Edgren, G.; Liang, L.; Adami, H.O.; Chang, E.T. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 2012, 27, 187–196.
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364.
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the role of angiogenesis in cancer ecosystems. Front. Oncol. 2018, 8, 248.
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84.
- De la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143.
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493.
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of Tumor Progression by Programmed Necrosis. Oxidative Med. Cell. Longev. 2018, 2018, 3537471.
- Tomes, L.; Emberley, E.; Niu, Y.; Troup, S.; Pastorek, J.; Strange, K.; Harris, A.; Watson, P.H. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res. Treat. 2003, 81, 61–69.
- Angiogenesis Inhibitors—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet (accessed on 14 February 2021).
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers 2020, 12, 1172.
- Sun, L.; Fan, G.; Shan, P.; Qiu, X.; Dong, S.; Liao, L.; Yu, C.; Wang, T.; Gu, X.; Li, Q.; et al. Regulation of energy homeostasis by the ubiquitin-independent REGγ 3 proteasome. Nat. Commun. 2016, 7, 1–15.
- Kania, E.; Pająk, B.; Orzechowski, A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed. Res. Int. 2015, 2015, 352794.
- Isoldi, M.; Visconti, M.; Castrucci, A. Anti-Cancer Drugs: Molecular Mechanisms of Action. Mini-Rev. Med. Chem. 2005, 5, 685–695.
- Kumar, B.; Singh, S.; Skvortsova, I.; Kumar, V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr. Med. Chem. 2017, 24, 4729–4752.
- Magalhaes, L.G.; Ferreira, L.L.G.; Andricopulo, A.D. Recent advances and perspectives in cancer drug design. Anais da Academia Brasileira de Ciências 2018, 1233–1250.
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770.
- Liu, J.; Ming, B.; Gong, G.H.; Wang, D.; Bao, G.L.; Yu, L.J. Current research on anti-breast cancer synthetic compounds. RSC Adv. 2018, 8, 4386–4416.
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological profile of natural and synthetic compounds with rigid adamantane-based scaffolds as potential agents for the treatment of neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2020, 529, 1225–1241.
- Ahuja, A.; Kim, J.H.; Kim, J.H.; Yi, Y.S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254.
- Lee, M.M.L.; Chan, B.D.; Wong, W.Y.; Leung, T.W.; Qu, Z.; Huang, J.; Zhu, L.; Lee, C.S.; Chen, S.; Tai, W.C.S. Synthesis and Evaluation of Novel Anticancer Compounds Derived from the Natural Product Brevilin A. ACS Omega 2020, 5, 14586–14596.
- Perks, C.M.; Keith, A.J.; Goodhew, K.L.; Savage, P.B.; Winters, Z.E.; Holly, J.M.P. Prolactin acts as a potent survival factor for human breast cancer cell lines. Br. J. Cancer 2004, 91, 305–311.
- Kozako, T.; Suzuki, T.; Yoshimitsu, M.; Arima, N.; Honda, S.I.; Soeda, S. Anticancer agents targeted to sirtuins. Molecules 2014, 19, 20295–20313.
- Paik, E.S.; Kim, T.H.; Cho, Y.J.; Ryu, J.; Choi, J.J.; Lee, Y.Y.; Kim, T.J.; Choi, C.H.; Kim, W.Y.; Sa, J.K.; et al. Preclinical assessment of the VEGFR inhibitor axitinib as a therapeutic agent for epithelial ovarian cancer. Sci. Rep. 2020, 10, 1–9.
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin A promotes oxidative stress and induces mitochondrial apoptosis in U87 glioblastoma cells. OncoTargets Ther. 2018, 11, 7031–7040.
- Chen, X.; Du, Y.; Nan, J.; Zhang, X.; Qin, X.; Wang, Y.; Hou, J.; Wang, Q.; Yang, J. Brevilin A, a Novel Natural Product, Inhibits Janus Kinase Activity and Blocks STAT3 Signaling in Cancer Cells. PLoS ONE 2013, 8, e63697.
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30.
- Vasir, J.K.; Vinod, L. Targeted Drug Delivery in Cancer Therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374.
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54.
- Modi, S.J.; Kulkarni, V.M. Vascular Endothelial Growth Factor Receptor (VEGFR-2)/KDR Inhibitors: Medicinal Chemistry Perspective. Med. Drug Discov. 2019, 2, 100009.
- Dratkiewicz, E.; Pietraszek-Gremplewicz, K.; Simiczyjew, A.; Mazur, A.J.; Nowak, D. Gefitinib or lapatinib with foretinib synergistically induce a cytotoxic effect in melanoma cell lines. Oncotarget 2018, 9, 18254–18268.
- Segovia-Mendoza, M.; González-González, M.E.; Barrera, D.; Díaz, L.; García-Becerra, R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of her2-positive breast cancer: Preclinical and clinical evidence. Am. J. Cancer Res. 2015, 5, 2531–2561.
- Harris, J.D.; Quatman, C.E.; Manring, M.M.; Siston, R.A.; Flanigan, D.C. How to write a systematic review. Am. J. Sports Med. 2014, 42, 2761–2768.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Reprint-Preferred Reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys. Ther. 2009, 89, 873–880.
- Marson, C.M. Saturated Heterocycles with Applications in Medicinal Chemistry. Adv. Heterocycl. Chem. 2016, 121, 13–33.
- Paudel, A.; Hamamoto, H.; Panthee, S.; Kaneko, K.; Matsunaga, S.; Kanai, M.; Suzuki, Y.; Sekimizu, K. A novel spiro-heterocyclic compound identified by the silkworm infection model inhibits transcription in Staphylococcus aureus. Front. Microbiol. 2017, 8, 712.
- Ali, S.M.; Siddiqui, R.; Ong, S.K.; Shah, M.R.; Anwar, A.; Heard, P.J.; Khan, N.A. Identification and characterization of antibacterial compound(s) of cockroaches (Periplaneta americana). Appl. Microbiol. Biotechnol. 2017, 101, 253–286.
- Khatoon, H.; Abdulmalek, E. Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules 2021, 26, 1055.
More
Information
Subjects:
Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




