
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikoletta Pechlivani | + 1498 word(s) | 1498 | 2021-11-23 04:07:43 | | | |
| 2 | Amina Yu | Meta information modification | 1498 | 2021-12-03 06:49:09 | | |
Video Upload Options
The antifibrinolytic proteins alpha-2 antiplasmin (α2AP), thrombin activatable fibrinolysis inhibitor (TAFI), complement C3 and plasminogen activator inhibitor-2 (PAI-2), can be incorporated into the fibrin clot by FXIIIa and affect fibrinolysis by different mechanisms. Therefore, these antifibrinolytic proteins are attractive targets for the development of novel therapeutics, both for the modulation of thrombosis risk, but also for potentially improving clot instability in bleeding disorders.
1. Introduction
The formation of obstructive intravascular thrombi remains a significant cause of morbidity and mortality worldwide [1]. These thrombi can form in arterial and venous vascular beds with the former having a rich presence of platelets [2][3]. This explains why it is mainly antiplatelet therapies that are chosen for the prevention of atherothrombotic disease, while anti-coagulants are used for the treatment and prevention of venous occlusion. However, the clinical management of arterial disease has undergone constant change over the past decade, as clinical outcome studies have shown that the combination of an antiplatelet and an anticoagulant is particularly effective at preventing atherothrombotic events [4][5]. The beneficial effects of combination therapies are not surprising given recent studies demonstrating that fibrin clot characteristics are predictors of clinical outcome in individuals at high risk of atherothrombosis [6][7][8]. Anticoagulants typically reduce fibrin network formation and can also make clots less robust, thus decreasing resistance to lysis, in turn reducing the risk of thrombotic vascular occlusion. A central difficulty in preventing vascular occlusion is the increased risk of bleeding events with more powerful anti-thrombotic agents. Therefore, there is a fine balance between inhibiting platelet function/fibrin network formation and ensuring bleeding risks are kept to a minimum. While newer antiplatelet and anticoagulant therapies are more effective at preventing thrombosis, risk of bleeding remains high. Rather than using powerful agents that have a “global effect” on platelet function and/or coagulation proteins, a more balanced strategy would be to target fibrin clot formation and breakdown, thus having agents with an improved efficacy/safety ratio. One of these potential pathways is targeting the factors responsible for hypofibrinolysis, given this is a known risk factor for thrombosis even with the use of powerful antiplatelet agents [6][7].
Altered incorporation of antifibrinolytic proteins into the fibrin network is an important mechanism that determines fibrinolysis potential [9]. Unlike the clinical use of warfarin (which inhibits synthesis of four coagulation proteins, factors II, VII, IX, and X) [10] or novel oral anticoagulants (NOAC) that inhibit factor IIa or Xa [11], the strategy of interfering with fibrinogen-bound or cross-linked antifibrinolytic proteins will offer the opportunity for a more targeted approach to improve the hypofibrinolytic environment with the real possibility of low risk of bleeding.
2. Interactions of Fibrinogen with Antifibrinolytic Proteins
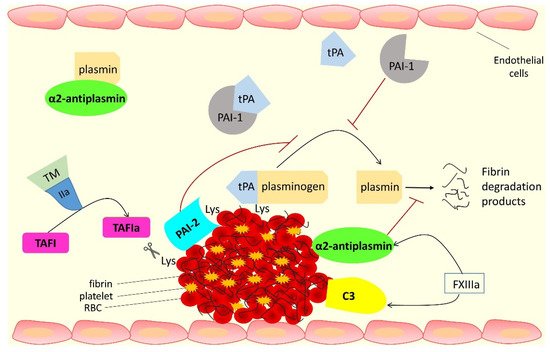
This entry focuses on the proteins that interact with fibrin(ogen) and are involved in the antifibrinolytic process; these are summarized in Figure 1 and Table 1 .
| α2AP | TAFI | C3 | PAI-2 | |
|---|---|---|---|---|
| Mass (kDa) | ~70 | 56 | 187 | 47 |
| Human gene | SERPINF2 | CPB2 | C3 | SERPINB2 |
| Synthesis/expression | Liver, kidney, and brain | Liver and megakaryocytes |
Liver and immune cells | Monocytes, macrophages, keratinocytes, fibroblasts, and placenta |
| Circulating plasma concentration | 70 µg/mL | 4–15 µg/mL | 1.2 mg/mL | Below detection limit |
| Antifibrinolytic function |
Direct binding to, and inhibition of, plasmin and cross-linking into the clot making it more resistant to lysis | Protects the clot from lysis by cleaving off C-terminal lysine residues from fibrin, which reduces plasminogen and tPA binding and subsequent plasmin generation | Incorporation into the fibrin clot causes prolongation of fibrinolysis | Cross-linking into fibrin at a site close to tPA binding site affects fibrin clot lysis |
3. Targeting the Antifibrinolytic Proteins for Developing Therapeutics
Antifibrinolytic proteins represent attractive targets for the development of therapeutics to modulate thrombosis risk. Various methodologies have been explored to inhibit the functions of antifibrinolytic proteins; most revolve around the production of protein-specific monoclonal antibodies.
Others have investigated inhibition of two pathways, using a heterodimer diabody against TAFI and PAI-1 in mouse models of thrombosis and stroke. The bispecific antibody was able to exhibit a profibrinolytic effect with low bleeding risk [12].
Future therapeutic strategies for the treatment of bleeding disorders such as haemophilia A and B are likely to focus on extended half-life coagulation factors, although adjunctive therapies targeting the antifibrinolytic proteins have the potential to improve efficacy of the replaced clotting factors and this remains an area for future research.
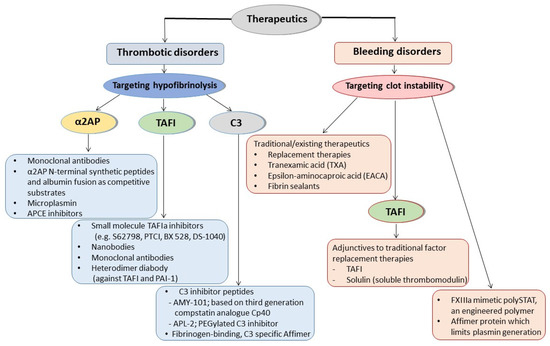
A schematic representation of the therapeutic approaches involving antifibrinolytic proteins targeting hypofibrinolysis or clot instability for thrombotic or bleeding disorders, respectively, is shown in Figure 2 .
4. Conclusions and the Future
While a large number of studies have investigated the role of fibrin-incorporated antifibrinolytic proteins in health and disease, characterization of their exact role in vascular occlusive disease is incompletely understood. This is likely related to the heterogeneity of the population studied, small numbers analysed, and/or the sensitivity of the methodologies applied. While more research in this area is needed, some of these antifibrinolytic proteins are emerging as potential therapeutic targets given their role in disease states and consistent effect on fibrinolysis. Perhaps the antifibrinolytic protein with the most evidence for use as a therapeutic target is α2AP; indeed, several approaches have been explored to modulate protein activity. Monoclonal antibodies against α2AP have been particularly effective at altering α2AP activity, and these have even been tested in phase I and phase II clinical studies, but none have made it into routine clinical practice to date. ΤAFI inhibitors have also been developed, with some showing early promising results, while there has been little investment in developing PAI-2 inhibitors given their inconsistent role in disease.
Interestingly, the use of antifibrinolytic proteins as therapeutic targets is not limited to thrombotic conditions, but also bleeding disorders, where they may prove to be effective as adjunctive therapies or even as main agents to stop blood loss.
Taken together, the molecular mechanisms involved in function of fibrin-incorporated antifibrinolytic proteins are largely understood, but more work is needed to fully elucidate the groups, or subgroups, of individuals who would benefit the most from antifibrinolytic-based therapies. While a number of approaches for modulating the function of antifibrinolytic proteins have been developed, more work is required to ensure that such therapies are effective in vivo (i.e., good efficacy/safety profile) and do not have unwanted “off target” effects. Overall, current evidence suggests that antifibrinolytic-directed therapies have the potential to be novel antithrombotic agents with a low risk of bleeding, while also being relevant to the discovery of agents that can be used in bleeding disorders. Appropriate collaborations between scientists, clinicians, and the pharmaceutical industry should help to make antifibrinolytic-directed therapies part of daily clinical practice.
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25.
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791.
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383.
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330.
- Steffel, J.; Eikelboom, J.W.; Anand, S.S.; Shestakovska, O.; Yusuf, S.; Fo, K.A.A. The COMPASS Trial: Net Clinical Benefit of Low-Dose Rivaroxaban Plus Aspirin as Compared With Aspirin in Patients With Chronic Vascular Disease. Circulation 2020, 142, 40–48.
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Impaired Fibrinolysis Predicts Adverse Outcome in Acute Coronary Syndrome Patients with Diabetes: A PLATO Sub-Study. Thromb. Haemost. 2020, 120, 412–422.
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Bertilsson, M.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: A PLATO substudy. Eur. Heart J. 2018, 39, 1078–1085.
- Kietsiriroje, N.; Ariens, R.A.S.; Ajjan, R.A. Fibrinolysis in Acute and Chronic Cardiovascular Disease. Semin. Thromb. Hemost. 2021, 47, 490–505.
- Kearney, K.; Tomlinson, D.; Smith, K.; Ajjan, R. Hypofibrinolysis in diabetes: A therapeutic target for the reduction of cardiovascular risk. Cardiovasc. Diabetol. 2017, 16, 34.
- Kuruvilla, M.; Gurk-Turner, C. A review of warfarin dosing and monitoring. Bayl. Univ. Med Cent. Proc. 2001, 14, 305–306.
- Mekaj, A.; Mekaj, Y.; Duci, S.; Miftari, E. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977.
- Wyseure, T.; Rubio, M.; Denorme, F.; De Lizarrondo, S.M.; Peeters, M.; Gils, A.; De Meyer, S.; Vivien, D.; Declerck, P.J. Innovative thrombolytic strategy using a heterodimer diabody against TAFI and PAI-1 in mouse models of thrombosis and stroke. Blood 2015, 125, 1325–1332.




