
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hemal Mehta | + 3132 word(s) | 3132 | 2021-11-29 04:32:23 | | | |
| 2 | Peter Tang | -10 word(s) | 3122 | 2021-12-02 02:53:12 | | |
Video Upload Options
Diabetic retinopathy (DR) is a leading cause of vision impairment in people aged 20–74 years. There is a trend of moving away from invasive (e.g., fundus fluorescein angiography) to non-invasive (e.g., wide-field optical coherence tomography (OCT), OCT angiography and colour fundus photography) imaging modalities to allow for more objective assessments that can be readily repeated in a time-efficient manner without compromising patient safety. Such quantitative assessments generating large amounts of data could benefit from artificial intelligence approaches to aid clinical decision making.
1. Introduction
|
DR Location or Feature |
Imaging Findings |
|---|---|
|
Retinal periphery |
Capillary dropout (reduced vessel density) Dilated and tortuous capillaries |
|
Choriocapillaris |
Flow voids increase as DR level worsens Flow voids do not correlate with outer retinal changes Reduced mean subfoveal choroidal and choriocapillaris thickness on OCT |
|
FAZ |
Enlargement Loss of circularity Slower blood flow velocity in perifoveal capillaries |
|
Capillary Integrity |
Loss of vessel density in peripapillary plexuses and in parafovea Non-perfused parafoveal areas Reduced FD of vascular tree in far peripheral retina on UWF angiography Central non-perfused areas associated with macular thickening on OCT Deep capillary plexus vessel density decreases with increasing severity of PDR Vessel changes in the superficial retinal layers are present in later stages of PDR |
|
Microaneurysms |
Less easily detected on OCTA compared to FA Turnover may be used as an objective measure for therapeutic response Counts decrease following anti-VEGF treatment Usually located in the deep plexus |
|
IRMA |
Do not breach the ILM Greater calibre than adjacent capillaries Some progress to NVE |
|
DME |
Chorioscleral interface may be not clearly identified in some areas OCTA flow areas in deep plexus correspond to cystic changes OCTA demontrates reperfusion following treatment with anti-VEGF Increased vessel density of microaneurysms in perifoveal retina |
|
NVD |
Vessels originate outside of physiologic cup Size and vascular pattern changes are visible in OCTA Arise from retinal arteries or veins, posterior ciliary arteries, or the choroid |
|
NVE |
Located adjacent to areas of non-perfusion on OCTA New classification systems based on NVE origin and branching pattern |
2. Invasive Fundus Imaging Modalities to Guide Diagnosis of PDR
2.1. Fundus Fluorescein Angiography (FFA)
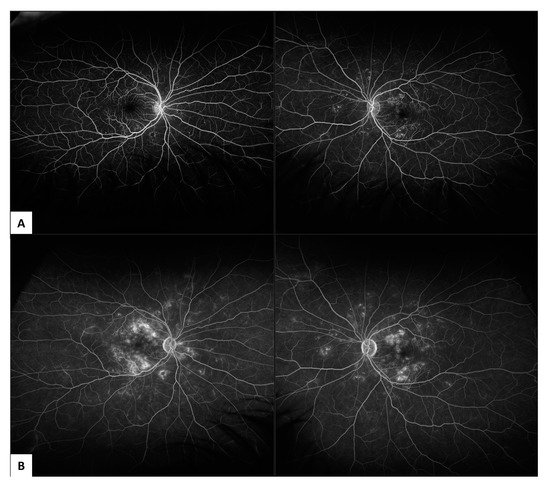
2.2. Indocyanine Green Angiography (ICG-A)
2.3. Ophthalmic B-Scan Ultrasonography
3. Non-Invasive Fundus Imaging Modalities to Guide Diagnosis and Treatment of PDR
3.1. Colour Fundus Imaging
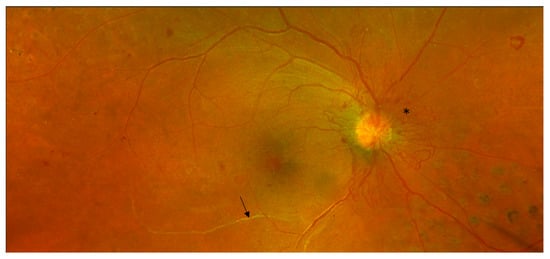
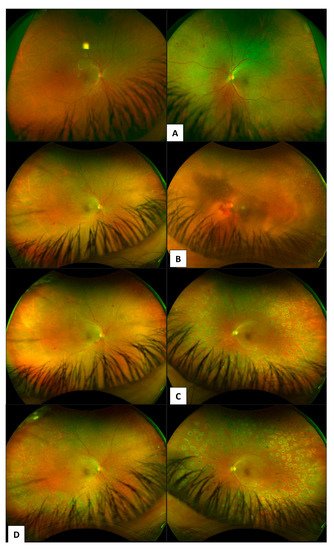
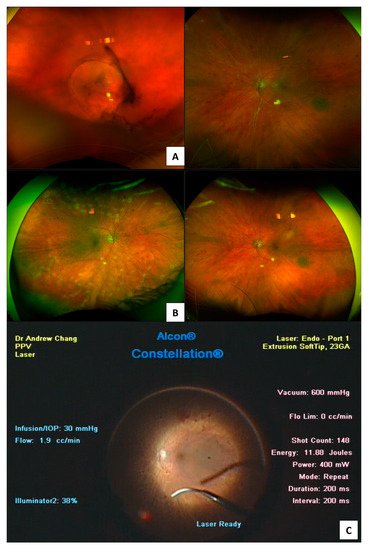
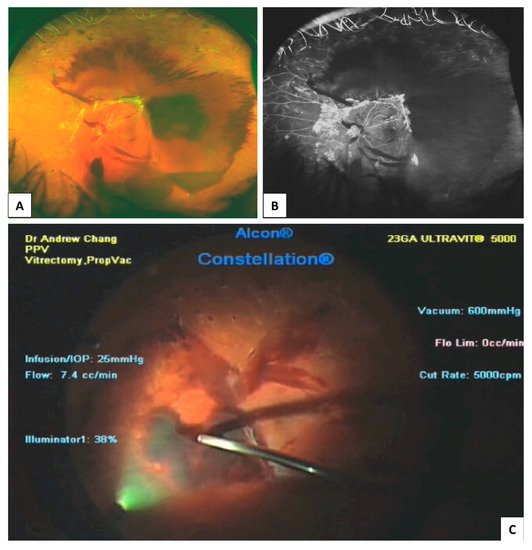
3.2. Optical Coherence Tomography (OCT)
3.3. OCTA
References
- Keel, S.; Xie, J.; Foreman, J.; van Wijngaarden, P.; Taylor, H.R.; Dirani, M. The Prevalence of Diabetic Retinopathy in Australian Adults with Self-Reported Diabetes: The National Eye Health Survey. Ophthalmology 2017, 124, 977–984.
- Mathur, R.; Bhaskaran, K.; Edwards, E.; Lee, H.; Chaturvedi, N.; Smeeth, L.; Douglas, I. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: A cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open 2017, 7, e014444.
- Amoaku, W.M.; Ghanchi, F.; Bailey, C.; Banerjee, S.; Banerjee, S.; Downey, L.; Gale, R.; Hamilton, R.; Khunti, K.; Posner, E.; et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye 2020, 34, 1–51.
- Ramchandran, R.; Bawany, M.H.; Ding, L.; Sharma, G.; Wykoff, C.C.; Kuriyan, A.E. Automated vessel density detection in fluorescein angiography images correlates with vision in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5312.
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in type 2 diabetes: Evidence from spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338.
- Duh, E.J.; Sun, J.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751.
- Fan, W.; Nittala, M.G.; Velaga, S.B.; Hirano, T.; Wykoff, C.C.; Ip, M.; Lampen, S.I.; van Hemert, J.; Fleming, A.; Verhoek, M.; et al. Distribution of Nonperfusion and Neovascularization on Ultrawide-Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am. J. Ophthalmol. 2019, 206, 154–160.
- Pan, J.; Chen, D.; Yang, X.; Zou, R.; Zhao, K.; Cheng, D.; Huang, S.; Zhou, T.; Yang, Y.; Chen, F. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 192, 146–156.
- Rush, R.B.; Del Valle Penella, A.; Reinauer, R.M.; Rush, S.W.; Bastar, P.G. Internal Limiting Membrane Peeling during Vitrectomy for Diabetic Vitreous Hemorrhage: A Randomized Clinical Trial. RETINA 2020, 41, 1118–1126.
- Marcus, D.M.; Singh, H.; Farooq, A.; Starnes, D.; Walia, H. Endolaserless vitrectomy with intravitreal aflibercept injection (IAI) for proliferative diabetic retinopathy (PDR)-related vitreous hemorrhage (LASER LESS TRIAL). Investig. Ophthalmol. Vis. Sci. 2017, 58, 5036.
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508.
- Tan, B.; Chua, J.; Lin, E.; Cheng, J.; Gan, A.; Yao, X.; Wong, D.W.; Sabanayagam, C.; Wong, D.; Chan, C.M.; et al. Quantitative Microvascular Analysis with Wide-Field Optical Coherence Tomography Angiography in Eyes with Diabetic Retinopathy. JAMA Netw. Open 2020, 3, e1919469.
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology 1991, 98, 786–806.
- The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy: Two-year results of a randomized trial—Diabetic Retinopathy Vitrectomy Study report 2. Arch. Ophthalmol. 1985, 103, 1644–1652.
- Silva, P.S.; Cruz, A.J.D.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, J.; Aiello, L.M.; Sun, J.K. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472.
- Wilkinson, C.; Ferris, F.; Klein, R.; Lee, P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682.
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2018, 136, 1138–1148.
- Preti, R.C.; Vasquez Ramirez, L.M.; Ribeiro Monteiro, M.L.; Pelayes, D.E.; Takahashi, W.Y. Structural and functional assessment of macula in patients with high-risk proliferative diabetic retinopathy submitted to panretinal photocoagulation and associated intravitreal bevacizumab injections: A comparative, randomised, controlled trial. Ophthalmologica 2013, 230, 1–8.
- Nikkhah, H.; Ghazi, H.; Razzaghi, M.R.; Karimi, S.; Ramezani, A.; Soheilian, M. Extended targeted retinal photocoagulation versus conventional pan-retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. Int. Ophthalmol. 2017, 38, 313–321.
- The Diabetic Retinopathy Study Research Group. Photocoagulation Treatment of Proliferative Diabetic Retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 1981, 88, 583–600.
- Silva, P.S.; Cavallerano, J.; Haddad, N.M.N.; Kwak, H.; Dyer, K.H.; Omar, A.F.; Shikari, H.; Aiello, L.M.; Sun, J.K. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015, 122, 949–956.
- Elnahry, A.G.; Ramsey, D.J. Automated Image Alignment for Comparing Microvascular Changes Detected by Fluorescein Angiography and Optical Coherence Tomography Angiography in Diabetic Retinopathy. Semin. Ophthalmol. 2021, 36, 757–764.
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Takahashi, A.; Omae, T.; Song, Y.S.; Takahashi, T.; Yoshida, A. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6247–6255.
- Chang, A.A.; Morse, L.; Handa, J.T.; Morales, R.B.; Tucker, R.; Hjelmeland, L.; A Yannuzzi, L. Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology 1998, 105, 1060–1068.
- Shiragami, C.; Shiraga, F.; Matsuo, T.; Tsuchida, Y.; Ohtsuki, H. Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 436–442.
- Costa, R.A.; Calucci, D.; Orefice, J.L. Indocyanine Green Angiography for The Detection Of Macular “Treatable Lesions” In Patients with Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 583.
- Mohamed, I.E.; Mohamed, M.A.; Yousef, M.; Mahmoud, M.Z.; Alonazi, B. Use of ophthalmic B-scan ultrasonography in determining the causes of low vision in patients with diabetic retinopathy. Eur. J. Radiol. Open 2018, 5, 79–86.
- Diabetic Retinopathy Vitrectomy Study Research Group. Early Vitrectomy for Severe Vitreous Hemorrhage in Diabetic Retinopathy. Four-year results of a randomized trial. Diabetic Retinopathy Study report 5. Arch. Ophthalmol. 1990, 108, 958–964.
- Flynn, H.W.; Chew, E.Y.; Simons, B.D.; Barton, F.B.; Remaley, N.A.; Ferris, F.L. Pars Plana Vitrectomy in the Early Treatment Diabetic Retinopathy Study. Ophthalmology 1992, 99, 1351–1357.
- Babiuch, A.; Wykoff, C.C.; Hach, J.; Srivastava, S.; E Talcott, K.; Yu, H.J.; Nittala, M.; Sadda, S.; Ip, M.S.; Le, T.; et al. Longitudinal panretinal microaneurysm dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Br. J. Ophthalmol. 2020, 105, 1111–1115.
- Shimura, M.; Kitano, S.; Muramatsu, D.; Fukushima, H.; Takamura, Y.; Matsumoto, M.; Kokado, M.; Kogo, J.; Sasaki, M.; Morizane, Y.; et al. Real-world management of treatment-naïve diabetic macular oedema: 2-year visual outcome focusing on the starting year of intervention from STREAT-DMO study. Br. J. Ophthalmol. 2020, 104, 1755–1761.
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A randomized clinical trial. JAMA 2015, 314, 2137–2146.
- Su, C.C.; Yang, C.H.; Yeh, P.T.; Yang, C.M. Macular tractional retinoschisis in proliferative diabetic retinopathy: Clinical characteristics and surgical outcome. Ophthalmologica 2013, 231, 23–30.
- Cruz-Inigo, Y.J.; Acaba, L.A.; Berrocal, M.H. Surgical management of retinal diseases: Proliferative diabetic retinopathy and traction retinal detachment. Dev. Ophthalmol. 2014, 54, 196–203.
- Ophir, A.; Martinez, M.R.; Mosqueda, P.; Trevino, A. Vitreous traction and epiretinal membranes in diabetic macular oedema using spectral-domain optical coherence tomography. Eye 2010, 24, 1545–1553.
- Chhablani, J.K.; Kim, J.S.; Cheng, L.; Kozak, I.; Freeman, W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1415–1420.
- Mishra, D.K.; Shanmugam, M.P.; Ramanjulu, R.; Sagar, P. Comparison of standard and “innovative wide-field” optical coherence tomography images in assessment of vitreoretinal interface in proliferative diabetic retinopathy: A pilot study. Indian J. Ophthalmol. 2020, 69, 99–102.
- Um, T.; Seo, E.J.; Kim, Y.J.; Yoon, Y.H. Optical coherence tomography angiography findings of type 1 diabetic patients with diabetic retinopathy, in comparison with type 2 patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 281–288.
- Pan, J.; Chen, F.; Chen, D.; Yang, X.; Wang, J.; Chen, Z.; He, X.; Zhou, T.; Zheng, J.; Chen, H. Novel Three Types of Neovascularization Elsewhere Determine the Differential Clinical Features of Proliferative Diabetic Retinopathy. Retina 2020, 41, 1265–1274.
- Stefansson, E. Physiology of retinal oxygenation. Acta Ophthalmol. 2015, 93.
- Stefánsson, E. Ocular Oxygenation and the Treatment of Diabetic Retinopathy. Surv. Ophthalmol. 2006, 51, 364–380.
- Sharma, T.; Fong, A.; Lai, T.Y.; Lee, V.; Das, S.; Lam, D. Surgical treatment for diabetic vitreoretinal diseases: A review. Clin. Exp. Ophthalmol. 2016, 44, 340–354.




