Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oscar Alejandro Aguilar Jiménez | + 2097 word(s) | 2097 | 2021-11-12 07:07:57 | | | |
| 2 | Jason Zhu | Meta information modification | 2097 | 2021-12-02 02:31:53 | | | | |
| 3 | Jason Zhu | Meta information modification | 2097 | 2021-12-02 02:33:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aguilar Jiménez, O.; Contreras Naranjo, J.E. Electrochemical Immunosensor. Encyclopedia. Available online: https://encyclopedia.pub/entry/16648 (accessed on 08 February 2026).
Aguilar Jiménez O, Contreras Naranjo JE. Electrochemical Immunosensor. Encyclopedia. Available at: https://encyclopedia.pub/entry/16648. Accessed February 08, 2026.
Aguilar Jiménez, Oscar, Jesús Eduardo Contreras Naranjo. "Electrochemical Immunosensor" Encyclopedia, https://encyclopedia.pub/entry/16648 (accessed February 08, 2026).
Aguilar Jiménez, O., & Contreras Naranjo, J.E. (2021, December 01). Electrochemical Immunosensor. In Encyclopedia. https://encyclopedia.pub/entry/16648
Aguilar Jiménez, Oscar and Jesús Eduardo Contreras Naranjo. "Electrochemical Immunosensor." Encyclopedia. Web. 01 December, 2021.
Copy Citation
Electrochemical immunosensors (EI) are systems that combine the analytical power of electrochemical techniques and the high selectivity and specificity of antibodies in a solid phase immunoassay for a target analyte.
Electrochemical immunosensors
Screen Printed Electrodes
Chemistry
sensor
1. Introduction
The International Union of Pure and Applied Chemistry (IUPAC) defined in 2011 an electrochemical immunosensor (EI), as an integrated analytical device where the biorecognition event is based on an antigen/antibody reaction, which can transduce the product molecules of this reaction into an electric signal through the electrode surface (transducer) and quantify the amount of antigen present in the sample [[1]]. EIs can be applied in several areas of the knowledge and industry. In the last decade, the interest in building new sensor platforms that advance into commercial sensing devices for early clinical diagnosis of cancer and heart diseases has increased [[2]]. So, in this sense, a wide variety of studies about EIs have been carried out, mainly focusing on the improvement of the analytical efficiency of the device. In other words, the goal in the design and construction of sensing platforms is to measure the smallest possible amount of the target analyte present in a real complex sample with high precision and accuracy [[3]]. To achieve this goal, it is necessary to create simple and innovative sensor platforms able to suppress non-specific binding (NSB) of undesirable proteins or molecules in the electrode surface [[4]].
Various strategies have been reported for minimizing NSB and are classified in two groups: physical and chemical surface modifications. Physical modification strategies are performed attaching molecules directly to the surface, e.g., blocking buffer solutions, or by forming a complex with other particles, e.g., avidin coated surfaces. Such physical protein adsorption is governed by van der Waals forces, hydrophobic interactions, electrostatic interactions and hydrogen bonding. On the other hand, chemical modification strategies are more specific than physical adsorption and include chemical reaction between different residues (e.g., amine, thiol, carboxyl) of interacting molecules. Chemical modification strategies include: i) allotropic modification of carbon, e.g., carbon nanostructures, ii) modification by metal nanoparticles, e.g., metallic Au/Ag nanoparticles and magnetic beads, iii) polymerization, e.g., polyethylene glycol (PEG), oligo (ethylene glycol) (oEG) and conducting polymers, iv) self-assembled monolayers (SAMs), v) diazonium salt surface chemistry and vi) sol-gel chemistry modification [[5],[6]]. The efficiency of this strategy depends on the chemical properties of the sample molecules and the molecular arrangements over the electrode.
2. Electrochemical Immunosensors, EIs
According to the IUPAC, a biosensor is an analytical device that incorporates a biological element immobilized on the surface of a physicochemical transducer, which recognizes the target molecule or analyte present in a sample and, by means of the transducer, this event of biorecognition is converted into a discrete or continuous electric signal, directly proportional to the amount of analyte in the sample [[7],[8],[9],[10]]. Finally, the electrical signal is shown as a response in a computer work station or a digital display (Figure 1). In general, biosensors are classified by their biorecognition element or their transduction principle [[11],[12]]. Antibodies, enzymes, cells/tissues, nucleic acids and aptamers are some of the elements of biorecognition frequently used. The principal types of transducers are: optical [[13],[14]], electrochemical [[15],[16],[17]], calorimetric [[18],[19]] and piezoelectric [[20],[21]].
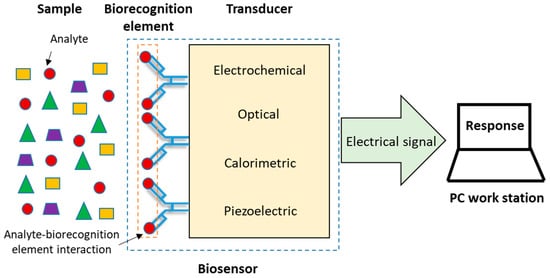
Figure 1. Components of a biosensor [[2]].
The most outstanding characteristics of these devices, which make them highly attractive options as analytical tools, are: their selectivity and specificity, high sensitivity, responsiveness that leads to a short analysis time, their ability to be included in integrated systems, ease of automation, ability to work in real time, its versatility and low cost, among others [[22],[23]]. In the EI, an affinity biosensor, the immobilized biological entities are antibodies (Abs) that recognize the target analyte (antigen, Ag), while an electrochemical transducer converts the binding event between the antigen and antibody, resulting in the formation of the complex antigen-antibody (Ag-Ab), into a useful electrical signal, either an electrical current (amperometric immunosensors), a potential difference (potentiometric immunosensors) or a change in resistivity (conductimetric and impedimetric immunosensors) [[24],[25]].
Antibodies, specifically monoclonal antibodies, are a very useful biorecognition element in EIs due to the high affinity and specificity that they show for the analyte of interest, additionally, they show good stability and versatility and a relative low cost compared to other elements of biorecognition such as polyclonal antibodies, enzymes, whole cells, nucleic acids and molecularly imprinted polymers (MIPs) [[26],[27]]. Monoclonal antibodies present some advantages compared to polyclonal antibodies., among these, its high specificity for a single epitope in a multivalent antigen, contrary to what happens in polyclonal antibodies that recognize and bind to the same antigen but can be combined with different epitopes. Another advantage of monoclonal antibodies is that the hybridoma cell line that produces them is potentially "immortal" and can produce the same antibodies in a constant and indefinite manner [[28],[29]].
EIs combine the high sensitivity of electrochemical techniques and the high selectivity and specificity of antibodies to the target analyte. The most common type of amperometric immunosensors is the ELISA sandwich immunoassay with electrochemical detection. Usually, a sandwich immuno-complex (BtnAb-Ag-AbHRP) is composed of a biotinylated primary antibody, also called capture antibody (BtnAb), the analyte, also called antigen (Ag) and a second antibody (detection antibody) labelled with a redox enzyme, e.g., horseradish peroxidase, HRP (AbHRP) adsorbed onto an avidin-coated electrode surface due to the high affinity of the avidin-biotin system (or its analogous streptavidin and neutravidin).
The purpose of this test is to quantify the number of antigens (target analyte) present in complex samples such as human serum through the detection of the immuno-complex, via an electron transfer reaction between the redox enzyme, the enzyme substrate (S), e.g., hydrogen peroxide (H2O2), and the redox mediator, e.g., 3,3′,5,5′-tetramethylbenzidine (TMB). The HRP oxidize the TMB into TMB+ in the presence of H2O2, releasing one electron per mole of TMB oxidized. TMB+ is reduced to TMB by the imposition of a potential. Thus, as a consequence of the oxidation and reduction of the electroactive product (P) in the electrode surface, a steady-state current is established in the process (Figure 2). If the BtnAb-Ag-AbHRP complex does not form, the measurable current values will be considerably low, which means a negative result. In contrast, high current values indicate a positive result [[30],[31],[32],[33]].
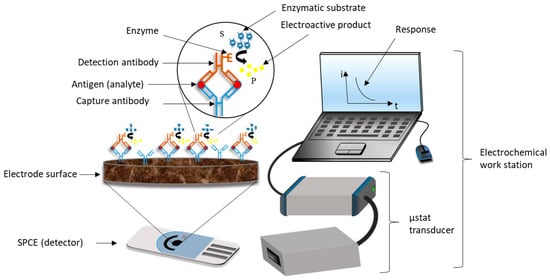
Figure 2. Basic analytical principle of an ELISA sandwich amperometric immunosensor using screen printed carbon electrodes (SPCEs) [[32]].
An example of an electrochemical ELISA sandwich immunoassay was proposed by Zhang et al. in 2015 for the quantification of prostate-specific antigen (PSA) using redox and catalysis “all-in-one” infinite coordination polymer (PtNP@ICP) as a signal tag for the label of PSA on the polyamidoamine dendrimers-modified glassy carbon electrode interface. Their results showed a limit of detection (LOD) of 0.3 pg/mL at 3sB [[34]].
As it is known, EIs are based on the antibody-antigen interaction and possess high specificity and selectivity for the analyte of interest. Since the antibody is immobilized on small electrode surfaces, this type of biosensors can be miniaturized and easily converted into portable devices with the help of microelectrodes (where the immunological reaction will be carried out), among which SPEs are of great importance [[29],[30]]. Immunosensors have a wide variety of applications (e.g., biomedical, clinical diagnosis, environmental and food industry) compared to enzyme-based sensors, given that antibodies are universal in contrast to enzymes, which depend on each substrate [[35]].
The advantages that EIs present over immunoassays, specifically in relation to the commercial sandwich ELISA test, are their good portability (easy miniaturization) in order to carry out clinical diagnosis in the place where the patient is located (point of care testing, POCT) with a minimum of training required, good sensitivity, low cost, rapid analysis, instrumental simplicity and precise measurements, while maintaining the optical approximation of the ELISA test. On the other hand, adaptation of the ELISA assay to an electrochemical approach is not an easy task, because many factors can affect the signal response and hence the performance analysis regarding sensitivity. A poor signal response may be due to the denaturation or loss of affinity and specificity of antibodies and proteins when they are adsorbed onto the electrode surface, an incorrect orientation of antibodies in its adsorbed state which leads to an increase of the steric hindrance, and cross-reactivity from antibodies to other non-specific molecules present in the sample instead of the antigen of interest. Also, in EIs, it is desirable to generate the enzymatic product directly on the electrode surface to favor the electron transfer rate; moreover, direct adsorption of interacting biomolecules may passivate or poison the electrode surface, thus affecting the electrochemical behavior of the sensor. These are the most common concerns in EIs in order to achieve performances comparable or even better than those of the commercial ELISA optical approach [[36],[37],[38],[39]].
Methodologies oriented to improve the electrochemical detection of biomolecules of interest in the development of more sensitive platforms employ magnetic beads, nanostructures (carbon nanotubes, CNTs, multiwall carbon nanotubes MWCNTs, Quantum dots), and nanoparticle labels [[40],[41],[42],[43]].
3. Screen Printed Electrodes, SPEs
A wide variety of electrodes have been used as a support in the fabrication of immunosensor devices: carbon paste electrodes, glassy carbon electrodes and gold electrodes. Recently, most of the sensor devices have been constructed onto SPEs. Nowadays, the screen printing microfabrication technology is well stablished, offers high-mass production of solid, planar, small size, low cost, disposable, mechanically robust and highly reliable thick film electrodes; allowing the construction of portable, low cost and pocket size devices for on-site diagnosis [[44],[45]]. A detailed description of the fabrication process of SPEs was reported by Li et al. in 2012 [[46]].
In summary, fabrication of SPEs consists of various steps: selection of the screen or mesh which will define the thick layer film, geometry and size of the SPE, selection and preparation of the inks for the reference, counter and working electrode, selection of the substrate, sequential deposition of layers of ink onto the substrate, drying and curing steps between each layer deposition of the ink [[47],[48],[49]]. Even though the exact formulation of the inks is according to the manufacturer as copyright information, it is well stablished that the ink is made mainly by synthetic grade graphite, vinyl or epoxy-based polymeric binder and solvents. Graphite is the electrode material, the binder increases adhesion and mechanical strength, and solvents allow to control the ink viscosity [[50]]. The control of the ink composition is very important, because any minimal change strongly affects the electron transfer process and changes the overall performance of the sensor device [[51],[52]].
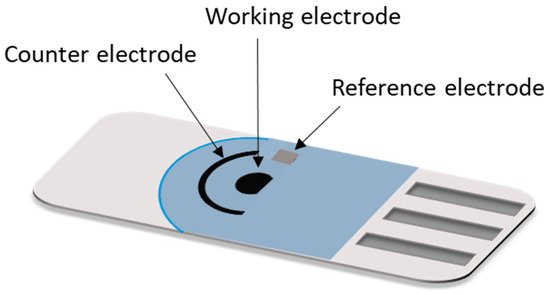
The SPE substrate usually is a non-conducting solid surface material such as alumina, glass, ceramic, plastic, etc. and the electrode conducting parts are made of carbon ink/paste, platinum, gold or other metal pastes. The improvement of the EIs is intimately related to the material of the working electrode on which the reactions of interest occurs. Carbon or other forms of carbon such as graphene, graphite, fullerene, carbon nanotubes, and single/multi-wall carbon nanotubes are most commonly employed as a material in the fabrication of the working electrode. Some characteristics that made carbon suitable for this purpose are its low cost (compare to metals e.g., gold, silver or platinum; which have been also utilized as a working electrode materials and present higher fabrication costs), easiness to modify, chemically inert, good electrical and thermal conductivity, high mechanical and dimensional stability and for electrochemical activities its low background currents and wide potential operational window [[53],[54],[55]]. Usually, the material of the reference electrode is Ag/AgCl and the counter electrode generally is made from the same working electrode material [[54],[55]] (Figure 3).
A diversity of SPEs configurations are available nowadays in the market for a broad spectrum of applications which can be personalized according to the customer requirement. Configurations of two electrodes (working and reference electrodes, also called first generation SPEs), three electrodes (working, reference and auxiliary electrodes, also called second generation SPEs) are the most used [[56]]. In addition, SPEs with multiplex working electrodes, arrays of eight SPEs and of 96 SPEs in a 96-well plate have been developed for the simultaneous detection of multiple biomarkers [[57]].
Recently, the interest to replace rigid substrates with paper substrates for fabricating SPEs has strongly increased in the development of new electroanalytical tools. Various studies about SPEs based on paper and transparency in order to detect a broad spectrum of biomolecules have been reported [[58],[59]]. Further, wearable, tattoo-based wearable and skin worn electrochemical sensors and biosensors employing SPEs as a sensor platform are a very good alternative to measure in real time physical parameters such as heart rate, respiration rate, oxygenation of the blood, skin temperature, bodily motion, brain activity, and blood pressure, etc., without compromising user comfort. These are some examples of the great versatility of SPEs in the continuous development of bioanalytical devices [[60],[61]].
References
- Pan, M.; Gu, Y.; Yun, Y.; Li, M.; Jin, X.; Wang, S.; Nanomaterials for Electrochemical Immunosensing. Sensors 2017, 17, 1041, https://doi.org/10.3390/s17051041.
- Bahadır, E.B.; Sezgintürk, M.K.; Applications of electrochemical immunosensors for early clinical diagnostics. Talanta 2015, 132, 162, https://doi.org/10.1016/j.talanta.2014.08.063.
- Wang, X.-H.; Wang, S.; Sensors and Biosensors for the Determination of Small Molecule Biological Toxins. Sensors 2008, 8, 6045, https://doi.org/10.3390/s8096045.
- Piro, B.; Reisberg, S.; Recent Advances in Electrochemical Immunosensors. Sensors 2017, 17, 794, https://doi.org/10.3390/s17040794.
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H.; A review on amperometric-type immunosensors based on screen-printed electrodes.. Analyst 2014, 139, 2289, DOI https://doi.org/10.1039/C3AN02050A.
- Gopinath, S.C.B.; Tang, T.-H.; Citartan, M.; Chen, Y.; Lakshmipriya, T.; Current aspects in immunosensors. Biosensors and Bioelectronics 2014, 57, 292, https://doi.org/10.1016/j.bios.2014.02.029.
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S.; Electrochemical biosensors: Recommended definitions and classification.. Pure and Applied Chemistry 1999, 71, 2333, https://doi.org/10.1351/pac199971122333.
- Wang, Y.; Xu, H.; Zhang, J.; Li, G.; Electrochemical Sensors for Clinic Analysis.. Sensors 2008, 8, 2043, https://doi.org/10.3390/s8042043.
- Zhou, F.; Yao, Y.; Luo, J.; Zhang, X.; Zhang, Y.; Yin, D.; Gao, F.; Wang, P.; Proximity hybridization-regulated catalytic DNA hairpin assembly for electrochemical immunoassay based on in situ DNA template-synthesized Pd nanoparticles. Analytica Chimica Acta 2017, 969, 8, https://doi.org/10.1016/j.aca.2017.03.038.
- Gao, F.; Zhou, F.; Chen, S.; Yao, Y.; Wu, J.; Yin, D.; Geng, D.; Wang, P.; Proximity hybridization triggered rolling-circle amplification for sensitive electrochemical homogeneous immunoassay.. Analyst 2017, 142, 4308, DOI https://doi.org/10.1039/C7AN01434A.
- Wang, P.; Liu, Q.. Biomedical Sensors and Measurement; Zhejiang University Press; Springer: New York, NY, USA,, 2011; pp. 199.
- Neethirajan, S.; Ragavan, V.; Weng, X.; Chand, R.; Biosensors for Sustainable Food Engineering: Challenges and Perspectives. Biosensors 2018, 8, 23, ttps://doi.org/10.3390/bios8010023.
- Damborský, P.; Švitel, J.; Katrlík, J.; Optical biosensors.. Essays in Biochemistry 2016, 60, 91, https://doi.org/10.1042/EBC20150010.
- Long, F.; Zhu, A.; Shi, H.; Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning.. Sensors 2013, 13, 13928, https://doi.org/10.3390/s131013928.
- Jarocka, U.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H.; Electrochemical immunosensor for detection of antibodies against influenza A virus H5N1 in hen serum. Biosensors and Bioelectronics 2014, 55, 301, https://doi.org/10.1016/j.bios.2013.12.030.
- De la Escosura-Muñiz, A.; Merkoçi, A.; Electrochemical detection of proteins using nanoparticles: Applications to diagnostics.. Expert Opinion on Medical Diagnostics 2010, 4, 21, https://doi.org/10.1517/17530050903386661.
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P; Recent Advances in the Fabrication and Application of Screen-Printed Electrochemical (Bio)Sensors Based on Carbon Materials for Biomedical, Agri-Food and Environmental Analyses. Biosensors 2016, 6, 50, https://doi.org/10.3390/bios6040050.
- Zhang, Y.; Tadigadapa, S.; Calorimetric biosensors with integrated microfluidic channels. Biosensors and Bioelectronics 2004, 19, 1733, https://doi.org/10.1016/j.bios.2004.01.009.
- Vo-Dinh, T.; Cullum, B.; Biosensors and biochips: advances in biological and medical diagnostics. Analytical Chemistry 2000, 366, 540, https://doi.org/10.1007/s002160051549.
- Janshoff, A.; Galla, H.-J.; Steinem, C.; Piezoelectric Mass-Sensing Devices as Biosensors-An Alternative to Optical Biosensors?. Angewandte Chemie International Edition 2000, 39, 4004, 10.1002/1521-3773(20001117)39:223.0.CO;2-2.
- Raiteri, R.; Grattarola, M.; Butt, H.-J.; Skládal, P.; Micromechanical cantilever-based biosensors. Sensors and Actuators B: Chemical 2001, 79, 115, https://doi.org/10.1016/S0925-4005(01)00856-5.
- Presnova, G.V.; Rybcova, M.Y.; Egorov, A.M.; Electrochemical biosensors based on horseradish peroxidase. Russian Journal of General Chemistry 2008, 78, 2482, https://doi.org/10.1134/S1070363208120293.
- Chung, J.W.; Park, J.M.; Bernhardt, R.; Pyun, J.C.; Immunosensor with a controlled orientation of antibodies by using NeutrAvidin-protein A complex at immunoaffinity layer. Journal of Biotechnology. 2006, 126, 325, 10.1016/j.jbiotec.2006.05.010.
- Kokkinos, C.; Economou, A.; Prodromidis, M.I.; Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. Trends in Analytical Chemistry 2016, 79, 88, https://doi.org/10.1016/j.trac.2015.11.020.
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R.; Electrochemical biosensors. Chemical Society Reviews. 2010, 39, 1747, https://doi.org/10.1039/B714449K.
- Santoro, K.; Ricciardi, C.. Biosensors.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge: MA, USA., 2016; pp. 430.
- Piro, B.; Shi, S.; Reisberg, S.; Noël, V.; Anquetin, G.; Comparison of Electrochemical Immunosensors and Aptasensors for Detection of Small Organic Molecules in Environment, Food Safety, Clinical and Public Security. Biosensors 2016, 6, 7, https://doi.org/10.3390/bios6010007.
- García Merino, A.; Anticuerpos monoclonales. Aspectos básicosMonoclonal antibodies. Basic features. Neurología 2011, 26, 301, https://doi.org/10.1016/j.nrl.2010.10.005.
- Sharma, S.; Byrne, H.; O’Kennedy, R.J.; Antibodies and antibody-derived analytical biosensors. Essays in Biochemistry 2016, 60, 9, https://doi.org/10.1042/EBC20150002.
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E.; Electrochemical Biosensors - Sensor Principles and Architectures. Sensors 2008, 8, 1400, https://doi.org/10.3390/s80314000.
- Moina, C.; Ybarra, G.. Fundamentals and Applications of Immunosensors.; Chiu, N.H.L., Christopoulos, T.K., Eds.; InTech: Rijeka, Croatia, 2012; pp. 65.
- Felix, F.S.; Angnes, L.; Electrochemical immunosensors – A powerful tool for analytical applications. Biosensors and Bioelectronics 2018, 102, 470, https://doi.org/10.1016/j.bios.2017.11.029.
- Azam, M.S.; Rahman, M.R.T.; Lou, Z.; Tang, Y.; Raqib, S.M.; Jothi, J.S.; Review: Advancements and application of immunosensors in the analysis of food contaminants. Nusantara Bioscience. 2014, 6, 186.
- Zhang, B.; Liu, B.; Chen, G.; Tang, D.; Redox and catalysis ‘all-in-one’ infinite coordination polymer for electrochemical immunosensor of tumor markers. Biosensors and Bioelectronics 2015, 64, 6, https://doi.org/10.1016/j.bios.2014.08.024.
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H.; Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207, https://doi.org/10.3390/s18010207.
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G.; Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends in Analytical Chemistry 2016, 79, 114, https://doi.org/10.1016/j.trac.2016.01.032.
- Ricci, F.; Volpe, G.; Micheli, L.; Palleschi, G.; A review on novel developments and applications of immunosensors in food analysis. Analytica Chimica Acta 2007, 605, 111, https://doi.org/10.1016/j.aca.2007.10.046.
- Ricci, F.; Adornetto, G.; Palleschi, G.; A review of experimental aspects of electrochemical immunosensors. Electrochimica Acta 2012, 84, 74, https://doi.org/10.1016/j.electacta.2012.06.033.
- Viguier, C.; Crean, C.; O’Kennedy, R.. Trends and perspectives in immunosensors.; Meulenberg, E.P., Eds.; Bentham Science: Emirate of Sharjah, UAE, 2012; pp. 184.
- Wang, J.; Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosensors and Bioelectronics 2006, 21, 1887, https://doi.org/10.1016/j.bios.2005.10.027.
- Ahammad, A.J.S.; Lee, J.-J.; Rahman, M.A.; Electrochemical Sensors Based on Carbon Nanotubes. Sensors 2009 , 9, 2289, https://doi.org/10.3390/s90402289.
- Wang, J.; Nanoparticle-Based Electrochemical Bioassays of Proteins. Electroanalysis 2007, 19, 769, https://doi.org/10.1002/elan.200603789.
- Malhotra, B.D.; Kumar, S.; Pandey, C.M.; Nanomaterials based biosensors for cancer biomarker detection. https://doi.org/10.1088/1742-6596/704/1/012011 2016, 704, 012011, https://doi.org/10.1088/1742-6596/704/1/012011.
- Renedo, O.D.; Alonso-Lomillo, M.A.; Martínez, M.J.A.; Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202, https://doi.org/10.1016/j.talanta.2007.03.050.
- García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E.; Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018., 8, 53, 10.3390/bios8020053.
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T.; Recent developments and applications of screen-printed electrodes in environmental assays—A review. Analytica Chimica Acta 2012, 734, 31, https://doi.org/10.1016/j.aca.2012.05.018.
- Fähnrich, K.A.; Pravda, M.; Guilbault, G.G.; Disposable amperometric immunosensor for the detection of polycyclic aromatic hydrocarbons (PAHs) using screen-printed electrodes. Biosensors and Bioelectronics 2003, 18, 73, https://doi.org/10.1016/S0956-5663(02)00112-4.
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B.; Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta. 2016 , 146, 801, 10.1016/j.talanta.2015.06.011.
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M.; Screen-printed electrodes for biosensing: a review (2008–2013). Microchimica Acta 2014 , 181, 865, https://doi.org/10.1007/s00604-014-1181-1.
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A.; Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochimica Acta 2008, 53, 3635, https://doi.org/10.1016/j.electacta.2007.12.044.
- Fan, Z.; Ho, J.C.; Takahashi, T.; Yerushalmi, R.; Takei, K.; Ford, A.C.; Chueh, Y.-L.; Javey, A.; Toward the Development of Printable Nanowire Electronics and Sensors. Advanced Materials 2009 , 21, 3730, https://doi.org/10.1002/adma.200900860.
- Hayat, A.; Marty, J.L.; Disposable Screen Printed Electrochemical Sensors: Tools for Environmental Monitoring. Sensors 2014, 14, 10432, https://doi.org/10.3390/s140610432.
- Yamanaka, K.; Vestergaard, M.C.; Tamiya, E.; Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016 , 16, 1761, https://doi.org/10.3390/s16101761.
- Rama, E.C.; Costa-García, A.; Screen-printed Electrochemical Immunosensors for the Detection of Cancer and Cardiovascular Biomarkers. Electroanalysis 2016 , 28, 1700, https://doi.org/10.1002/elan.201600126.
- Liu, C.C.; Electrochemical Based Biosensors. Biosensors 2012, 2, 269, https://doi.org/10.3390/bios2030269.
- Tudorache, M.; Bala, C.; Biosensors based on screen-printing technology, and their applications in environmental and food analysis. Analytical and Bioanalytical Chemistry 2007, 388, 565, https://doi.org/10.1007/s00216-007-1293-0.
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M.; Multiplexed Electrochemical Immunosensors for Clinical Biomarkers. Sensors 2017, 17, 965, https://doi.org/10.3390/s17050965.
- Devarakonda, S.; Singh, R.; Bhardwaj, J.; Jang, J.; Cost-Effective and Handmade Paper-Based Immunosensing Device for Electrochemical Detection of Influenza Virus. Sensors 2017, 17, 2597., https://doi.org/10.3390/s17112597.
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S.; Recent Developments in Paper-Based Microfluidic Devices. Analytical Chemistry 2015, 87, 19, https://doi.org/10.1021/ac503968p.
- Windmiller, J.R.; Wang, J.; Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2012, 24, 1, https://doi.org/10.1002/elan.201200349.
- Bandodkar, A.J.; Jia,W.; Wang, J.; Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanalysis 2015 , 27, 1, https://doi.org/10.1002/elan.201400537.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.4K
Revisions:
3 times
(View History)
Update Date:
02 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No