
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prarthna Chandar Kulandaisamy | + 1180 word(s) | 1180 | 2021-11-30 09:00:25 | | | |
| 2 | Jessie Wu | Meta information modification | 1180 | 2021-12-02 03:21:14 | | | | |
| 3 | Jessie Wu | + 169 word(s) | 1349 | 2021-12-02 03:22:44 | | |
Video Upload Options
Overview of current guidelines and practices in the management of malignant pleural effusion
1. Background
Malignant pleural effusion (MPE) is the second most common cause of pleural exudate and affects 15% of all patients with cancer [1]. MPE accounts for greater than 125,000 admissions per year in the United States, with costs of around $5 billion for inpatient charges alone. It usually indicates an advanced stage or metastatic disease and hence portends a poor prognosis. The average life expectancy of patients presenting with MPE is 3–12 months depending on the underlying tumor type and patient comorbidities [2]. Lung cancer, breast cancer, and lymphoma contribute to the majority of these effusions, followed by gynecological malignancies and malignant mesothelioma [3]. Patients frequently experience debilitating dyspnea and other symptoms which severely compromise their quality of life. Various therapies are currently available for the management of these effusions. The management of MPE is challenging and is mainly focused on the relief of symptoms and the improvement of the patients’ quality of life. The therapy needs to be tailored to individual patients taking into account their preferences, life expectancy, affordability, presence of trapped lungs, resources available, and the experience of the treatment team.
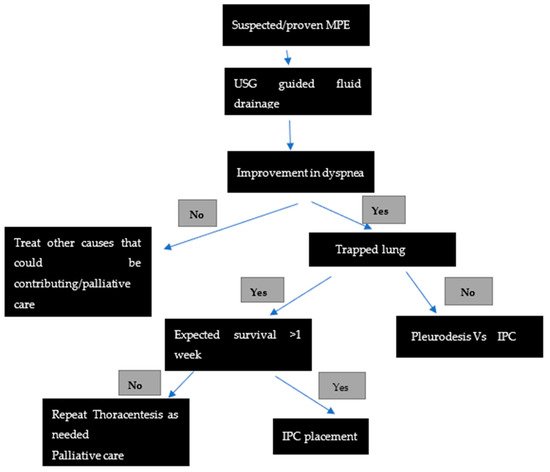
2. Management of Malignant Pleural Effusion
| PICO 1 | RECOMMENDATIONS |
|---|---|
|
Yes |
|
No |
|
Yes. Manometry if pleurodesis is contemplated to assess for lung re-expansion |
|
Yes |
|
Yes, there is no difference in the efficacy between the two |
|
IPC is the preferred method of choice over chemical pleurodesis |
|
Not unless infection does not improve |
2.1. Thoracic Drainage and Pleurodesis
2.2. A Thoracoscopy with Pleurodesis
References
- Feller-Kopman, D.J.; Reddy, C.B.; DeCamp, M.M.; Diekemper, R.L.; Gould, M.K.; Henry, T.; Iyer, N.P.; Lee, Y.G.; Lewis, S.Z.; Maskell, N.A.; et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 839–849.
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.; et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. J. Cardiothorac. Surg. 2019, 55, 116–132.
- Penz, E.; Watt, K.N.; Hergott, C.A.; Rahman, N.M.; Psallidas, I. Management of malignant pleural effusion: Challenges and solutions. Cancer Manag. Res. 2017, 9, 229–241.
- Pereyra, M.F.; Ferreiro, L.; Valdés, L. Unexpandable lung. Arch. Bronconeumol. 2013, 49, 63–69.
- Clive, A.O.; Kahan, B.C.; Hooper, C.E.; Bhatnagar, R.; Morley, A.J.; Zahan-Evans, N.; Bintcliffe, O.J.; Boshuizen, R.C.; Fysh, E.T.; Tobin, C.L.; et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax 2014, 69, 1098–1104.
- Metintas, M.; Ak, G.; Dundar, E.; Yildirim, H.; Ozkan, R.; Kurt, E.; Erginel, S.; Alatas, F.; Metintas, S. Medical thoracoscopy vs. CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions. Chest 2010, 137, 1362–1368.
- Havelock, T.; Teoh, R.; Laws, D.; Gleeson, F. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65, i61–i76.
- Lee, Y.C.G.; Baumann, M.H.; Maskell, N.A.; Waterer, G.W.; Eaton, T.E.; Davies, R.J.O.; Heffner, J.E.; Light, R.W. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003, 124, 2229–2238.
- Bhatnagar, R.; Piotrowska, H.E.; Laskawiec-Szkonter, M.; Kahan, B.C.; Luengo-Fernandez, R.; Pepperell, J.C.T.; Evison, M.D.; Holme, J.; Al-Aloul, M.; Psallidas, L.; et al. Effect of Thoracoscopic Talc Poudrage vs. Talc Slurry via Chest Tube on Pleurodesis Failure Rate among Patients with Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020, 323, 60–69.
- Janssen, J.P.; Collier, G.; Astoul, P.; Tassi, G.F.; Noppen, M.; Rodriguez-Panadero, F.; Loddenkemper, R.; Herth, F.J.; Gasparini, S.; Marquette, C.H.; et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: A prospective cohort study. Lancet 2007, 369, 1535–1539.
- Shojaee, S.; Lee, H.J. Thoracoscopy: Medical versus surgical—In the management of pleural diseases. J. Thorac. Dis. 2015, 7 (Suppl. S4), S339–S351.




