
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joe Poh Sheng Yeong | + 2649 word(s) | 2649 | 2021-08-23 10:36:38 |
Video Upload Options
Triple-negative breast cancer (TNBC) has been associated with worse prognoses due to the limited treatment options. Thus, there is a need to characterise new biomarkers or treatment targets to improve patient outcomes. Cancer testis antigens (CTAs) are a group of antigens that are preferentially expressed in tumours and exhibit strong immunogenicity, as such, CTAs hold great promise as potential treatment targets and biomarkers in cancer.
1. Introduction
Breast cancer is a heterogeneous disease that can be classified based on the clinical, morphological and biological characteristics [1][2]. Triple-negative breast cancers (TNBC), a special class of breast cancers that are negative for oestrogen receptor (ER), progesterone receptor (PR) and HER2 (cerbB2), represent 9–17% of all breast carcinomas, depending on the threshold for ER, PR and HER2 positivity [1][3][4][5]. TNBC are also of high histological grade, displaying aggressive clinical behaviour with poorer prognosis and accompanied by frequent metastasis to the brain and lungs, with a shorter time to recurrence and death [3][5].
Cancer testis antigens (CTAs) are tumour antigens that are expressed normally in embryonic stem cells and testicular germ cells and minimally expressed in most other tissues. CTAs are aberrantly found in various cancers, especially advanced cancers with stem-cell-like characteristics [6][7]. CTAs were first identified in 1991 in a study showing that the presence of MAGEA1 caused resistant tumour cell clones to be sensitised to killing by autologous cytotoxic T-lymphocytes [8]. Since then, there has been an explosion of CTA-related research and the discovery of more CTAs, including NY-ESO1, which is the most successful target to date for cancer immunotherapy [9][10]. The number of CTAs identified has also increased exponentially over the years. In 2009, a database was created of about 70 families and more than 200 members of CTAs [11]. With the advent of next-generation sequencing, there has been a huge increase in genomic data. By integrating transcriptomic data from multiple databases, Wang et al. systematically identified 876 new CTAs in 19 cancers [12], and a different research group found an additional 201 new CTAs [13].
2. Expression of CTAs in Triple-Negative Breast Cancer
Receptor tyrosine kinase-like orphan receptor 2 (ROR2), a novel Wnt receptor, belongs to the tyrosine kinase receptor family, which is important in regulating skeletal and neuronal development, cell migration and cell polarity ( Table 1 ) [14]. Breast cancer patients, including those with TNBC expressing ROR2, experienced a significantly worse prognosis with shorter overall survival compared to those lacking ROR2 [15].
Table 1. Summary of CTAs in TNBC.
| CTAs | Cellular Function | Institute | Cohort | Prevalence of CTAs in TNBC | Type of Assay | Antibodies | Role in TNBC | Ref. |
|---|---|---|---|---|---|---|---|---|
| CTAs associated with worse prognosis in TNBC | ||||||||
| A-kinase anchoring proteins (AKAP3) | Sperm function | Breast Cancer Research Centre (Tehran, Iran) [16] |
Asian | 20% (n = 25) |
Real-Time Polymerase Chain Reaction (RT-PCR) | Loss of expression in TNBC. Breast cancer patients who were positive for AKAP3 had better 5-year disease-free survival. | [16][17] | |
| Melanoma antigen gene (MAGE) | Not known. May promote tumourigenesis and metastasis. | Italian National Cancer Institute [18] | Caucasian | MAGE-A: 23% (n = 44) |
IHC | MAGE-A Antibody (6C1) | Frequently overexpressed in TNBC. Higher expression of MAGE-A was reported to define a very aggressive subtype of TNBC and correlated with poor prognosis of patients. MAGE-A3, -A6 and -C2 expression in breast cancers was significantly associated with negative ER or negative PR status, higher-grade tumours and correlated with worse outcomes. MAGE-A10 expression was associated with ER-negative, PR-negative and HER2-negative status. | [18][19][20][21][22][23][24][25][26][27][28][29] |
| Royal Brisbane Women’s Hospital [26] | Caucasian | MAGE-A: 47% (n = 65) |
IHC | MAGE-A Antibody (6C1), Santa Cruz Biotechnology(USA) | ||||
| Affiliated Tumour Hospital of Xinjiang Medical University [27] | Asian | MAGE-C: 38.2% (n = 110) |
IHC | Rabbit polyclonal MAGE-C2 Antibody, Sigma-Aldrich (USA) | ||||
| Centre of Breast Cancer of The Fourth Hospital of Hebei Medical University (Shijiazhuang Hebei) [28] | Asian | MAGE-A: 76.5% (n = 17) |
IHC | MAGE-A Antibody (6C1), Santa Cruz Biotechnology(USA) | ||||
| University Hospital Center Zagreb [25] | Caucasian | MAGE-A: 85.7% (n = 49) |
IHC | 3DA3 Monoclonal Antibody | ||||
| Split University Hospital Centre, Croatia [30] | Caucasian | MAGE-A1 Specific: 69.2% (n = 81) |
IHC | Monoclonal Antibody 77B | ||||
| Multi-MAGE: 58% (n = 81) |
IHC | Monoclonal Antibody 57B | ||||||
| MAGE-A10: 16% (n = 81) |
IHC | Monoclonal Antibody 3GA11 | ||||||
| European Institute of Oncology (Milan, Italy) [19] | Caucasian | MAGE-A: 32% (n = 50) |
IHC | Antibody cocktail of monoclonal antibodies 6C1, MA454, M3H67 and 57B | ||||
| Copenhagen University Hospital [31] | Caucasian | MAGE-A: 33% (n = 78) |
IHC | Rabbit polyclonal anti-peptide antibody EP101638 (rab Ab 1982) raised against Mage-4, Eurogentec (Belgium) | ||||
| National Cancer Institute (Milan, Italy) [29] | Caucasian | MAGE-A: 85.7–93% (n = 21) |
IHC | MAGE-A3 (Clone 60054-1-Ig) Monoclonal Antibody, Proteinthec (USA) | ||||
| Mesothelin (MSLN) | GPI-anchored membrane protein | Perelman School of Medicine, University of Pennsylvania [32] | Caucasian | 67% (n = 99) |
IHC | Mesothelin Monoclonal Antibody (clone 5B2), Thermo Scientific (USA) | MSLN is significantly expressed in TNBC compared to non-TNBC and is an independent prognostic marker associated with distant metastasis and worse survival. | [32][33][34] |
| University of Texas MD Anderson Cancer Center [34] | Caucasian | 34% (n = 109) |
IHC | Mesothelin Monoclonal Antibody (clone 5B2), Novocastra (USA) | ||||
| Prostate stem cell antigen (PSCA) | GPI-anchored membrane protein | University Hospital of Dresden, Germany [35] | Caucasian | 17% (n = 90) |
IHC | PSCA antibody MB1 | Distribution of PSCA expression among TNBC was comparable to the total population. Patients with PSCA-positive invasive micropapillary carcinoma (IMPC) of the breast had decreased disease-free survival. | [35][36] |
| Receptor tyrosine kinase-like orphan receptor 2 (ROR2) | Tyrosine kinase receptor family | University of New South Wales [15] | Caucasian | 87% (n = 295, breast cancer including triple- negative) |
IHC | Human ROR2 polyclonal antibody, Sigma-Aldrich (Australia) | Breast cancer patients including TNBC expressing ROR2 had significantly worse prognoses with shorter overall survival compared to those lacking ROR2. | [15] |
| Sperm protein associated with the nucleus X-linked (SPANX) | Sperm function | University of Texas Health Science Center [37] | Caucasian | 73% (n = 15) |
IHC | SPANXB1 (#H00728695), Abnova (Taiwan) | SPANXB1 was frequently overexpressed in human primary and metastatic TNBC. In ER-negative patients, elevated SPANX-A/C/D was correlated with shorter distant metastasis-free survival time. | [37][38] |
| CTAs associated with better prognosis in TNBC | ||||||||
| New York oesophageal squamous cell carcinoma-1 (NY-ESO-1) | Unknown; might be involved in cell cycle progression and growth | New York Presbyterian Hospital-Weill Cornell Medical Center and UCSF Medical Center [21] | Caucasian | 19.2% (n = 50) |
IHC | NY-ESO-1 Monoclonal Antibody(E978) produced in author’s laboratory | Higher expression of NY-ESO-1 was detected in TNBC. NY-ESO-1 expression was correlated with tumour-infiltrating lymphocytes and associated with good prognosis. | [19][21][25][26][29][39][40][41][42] |
| University Hospital Center Zagreb [25] | Caucasian | 10% (n = 50) |
IHC | NY-ESO-1 Monoclonal Antibody (B9.8.1.1) | ||||
| Roswell Park Cancer Institute [39] | Caucasian | 16% (n = 168) |
IHC | NY-ESO-1 Mouse Monoclonal, Zymed/Invitrogen (USA) | ||||
| Asan Medical Centre, Korea [41] | Asian | 9.3% (n = 172) |
IHC | NY-ESO-1 Monoclonal Antibody (E978), Invitrogen (USA) | ||||
| Royal Brisbane Women’s hospital [26] | Caucasian | ~20% (n = 65) |
IHC | NY-ESO-1 Antibody (E978), Santa Cruz Biotechnology(USA) | ||||
| National Cancer Institute (Milan, Italy) [29] | Caucasian | 28.6% (n = 21) |
IHC | NY-ESO-1 Monoclonal Antibody (E978), Invitrogen (USA) | ||||
| European Institute of Oncology (Milan, Italy) [42] | Caucasian | 16% (n = 50) |
IHC | NY-ESO-1 Monoclonal antibody (E978) provided by Ludwig Institute for Cancer Research | ||||
| CTAs with oncogenic potential | ||||||||
| Melanoma antigen gene (MAGE) | Not known. May promote tumourigenesis and metastasis. | See Above | Promote tumourigenesis and metastasis via various mechanisms such as acting as master regulator of E3 RING ubiquitin ligase, inhibiting p53 tumour suppressor or by enhancing cell motility. | [19][20][21][22][23][24][25] | ||||
| New York oesophageal squamous cell carcinoma-1 (NY-ESO-1) | Unknown; might be involved in cell cycle progression and growth | See Above | Might be involved in cellular proliferation and growth. | [9] | ||||
| Preferentially expressed antigen of melanoma (PRAME) | Membrane-bound protein | National Cancer Institute (Milan, Italy) [29] | Caucasian | 85.7–96.6% (n = 21) | IHC | PRAME Polyclonal Antibody (Clone NBP1-85418), Novus Boilogicals (USA) | Role in EMT reprogramming. Expression of PRAME was associated with negative ER status. |
[43][44][45] |
| Sperm-associated antigen 9 (SPAG9) | Sperm function | National Institute of Immunology, Aruna Asaf Ali Marg, (New Delhi, India) [46] | Asian | NA | IHC | Polyclonal antibody to SPAG9 was prepared in authors’ laboratory | Analysis of 100 breast cancer tissues (94 infiltrating ductal carcinomas [IDC], 2 ductal carcinomas in situ [DCIS] and 4 invasive lobular carcinomas [ILC]) revealed that 88% of samples stained positive for SPAG9. Role in invasiveness of breast cancer. Downregulation could reduce invasive potential of TNBC. | [46][47] |
| Sperm protein associated with the nucleus X-linked (SPANX) | Sperm function | See Above | Required for metastasis. Interacts with lamin A/C at the inner nuclear membrane and involved in the formation of actin-rich cellular protrusions that reorganise the extracellular matrix. | [37][38] | ||||
| Testes-specific protease 50 (TSP50) | Oncogene | Northeast Normal University (Changchun, China) | Caucasian | NA | IHC | TSP50 Monoclonal Antibody was prepared in authors’ laboratory | Analysis of 88 clinical breast cancer tissue microarrays (BR955 and BR 1101 from US Biomax, Rockville, MD, USA) revealed that 90.9% of specimens stained positive for TSP50 compared to 10% of adjacent normal tissues. Role in cell growth. Knockdown of TSP50 in breast cancer cells significantly inhibits cellular proliferation. TSP50-positive tumours were associated with negative ER expression and higher grade. | [48][49] |
| Zinc-finger protein 165 (ZNF165) | Gene regulation | Simmons Comprehensive Cancer Center, UT-Southwestern Medical Center, Dallas [50] | Caucasian | 90% (n = 10) |
IHC | ZNF165 (H00007718), Novus Biologicals (USA) | Enhances growth and survival of human TNBC cells both in vitro and in vivo by regulating TGF-β signalling. Frequently overexpressed in TNBC. | [50][51] |
| Tripartite motif containing 27 (TRIM27) | Gene regulation | Simmons Comprehensive Cancer Center, UT-Southwestern Medical Center, Dallas [50] | Caucasian | NA | TCGA | TRIM27 expression was significantly elevated in TNBC compared to normal breast tissue based on TCGA data. Displayed difference in cellular localisation, as it was mainly cytoplasmic in normal breast epithelia and more nuclear in TNBC tissues. Regulates TGFβ-dependent transcription in complex with ZNF165, ZNF446 and SMAD in TNBC. |
[50][51] | |
| Other CTAs with increased expression in TNBC | ||||||||
| Actin like 8 (ACTL8) | Cellular architecture | National Centre for Tumour Diseases (Heidelberg, Germany) [52] | Caucasian | 57% (n = 98, TCGA) |
TCGA | Frequently expressed in TNBC based on in silico analysis. | [52] | |
| Chromosome X open reading frame 6/ mastermind-like domain containing 1/Kita-Kyu-Shu lung cancer antigen-1 (CXorf6/MAMDL1/KK-LC-1/CT83) | Development of male genitalia Not known |
Johannes Gutenberg-University (Mainz, Germany) [53] | Caucasian | 64.7% (n = 17, from commercial vendor) |
IHC | Anti-CXorf61-A polyclonal antibody | Frequently expressed in TNBC. | [53] |
| Kitasato University Medical Center (Japan) [54] | Asian | 100% (n = 8) |
IHC | Mouse monoclonal antibody was prepared by CLEA Japan (Japan) | Frequently expressed in TNBC based on in silico analysis. Frequently overexpressed in TNBC and tumours without ER expression. | [52][54] | ||
| Sperm protein 17 (SP17) | Sperm function | University of Texas MD Anderson Cancer [55] | Caucasian | 47.2% (n = 36) |
IHC | Antibody against SP17 | SP17 is frequently expressed in primary breast tumours and in TNBC. | [55] |
| Wilms tumour-1 (WT-1) | Transcription factor | European Institute of Oncology (Milan, Italy) [42] | Caucasian | 54% (n = 27) |
IHC | WT1 Monoclonal Antibody (Clone WT49), Monosan (Netherlands) | Highest expression in TNBC compared to other breast cancer subtypes. | [42] |
SPANX-A/C/D was discovered as an important factor for the metastasis of breast cancer cells; it was hypothesised to act via interactions with components of the cytoskeleton at the inner nuclear membrane and is required to produce actin-rich cellular protrusions during modelling of the extracellular matrix ( Table 1 ). Sperm-associated antigen 9 (SPAG9) mRNA and protein expression was found in the cytoplasm of all examined breast cancer cells, including TNBC cells ( Table 1 ) [47]. Interestingly, the downregulation of SPAG9 ameliorated the invasiveness of TNBC [47].
Testes-specific protease 50 ( TSP50 ) is an oncogene that promotes breast cancer survival, invasion and metastasis via the activation of the NF-κB signalling pathway ( Table 1 ) [56]. Knockdown of TSP50 in breast cancer cells significantly inhibited cellular proliferation [49]. The levels of TSP50 together with the expression of p65 and matrix metalloproteinase 9 (MMP9) were analysed in conjunction with clinicopathological features, such as tumour size, pathologic grade, ER and PR levels, in breast cancer tissues [48], and the majority of TSP50+/ p65+ tumours (72%) and TSP50+/MMP9+(78%) tumours were negative for ER expression and tended to be of a higher grade [48].
Sperm protein 17 (SP17) is involved in various stages of spermiogenesis, and aberrant SP17 expression has been linked to cancers such as ovarian, oesophagus, central nervous system, multiple myeloma and esthesioneuroblastoma ( Table 1 ) [57][58][59][60][61][62]. The exact role of SP17 in cancer cells has not yet been elucidated, with some reports suggesting that SP17 promotes cell–cell adhesion in malignant B-lymphocytes via interaction with heparan-sulphate and enhances cell movement and drug resistance in ovarian cells [63][64]. SP17 was preferentially expressed in breast cancer cell lines and primary breast tumours, including TNBC, compared to non-tumoural breast tissue [55]. Specific anti-SP17 antibodies were also discovered in patients’ sera, and the generation of SP17-specific, HLA class I-restricted, cytotoxic T-lymphocytes led to the death of breast cancer cells, opening the possibility that SP17 could be a valid target for TNBC immunotherapy [55].
3. Future Potential Application of CTAs Clinically
Multiple laboratories have utilised mIHC/IF to evaluate biomarkers in several cancers. Systematic review and meta-analysis of tumour patients from 8135 patients revealed that mIHC/IF had superior diagnostic accuracy in predicting clinical response to anti-PD-1/PD-L1 therapy than PD-L1 IHC, tumour mutational burden or gene expression profiling [65]. An optimised mIHC /IF protocol was developed for PD-L1 testing in TNBC which demonstrated good correlation with conventional IHC, thus providing further evidence for the feasibility of incorporating mIHC/IF in clinical practice [66]. A recent multisite study comparing mIF on PD-1/PD-L1 axis on tonsil and breast carcinoma and non-small cell lung cancer (NSCLC) demonstrated good reproducibility and sensitivity across multiple institutions which included Johns Hopkins University, Yale University, MD Anderson Cancer Center, Earle A. Chiles Research Institute, Akoya Biosciences and Bristol-Myers Squibb [67]. Standardisation, validation and reproducibility of end-to-end workflow across multiple sites and clinical laboratory processes are important to promote translation of mIHC/IF technology to clinical practice. The evidence from feasibility studies have been encouraging, and more research could be done in this area to translate emerging technologies to clinical practice. Recently, a high throughput system to analyse tissue microarrays of breast cancer samples using multiplexed microfluidic IHC to generate biomarker barcodes have been developed [68]. The biomarker barcode of breast cancer patient-derived tissue microarrays was compared to traditional method of breast cancer diagnosis, thus opening the possibility of high-throughput screening with diagnostic capability.
CTAs such as NY-ESO-1, MAGE, MSLN, SPANXB1, PRAME, ZNF165, TRIM27, KK-LC-1 , SP17 and WT-1 were found to be enriched in TNBC compared to non-TNBC tissue ( Table 1 ). The specific enrichment of CTAs opens the possibility of CTAs as targets for personalised treatments or for identifying subtypes of patients with better or worse prognosis. In addition, CTAs are immunogenic, thus providing opportunities for development of therapeutic vaccines and are attractive targets for immunotherapy. Given the growing evidence of their roles in TNBC, CTAs are potential targets for therapeutic intervention. Several clinical trials are currently being conducted to evaluate the effectiveness of CTAs, such as NY-ESO-1, MAGE, MSLN, PRAME, PSCA, ROR2 and WT1, as treatment targets in breast cancers and other solid tumours. CTAs are being widely developed as cancer vaccine, T-cell immunotherapy and antibody-based therapy in breast cancer including TNBC. Other CTAs are also evaluated in clinical trials as single therapy or combination therapy in other cancer types, highlighting the attractiveness of CTAs as potential therapeutic strategy for cancers [69].
Further research could be performed to analyse expressions of CTAs with different immune infiltrates and correlate with clinicopathological characteristics, given the growing evidence of CTA having immunogenic and oncogenic properties in TNBC and promoting tumourigenesis or invasiveness ( Table 1 ). Several CTAs are co-expressed in TNBC, and further research could be performed to delineate different CTAs molecular signatures and its prognostic value for TNBC clinically. More research could be performed to understand mechanistically the underlying biological and cellular functions of CTAs in TNBC, as well as the relationships between CTAs and hormone receptor or HER2 signalling pathways. Current evidence suggests that some CTAs reduced the effectiveness of responses to chemotherapy, and a detailed understanding of the important signalling pathways in TNBC could help in designing more efficacious drugs.
A key aspect for investigation is whether CTAs are abnormally expressed in breast tissue before malignancy occurs and if they could be used as potential biomarkers for TNBC. This would help clinicians to identify women at high risk for cancer development, thus improving screening protocols for the early detection of TNBC ( Figure 1 ).
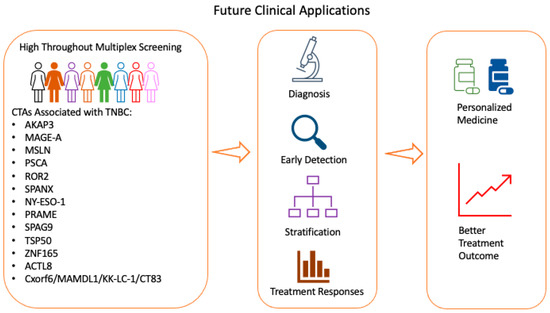
4. Conclusions
TNBC frequently expresses CTAs, and some CTA expression correlates with overall survival and prognosis. CTAs show biased expression in cancer and robust immunogenicity, thus serving as ideal targets for cancer immunotherapy. Multiple clinical trials have been conducted or are currently on-going to investigate the role of CTAs as treatment targets in advanced cancers, such as TBNC. Further research could be conducted to delineate the mechanism of action of CTAs in TNBC, increasing the efficacy of CTAs or in combination with other immunotherapies, identifying patients that would benefit most from the treatment and devising better drug delivery. With the collation of more data, CTAs may also be incorporated in routine screening protocols for TNBC.
References
- Thike, A.A.; Cheok, P.Y.; Jara-Lazaro, A.R.; Tan, B.; Tan, P.; Tan, P.H. Triple-negative breast cancer: Clinicopathological characteristics and relationship with basal-like breast cancer. Mod. Pathol. 2010, 23, 123–133.
- Badve, S.; Dabbs, D.J.; Schnitt, S.J.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Mod. Pathol. 2011, 24, 157–167.
- Teng, Y.H.; Thike, A.A.; Wong, N.S.; Tan, P.H. Therapeutic targets in triple negative breast cancer—Where are we now? Recent Pat. Anti Cancer Drug Discov. 2011, 6, 196–209.
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313, 165–173.
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948.
- Scanlan, M.J.; Gordon, C.M.; Williamson, B.; Lee, S.Y.; Chen, Y.T.; Stockert, E.; Jungbluth, A.; Ritter, G.; Jager, D.; Jager, E.; et al. Identification of cancer/testis genes by database mining and mRNA expression analysis. Int. J. Cancer 2002, 98, 485–492.
- Simpson, A.J.; Caballero, O.L.; Jungbluth, A.; Chen, Y.T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 2005, 5, 615–625.
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647.
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947.
- D’Angelo, S.P.; Melchiori, L.; Merchant, M.S.; Bernstein, D.; Glod, J.; Kaplan, R.; Grupp, S.; Tap, W.D.; Chagin, K.; Binder, G.K.; et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018, 8, 944–957.
- Almeida, L.G.; Sakabe, N.J.; deOliveira, A.R.; Silva, M.C.; Mundstein, A.S.; Cohen, T.; Chen, Y.T.; Chua, R.; Gurung, S.; Gnjatic, S.; et al. CTdatabase: A knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009, 37, D816–D819.
- Wang, C.; Gu, Y.; Zhang, K.; Xie, K.; Zhu, M.; Dai, N.; Jiang, Y.; Guo, X.; Liu, M.; Dai, J.; et al. Systematic identification of genes with a cancer-testis expression pattern in 19 cancer types. Nat. Commun. 2016, 7, 10499.
- Da Silva, V.L.; Fonseca, A.F.; Fonseca, M.; da Silva, T.E.; Coelho, A.C.; Kroll, J.E.; de Souza, J.E.S.; Stransky, B.; de Souza, G.A.; de Souza, S.J. Genome-wide identification of cancer/testis genes and their association with prognosis in a pan-cancer analysis. Oncotarget 2017, 8, 92966–92977.
- Masiakowski, P.; Carroll, R.D. A novel family of cell surface receptors with tyrosine kinase-like domain. J. Biol. Chem. 1992, 267, 26181–26190.
- Henry, C.; Quadir, A.; Hawkins, N.J.; Jary, E.; Llamosas, E.; Kumar, D.; Daniels, B.; Ward, R.L.; Ford, C.E. Expression of the novel Wnt receptor ROR2 is increased in breast cancer and may regulate both beta-catenin dependent and independent Wnt signalling. J. Cancer Res. Clin. Oncol. 2015, 141, 243–254.
- Esmaeili, R.; Majidzadeh, A.K.; Farahmand, L.; Ghasemi, M.; Salehi, M.; Khoshdel, A.R. AKAP3 correlates with triple negative status and disease free survival in breast cancer. BMC Cancer 2015, 15, 681.
- Sharma, S.; Qian, F.; Keitz, B.; Driscoll, D.; Scanlan, M.J.; Skipper, J.; Rodabaugh, K.; Lele, S.; Old, L.J.; Odunsi, K. A-kinase anchoring protein 3 messenger RNA expression correlates with poor prognosis in epithelial ovarian cancer. Gynecol. Oncol. 2005, 99, 183–188.
- Hamai, A.; Memeo, L.; Colarossi, C.; Canzonieri, V.; Perin, T.; Ayyoub, M.; Valmori, D. Expression of MAGE-A antigens is frequent in triple-negative breast cancers but does not correlate with that of basal-like markers. Ann. Oncol. 2011, 22, 986–987.
- Curigliano, G.; Viale, G.; Ghioni, M.; Jungbluth, A.A.; Bagnardi, V.; Spagnoli, G.C.; Neville, A.M.; Nole, F.; Rotmensz, N.; Goldhirsch, A. Cancer-testis antigen expression in triple-negative breast cancer. Ann. Oncol. 2011, 22, 98–103.
- Grigoriadis, A.; Caballero, O.L.; Hoek, K.S.; da Silva, L.; Chen, Y.T.; Shin, S.J.; Jungbluth, A.A.; Miller, L.D.; Clouston, D.; Cebon, J.; et al. CT-X antigen expression in human breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 13493–13498.
- Chen, Y.T.; Ross, D.S.; Chiu, R.; Zhou, X.K.; Chen, Y.Y.; Lee, P.; Hoda, S.A.; Simpson, A.J.; Old, L.J.; Caballero, O.; et al. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS ONE 2011, 6, e17876.
- Karn, T.; Pusztai, L.; Ruckhaberle, E.; Liedtke, C.; Muller, V.; Schmidt, M.; Metzler, D.; Wang, J.; Coombes, K.R.; Gatje, R.; et al. Melanoma antigen family A identified by the bimodality index defines a subset of triple negative breast cancers as candidates for immune response augmentation. Eur. J. Cancer 2012, 48, 12–23.
- Ayyoub, M.; Scarlata, C.M.; Hamai, A.; Pignon, P.; Valmori, D. Expression of MAGE-A3/6 in primary breast cancer is associated with hormone receptor negative status, high histologic grade, and poor survival. J. Immunother. 2014, 37, 73–76.
- Yang, F.; Zhou, X.; Miao, X.; Zhang, T.; Hang, X.; Tie, R.; Liu, N.; Tian, F.; Wang, F.; Yuan, J. MAGEC2, an epithelial-mesenchymal transition inducer, is associated with breast cancer metastasis. Breast Cancer Res. Treat. 2014, 145, 23–32.
- Badovinac Crnjevic, T.; Spagnoli, G.; Juretic, A.; Jakic-Razumovic, J.; Podolski, P.; Saric, N. High expression of MAGE-A10 cancer-testis antigen in triple-negative breast cancer. Med. Oncol. 2012, 29, 1586–1591.
- Raghavendra, A.; Kalita-de Croft, P.; Vargas, A.C.; Smart, C.E.; Simpson, P.T.; Saunus, J.M.; Lakhani, S.R. Expression of MAGE-A and NY-ESO-1 cancer/testis antigens is enriched in triple-negative invasive breast cancers. Histopathology 2018, 73, 68–80.
- Zhao, Q.; Xu, W.T.; Shalieer, T. Pilot Study on MAGE-C2 as a Potential Biomarker for Triple-Negative Breast Cancer. Dis. Markers 2016, 2016, 2325987.
- Wang, H.; Sang, M.; Geng, C.; Liu, F.; Gu, L.; Shan, B. MAGE-A is frequently expressed in triple negative breast cancer and associated with epithelial-mesenchymal transition. Neoplasma 2016, 63, 44–56.
- Tessari, A.; Pilla, L.; Silvia, D.; Duca, M.; Paolini, B.; Carcangiu, M.L.; Mariani, L.; de Braud, F.G.; Cresta, S. Expression of NY-ESO-1, MAGE-A3, PRAME and WT1 in different subgroups of breast cancer: An indication to immunotherapy? Breast 2018, 42, 68–73.
- Mrklic, I.; Spagnoli, G.C.; Juretic, A.; Pogorelic, Z.; Tomic, S. Co-expression of cancer testis antigens and topoisomerase 2-alpha in triple negative breast carcinomas. Acta Histochem. 2014, 116, 740–746.
- Cabezon, T.; Gromova, I.; Gromov, P.; Serizawa, R.; Timmermans Wielenga, V.; Kroman, N.; Celis, J.E.; Moreira, J.M. Proteomic profiling of triple-negative breast carcinomas in combination with a three-tier orthogonal technology approach identifies Mage-A4 as potential therapeutic target in estrogen receptor negative breast cancer. Mol. Cell. Proteom. 2013, 12, 381–394.
- Tchou, J.; Wang, L.C.; Selven, B.; Zhang, H.; Conejo-Garcia, J.; Borghaei, H.; Kalos, M.; Vondeheide, R.H.; Albelda, S.M.; June, C.H.; et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res. Treat. 2012, 133, 799–804.
- Tozbikian, G.; Brogi, E.; Kadota, K.; Catalano, J.; Akram, M.; Patil, S.; Ho, A.Y.; Reis-Filho, J.S.; Weigelt, B.; Norton, L.; et al. Mesothelin expression in triple negative breast carcinomas correlates significantly with basal-like phenotype, distant metastases and decreased survival. PLoS ONE 2014, 9, e114900.
- Parinyanitikul, N.; Blumenschein, G.R.; Wu, Y.; Lei, X.; Chavez-Macgregor, M.; Smart, M.; Gonzalez-Angulo, A.M. Mesothelin expression and survival outcomes in triple receptor negative breast cancer. Clin. Breast Cancer 2013, 13, 378–384.
- Link, T.; Kuithan, F.; Ehninger, A.; Kuhlmann, J.D.; Kramer, M.; Werner, A.; Gatzweiler, A.; Richter, B.; Ehninger, G.; Baretton, G.; et al. Exploratory investigation of PSCA-protein expression in primary breast cancer patients reveals a link to HER2/neu overexpression. Oncotarget 2017, 8, 54592–54603.
- Meng, F.; Liu, B.; Xie, G.; Song, Y.; Zheng, X.; Qian, X.; Li, S.; Jia, H.; Zhang, X.; Zhang, L.; et al. Amplification and overexpression of PSCA at 8q24 in invasive micropapillary carcinoma of breast. Breast Cancer Res. Treat. 2017, 166, 383–392.
- Kannan, A.; Philley, J.V.; Hertweck, K.L.; Ndetan, H.; Singh, K.P.; Sivakumar, S.; Wells, R.B.; Vadlamudi, R.K.; Dasgupta, S. Cancer Testis Antigen Promotes Triple Negative Breast Cancer Metastasis and is Traceable in the Circulating Extracellular Vesicles. Sci. Rep. 2019, 9, 11632.
- Maine, E.A.; Westcott, J.M.; Prechtl, A.M.; Dang, T.T.; Whitehurst, A.W.; Pearson, G.W. The cancer-testis antigens SPANX-A/C/D and CTAG2 promote breast cancer invasion. Oncotarget 2016, 7, 14708–14726.
- Ademuyiwa, F.O.; Bshara, W.; Attwood, K.; Morrison, C.; Edge, S.B.; Karpf, A.R.; James, S.A.; Ambrosone, C.B.; O’Connor, T.L.; Levine, E.G.; et al. NY-ESO-1 cancer testis antigen demonstrates high immunogenicity in triple negative breast cancer. PLoS ONE 2012, 7, e38783.
- Bandic, D.; Juretic, A.; Sarcevic, B.; Separovic, V.; Kujundzic-Tiljak, M.; Hudolin, T.; Spagnoli, G.C.; Covic, D.; Samija, M. Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: Retrospective immunohistochemical study. Croat. Med. J. 2006, 47, 32–41.
- Lee, H.J.; Kim, J.Y.; Song, I.H.; Park, I.A.; Yu, J.H.; Gong, G. Expression of NY-ESO-1 in Triple-Negative Breast Cancer Is Associated with Tumor-Infiltrating Lymphocytes and a Good Prognosis. Oncology 2015, 89, 337–344.
- Curigliano, G.; Bagnardi, V.; Ghioni, M.; Louahed, J.; Brichard, V.; Lehmann, F.F.; Marra, A.; Trapani, D.; Criscitiello, C.; Viale, G. Expression of tumor-associated antigens in breast cancer subtypes. Breast 2020, 49, 202–209.
- Epping, M.T.; Hart, A.A.; Glas, A.M.; Krijgsman, O.; Bernards, R. PRAME expression and clinical outcome of breast cancer. Br. J. Cancer 2008, 99, 398–403.
- Yao, J.; Caballero, O.L.; Yung, W.K.; Weinstein, J.N.; Riggins, G.J.; Strausberg, R.L.; Zhao, Q. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol. Res. 2014, 2, 371–379.
- Al-Khadairi, G.; Naik, A.; Thomas, R.; Al-Sulaiti, B.; Rizly, S.; Decock, J. PRAME promotes epithelial-to-mesenchymal transition in triple negative breast cancer. J. Transl. Med. 2019, 17, 9.
- Kanojia, D.; Garg, M.; Gupta, S.; Gupta, A.; Suri, A. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 630–639.
- Sinha, A.; Agarwal, S.; Parashar, D.; Verma, A.; Saini, S.; Jagadish, N.; Ansari, A.S.; Lohiya, N.K.; Suri, A. Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells: Possible implications in targeted therapy. J. Exp. Clin. Cancer Res. 2013, 32, 69.
- Song, Z.B.; Ni, J.S.; Wu, P.; Bao, Y.L.; Liu, T.; Li, M.; Fan, C.; Zhang, W.J.; Sun, L.G.; Huang, Y.X.; et al. Testes-specific protease 50 promotes cell invasion and metastasis by increasing NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell Death Dis. 2015, 6, e1703.
- Wang, M.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Meng, X.Y.; Huang, Y.X.; Sun, Y.; Zheng, L.H.; Li, Y.X. Basic FGF downregulates TSP50 expression via the ERK/Sp1 pathway. J. Cell. Biochem. 2010, 111, 75–81.
- Maxfield, K.E.; Taus, P.J.; Corcoran, K.; Wooten, J.; Macion, J.; Zhou, Y.; Borromeo, M.; Kollipara, R.K.; Yan, J.; Xie, Y.; et al. Comprehensive functional characterization of cancer-testis antigens defines obligate participation in multiple hallmarks of cancer. Nat. Commun. 2015, 6, 8840.
- Gibbs, Z.A.; Reza, L.C.; Cheng, C.C.; Westcott, J.M.; McGlynn, K.; Whitehurst, A.W. The testis protein ZNF165 is a SMAD3 cofactor that coordinates oncogenic TGFbeta signaling in triple-negative breast cancer. eLife 2020, 9.
- Kaufmann, J.; Wentzensen, N.; Brinker, T.J.; Grabe, N. Large-scale in-silico identification of a tumor-specific antigen pool for targeted immunotherapy in triple-negative breast cancer. Oncotarget 2019, 10, 2515–2529.
- Paret, C.; Simon, P.; Vormbrock, K.; Bender, C.; Kolsch, A.; Breitkreuz, A.; Yildiz, O.; Omokoko, T.; Hubich-Rau, S.; Hartmann, C.; et al. CXorf61 is a target for T cell based immunotherapy of triple-negative breast cancer. Oncotarget 2015, 6, 25356–25367.
- Kondo, Y.; Fukuyama, T.; Yamamura, R.; Futawatari, N.; Ichiki, Y.; Tanaka, Y.; Nishi, Y.; Takahashi, Y.; Yamazaki, H.; Kobayashi, N.; et al. Detection of KK-LC-1 Protein, a Cancer/Testis Antigen, in Patients with Breast Cancer. Anticancer. Res. 2018, 38, 5923–5928.
- Mirandola, L.; Pedretti, E.; Figueroa, J.A.; Chiaramonte, R.; Colombo, M.; Chapman, C.; Grizzi, F.; Patrinicola, F.; Kast, W.M.; Nguyen, D.D.; et al. Cancer testis antigen Sperm Protein 17 as a new target for triple negative breast cancer immunotherapy. Oncotarget 2017, 8, 74378–74390.
- Li, Y.; Li, J.; Wang, Y.; Zhang, Y.; Chu, J.; Sun, C.; Fu, Z.; Huang, Y.; Zhang, H.; Yuan, H.; et al. Roles of cancer/testis antigens (CTAs) in breast cancer. Cancer Lett. 2017, 399, 64–73.
- Grizzi, F.; Gaetani, P.; Franceschini, B.; Di Ieva, A.; Colombo, P.; Ceva-Grimaldi, G.; Bollati, A.; Frezza, E.E.; Cobos, E.; Rodriguez y Baena, R.; et al. Sperm protein 17 is expressed in human nervous system tumours. BMC Cancer 2006, 6, 23.
- Gupta, G.; Sharma, R.; Chattopadhyay, T.K.; Gupta, S.D.; Ralhan, R. Clinical significance of sperm protein 17 expression and immunogenicity in esophageal cancer. Int. J. Cancer 2007, 120, 1739–1747.
- Lim, S.H.; Wang, Z.; Chiriva-Internati, M.; Xue, Y. Sperm protein 17 is a novel cancer-testis antigen in multiple myeloma. Blood 2001, 97, 1508–1510.
- Nakazato, T.; Kanuma, T.; Tamura, T.; Faried, L.S.; Aoki, H.; Minegishi, T. Sperm protein 17 influences the tissue-specific malignancy of clear cell adenocarcinoma in human epithelial ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 426–432.
- Bumm, K.; Grizzi, F.; Franceschini, B.; Koch, M.; Iro, H.; Wurm, J.; Ceva-Grimaldi, G.; Dimmler, A.; Cobos, E.; Dioguardi, N.; et al. Sperm protein 17 expression defines 2 subsets of primary esthesioneuroblastoma. Hum. Pathol. 2005, 36, 1289–1293.
- Chiriva-Internati, M. Sperm protein 17: Clinical relevance of a cancer/testis antigen, from contraception to cancer immunotherapy, and beyond. Int. Rev. Immunol. 2011, 30, 138–149.
- Lacy, H.M.; Sanderson, R.D. Sperm protein 17 is expressed on normal and malignant lymphocytes and promotes heparan sulfate-mediated cell-cell adhesion. Blood 2001, 98, 2160–2165.
- Li, F.Q.; Han, Y.L.; Liu, Q.; Wu, B.; Huang, W.B.; Zeng, S.Y. Overexpression of human sperm protein 17 increases migration and decreases the chemosensitivity of human epithelial ovarian cancer cells. BMC Cancer 2009, 9, 323.
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1195–1204.
- Yeong, J.; Tan, T.; Chow, Z.L.; Cheng, Q.; Lee, B.; Seet, A.; Lim, J.X.; Lim, J.C.T.; Ong, C.C.H.; Thike, A.A.; et al. Multiplex immunohistochemistry/immunofluorescence (mIHC/IF) for PD-L1 testing in triple-negative breast cancer: A translational assay compared with conventional IHC. J. Clin. Pathol. 2020, 73, 557–562.
- Taube, J.M.; Roman, K.; Engle, E.L.; Wang, C.; Ballesteros-Merino, C.; Jensen, S.M.; McGuire, J.; Jiang, M.; Coltharp, C.; Remeniuk, B.; et al. Multi-institutional TSA-amplified Multiplexed Immunofluorescence Reproducibility Evaluation (MITRE) Study. J. Immunother. Cancer 2021, 9.
- Cho, C.H.; Cho, M.; Park, J.K. Biomarker barcodes: Multiplexed microfluidic immunohistochemistry enables high-throughput analysis of tissue microarray. Lab Chip 2021.
- Li, X.F.; Ren, P.; Shen, W.Z.; Jin, X.; Zhang, J. The expression, modulation and use of cancer-testis antigens as potential biomarkers for cancer immunotherapy. Am. J. Transl. Res. 2020, 12, 7002–7019.




