
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dimitrios Kontogiannatos | + 1055 word(s) | 1055 | 2021-11-23 05:04:14 | | | |
| 2 | Dimitrios Kontogiannatos | Meta information modification | 1055 | 2021-11-30 16:24:19 | | | | |
| 3 | Jessie Wu | Meta information modification | 1055 | 2021-12-03 09:27:08 | | |
Video Upload Options
Cordyceps militaris is an entomopathogenic ascomycete with similar pharmacological importance to that of the wild caterpillar fungus Ophiocordyceps sinensis C. militaris has attracted significant research and commercial interest due to its content in bioactive compounds beneficial to human health and the relative ease of cultivation under laboratory conditions.
1. Introduction
C. militaris has attracted significant research and commercial interest due to its content in bioactive compounds beneficial to human health and the relative ease of cultivation under laboratory conditions.Cordyceps is a large and diverse genus of the family Cordycipitaceae (Hypocreales, Ascomycota) comprising 627 species according to MycoBank (https://www.mycobank.org; 12 November 2021). They are parasitic fungi, mostly endoparasitoids of insects and other arthropods, while some of them are parasitic on other fungi [1]. Cordyceps was named after the Greek word “kordyle” meaning “club”, and the Latin stem “-ceps” meaning “head” [2]. Most species grow on larvae, pupae, or adults of insects of the orders Arachnida, Coleoptera, Lepidoptera, Hymenoptera, Hemiptera, Orthoptera, Diptera, and Isoptera [3]. Cordyceps militaris (L.) Fr. is a common endoparasitoid of insects, usually growing on pupae and less commonly found on larvae, and is widely distributed in North and South America, Europe, and Asia. Fruit bodies (ascomata) are variable in form and especially in size; this variability is associated with the type of food source(s) available for fungal growth, while it is also affected by the size of the host and the number of stromata formed [3].C. militaris mycelia and ascomata can be generated ex situ with or without the addition of alive and/or dead insect tissues. A wide range of methodologies to produce fruit bodies and mycelia of C. militaris are reported in pertinent literature including: (a) solid-state fermentation [4][5][6][7], (b) submerged static fermentation [6][8], (c) repeated batch or one-step non-static fermentation in liquid media [9][10][11], and (d) cultivation following larval or pupal infection [12][13].
2. Substrates, Nutrient Requirements and Treatments Related with the Cultivation of C. militaris
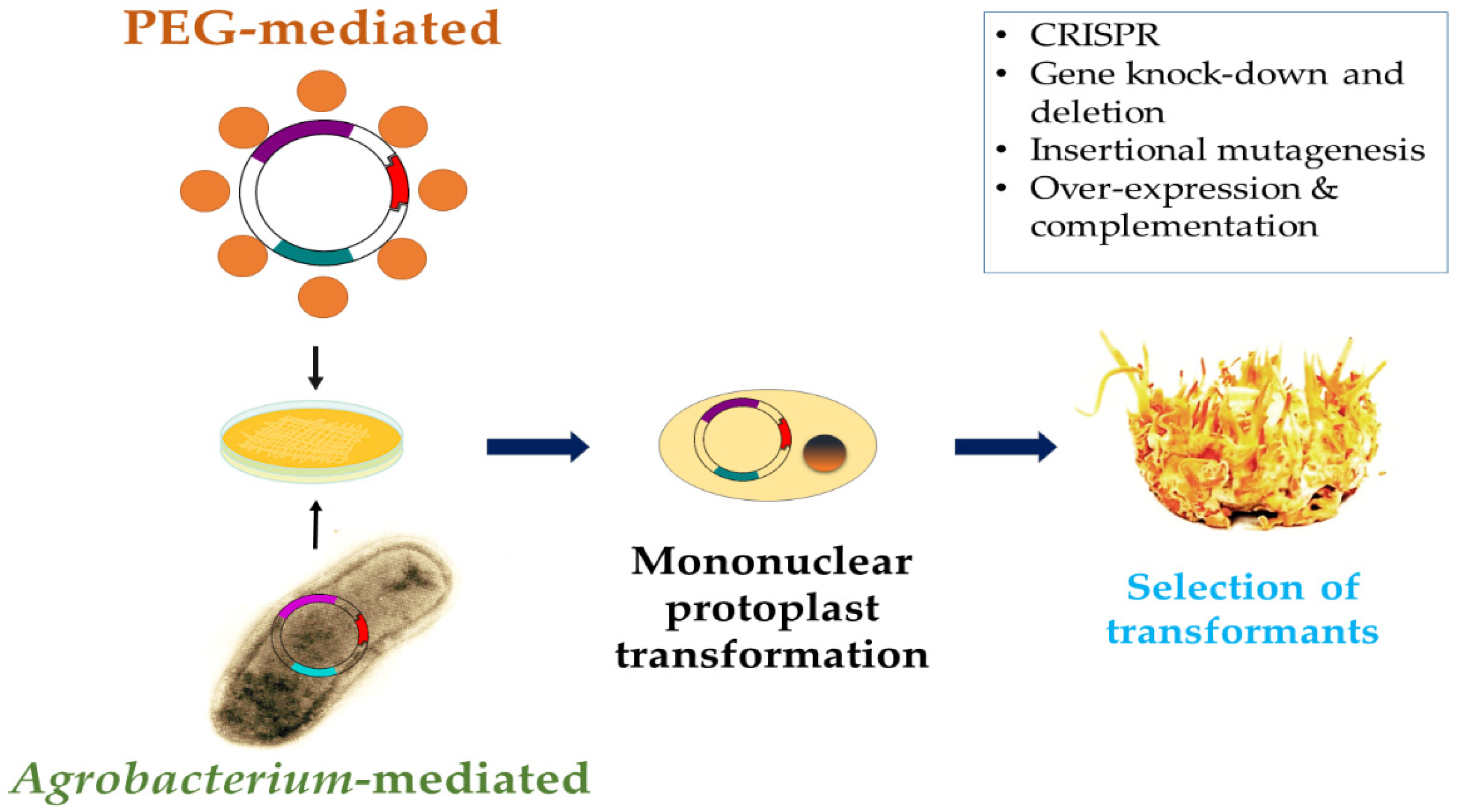
3. Genetics, Genomics, and Genetic Engineering of C. militaris
4. Molecular, Nutritional, and Environmental Aspects of Cordycepin Biosynthesis and Ascomata Formation
C. militaris produces a large number of bioactive metabolites, including ergothioneine, ergosterol, adenosine, polysaccharides, and cordycepin [9][20][21][22][23]. Among them, cordycepin exhibits a large range of health beneficial effects, such as broad-spectrum antibiotic activity and the ability to inhibit cell proliferation and to induce cell apoptosis, as well as having antioxidant, anticancer, and anti-inflammatory properties [24][25][26][27][28]. It is currently considered to be one of the most promising fungal compounds with respect to its pharmacological and therapeutic potential; therefore, the study of aspects related to cordycepin biosynthesis and ascomata production is essential to the commercial exploitation of this species. The basic metabolic route for cordycepin biosynthesis has been recently explored using a combination of in silico analyses and functional genomics approaches [29]. Four genes for cordycepin synthetase were identified in C. militaris genome and were designated as cns1–cns4; these genes contain different conserved domains, such as the oxidoreductase/dehydrogenase domain in Cns1, the HDc family of metal-dependent phosphohydrolase domain in Cns2, and the N-terminal nucleoside/nucleotide kinase (NK) and C-terminal HisG domains in Cns3, while Cns4 is a putative ATP-binding cassette type of transporter. Using Agrobacterium-mediated single and joint gene deletion mutants of the aforementioned genes, Xia et al. [29] demonstrated that Cns1 and Cns2 are required for cordycepin biosynthesis, while Cns3 is implicated in the biosynthesis of an additional “safeguard” molecule, i.e., pentostatin. In addition, the same authors reported that cordycepin biosynthesis (starting from adenosine and proceeding through the stepwise reactions of phosphorylation, dephosphorylation, and reduction) is catalyzed by the Cns1/Cns2 complex in parallel with the biosynthesis of pentostatin. This dual production is similar to the bacterial “protector-protégé strategy” of purine metabolism in which the safeguarded cordycepin can be deaminated to 30-deoxyinosine once the former reaches a self-toxic level in fungal cells [29].
5. Conclusions
The ethnopharmacological importance of C. militaris has been widely analyzed in the past by many researchers [2][5][8][30][31][32][33] since this fungus is considered to be a valuable source of metabolites that act directly on various human metabolic pathways. For example, the methanolic extract of C. militaris presented antioxidant, antibacterial, antifungal, and antiproliferative properties in different human tumor cell lines [31], while cordycepin, the major bioactive compound of C. militaris, presented potent anti-inflammatory, anticancer, anti-metastatic, and immune-modulatory activities [32][33][34][35][36]. Although C. militaris can be easily cultivated in rice-based media, particular requirements related to the physiology and genetic makeup of this fungus impose serious obstacles in commercial applications. Hence, strain degeneration can completely hinder fruit body production at a commercial-scale, while improper cultivation techniques can downgrade metabolite production. Because of the entomopathogenic nature of C. militaris, the use of insect-based substrates in large-scale cultivation projects must be taken into consideration. During the past few years, insect mass production systems have become a contemporary trend in organic waste treatment and biotransformation [37], while the insect-based pet feed industry is growing exponentially. Considering the increasing availability of such by-products, the cultivation practices of C. militaris could exploit this type of waste material in the frame of the circular economy concept.
References
- Nikoh, N.; Fukatsu, T. Interkingdom Host Jumping Underground: Phylogenetic Analysis of Entomoparasitic Fungi of the Genus Cordyceps. Mol. Biol. Evol. 2000, 17, 629–638.
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The Genus Cordyceps: An Extensive Review of Its Traditional Uses, Phytochemistry and Pharmacology. Fitoterapia 2018, 129, 293–316.
- Mains, E.B. North American Entomogenous Species of Cordyceps. Mycologia 1958, 50, 169–222.
- Rao, Y.K.; Fang, S.-H.; Wu, W.-S.; Tzeng, Y.-M. Constituents Isolated from Cordyceps militaris Suppress Enhanced Inflammatory Mediator’s Production and Human Cancer Cell Proliferation. J. Ethnopharmacol. 2010, 131, 363–367.
- Chiu, C.-P.; Liu, S.-C.; Tang, C.-H.; Chan, Y.; El-Shazly, M.; Lee, C.-L.; Du, Y.-C.; Wu, T.-Y.; Chang, F.-R.; Wu, Y.-C. Anti-Inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548.
- Lee, J.; Cho, K.; Shin, S.G.; Bae, H.; Koo, T.; Han, G.; Hwang, S. Nutrient Recovery of Starch Processing Waste to Cordyceps militaris: Solid State Cultivation and Submerged Liquid Cultivation. Appl. Biochem. Biotechnol. 2016, 180, 274–288.
- Lin, Q.; Long, L.; Wu, L.; Zhang, F.; Wu, S.; Zhang, W.; Sun, X. Evaluation of Different Agricultural Wastes for the Production of Fruiting Bodies and Bioactive Compounds by Medicinal Mushroom Cordyceps militaris. J. Sci. Food Agric. 2017, 97, 3476–3480.
- Cui, J.D. Biotechnological Production and Applications of Cordyceps militaris, a Valued Traditional Chinese Medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484.
- Zeng, Y.; Han, Z.; Qiu, P.; Zhou, Z.; Tang, Y.; Zhao, Y.; Zheng, S.; Xu, C.; Zhang, X.; Yin, P. Salinity-Induced Anti-Angiogenesis Activities and Structural Changes of the Polysaccharides from Cultured Cordyceps militaris. PLoS ONE 2014, 9, e103880.
- Tang, J.; Qian, Z.; Wu, H. Enhancing Cordycepin Production in Liquid Static Cultivation of Cordyceps militaris by Adding Vegetable Oils as the Secondary Carbon Source. Bioresour. Technol. 2018, 268, 60–67.
- Wang, C.-C.; Wu, J.-Y.; Chang, C.-Y.; Yu, S.-T.; Liu, Y.-C. Enhanced Exopolysaccharide Production by Cordyceps militaris Using Repeated Batch Cultivation. J. Biosci. Bioeng. 2019, 127, 499–505.
- Guo, M.; Guo, S.; Huaijun, Y.; Bu, N.; Dong, C. Comparison of Major Bioactive Compounds of the Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes), Fruiting Bodies Cultured on Wheat Substrate and Pupae. Int. J. Med. Mushrooms 2016, 18, 327–336.
- Shi, K.; Yang, G.; He, L.; Yang, B.; Li, Q.; Yi, S. Purification, Characterization, Antioxidant, and Antitumor Activity of Polysaccharides Isolated from Silkworm Cordyceps. J. Food Biochem. 2020, 44, e13482.
- Lou, H.; Ye, Z.; Yun, F.; Lin, J.; Guo, L.; Chen, B.; Mu, Z. Targeted Gene Deletion in Cordyceps militaris Using the Split-Marker Approach. Mol. Biotechnol. 2018, 60, 380–385.
- Lou, H.-W.; Ye, Z.-W.; Yu, Y.-H.; Lin, J.-F.; Guo, L.-Q.; Chen, B.-X.; Tang, H.-B.; Wei, T.; Chen, L.-T.; Yun, F. The Efficient Genetic Transformation of Cordyceps militaris by Using Mononuclear Protoplasts. Sci. Hortic. 2019, 243, 307–313.
- Zheng, Z.; Huang, C.; Cao, L.; Xie, C.; Han, R. Agrobacterium tumefaciens-Mediated Transformation as a Tool for Insertional Mutagenesis in Medicinal Fungus Cordyceps militaris. Fungal Biol. 2011, 115, 265–274.
- Lou, H.-W.; Zhao, Y.; Chen, B.-X.; Yu, Y.-H.; Tang, H.-B.; Ye, Z.-W.; Lin, J.-F.; Guo, L.-Q. Cmfhp Gene Mediates Fruiting Body Development and Carotenoid Production in Cordyceps militaris. Biomolecules 2020, 10, 410.
- Lu, Y.; Xia, Y.; Luo, F.; Dong, C.; Wang, C. Functional Convergence and Divergence of Mating-Type Genes Fulfilling in Cordyceps militaris. Fungal Genet. Biol. 2016, 88, 35–43.
- Chen, B.-X.; Wei, T.; Ye, Z.-W.; Yun, F.; Kang, L.-Z.; Tang, H.-B.; Guo, L.-Q.; Lin, J.-F. Efficient CRISPR-Cas9 Gene Disruption System in Edible-Medicinal Mushroom Cordyceps militaris. Front. Microbiol. 2018, 9, 1157.
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical Composition and Nutritional and Medicinal Value of Fruit Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291.
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a Metabolic Product Isolated from Cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949.
- Paterson, R.R.M. Cordyceps—A Traditional Chinese Medicine and Another Fungal Therapeutic Biofactory? Phytochemistry 2008, 69, 1469–1495.
- Zhang, Q.; Liu, Y. The Strategies for Increasing Cordycepin Production of Cordyceps militaris by Liquid Fermentation. Fungal Genom. Biol. 2016, 6, 134.
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.-J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I. Cordycepin Inhibits Lipopolysaccharide-Induced Inflammation by the Suppression of NF-ΚB through Akt and P38 Inhibition in RAW 264.7 Macrophage Cells. Eur. J. Pharmacol. 2006, 545, 192–199.
- Noh, E.-M.; Kim, J.-S.; Hur, H.; Park, B.-H.; Song, E.-K.; Han, M.-K.; Kwon, K.-B.; Yoo, W.-H.; Shim, I.-K.; Lee, S.J. Cordycepin Inhibits IL-1β-Induced MMP-1 and MMP-3 Expression in Rheumatoid Arthritis Synovial Fibroblasts. Rheumatology 2009, 48, 45–48.
- Tian, X.; Li, Y.; Shen, Y.; Li, Q.; Wang, Q.; Feng, L. Apoptosis and Inhibition of Proliferation of Cancer Cells Induced by Cordycepin. Oncol. Lett. 2015, 10, 595–599.
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and Therapeutic Potential of Cordyceps with Special Reference to Cordycepin. 3 Biotech 2014, 4, 1–12.
- Zhou, X.; Cai, G.; He, Y.I.; Tong, G. Separation of Cordycepin from Cordyceps militaris Fermentation Supernatant Using Preparative HPLC and Evaluation of Its Antibacterial Activity as an NAD+-Dependent DNA Ligase Inhibitor. Exp. Ther. Med. 2016, 12, 1812–1816.
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal Cordycepin Biosynthesis Is Coupled with the Production of the Safeguard Molecule Pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e4.
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal Uses of the Mushroom Cordyceps militaris: Current State and Prospects. Fitoterapia 2010, 81, 961–968.
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. The Methanolic Extract of Cordyceps militaris (L.) Link Fruiting Body Shows Antioxidant, Antibacterial, Antifungal and Antihuman Tumor Cell Lines Properties. Food Chem. Toxicol. 2013, 62, 91–98.
- Chen, Y.-C.; Chen, Y.-H.; Pan, B.-S.; Chang, M.-M.; Huang, B.-M. Functional Study of Cordyceps sinensis and Cordycepin in Male Reproduction: A Review. J. Food Drug Anal. 2017, 25, 197–205.
- Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. Enrichment of Cordycepin for Cosmeceutical Applications: Culture Systems and Strategies. Appl. Microbiol. Biotechnol. 2019, 103, 1681–1691.
- Lee, C.-T.; Huang, K.-S.; Shaw, J.-F.; Chen, J.-R.; Kuo, W.-S.; Shen, G.; Grumezescu, A.M.; Holban, A.M.; Wang, Y.-T.; Wang, J.-S. Trends in the Immunomodulatory Effects of Cordyceps militaris: Total Extracts, Polysaccharides and Cordycepin. Front. Pharmacol. 2020, 11, 1824.
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and Antimetastatic Effects of Cordycepin, an Active Component of Cordyceps sinensis. J. Pharmacol. Sci. 2015, 127, 53–56.
- Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Properties of Cordycepin and Their Underlying Mechanisms. Int. J. Mol. Sci. 2018, 19, 3027.
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91.




