Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Nawrot | + 1598 word(s) | 1598 | 2021-11-23 08:54:53 | | | |
| 2 | Peter Tang | Meta information modification | 1598 | 2021-11-30 02:38:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nawrot, R.; Gracz-Bernaciak, J.; Mazur, O. Diversity and Role of Latex in Plant Physiology. Encyclopedia. Available online: https://encyclopedia.pub/entry/16530 (accessed on 05 March 2026).
Nawrot R, Gracz-Bernaciak J, Mazur O. Diversity and Role of Latex in Plant Physiology. Encyclopedia. Available at: https://encyclopedia.pub/entry/16530. Accessed March 05, 2026.
Nawrot, Robert, Joanna Gracz-Bernaciak, Oliwia Mazur. "Diversity and Role of Latex in Plant Physiology" Encyclopedia, https://encyclopedia.pub/entry/16530 (accessed March 05, 2026).
Nawrot, R., Gracz-Bernaciak, J., & Mazur, O. (2021, November 30). Diversity and Role of Latex in Plant Physiology. In Encyclopedia. https://encyclopedia.pub/entry/16530
Nawrot, Robert, et al. "Diversity and Role of Latex in Plant Physiology." Encyclopedia. Web. 30 November, 2021.
Copy Citation
Latex, a sticky emulsion produced by specialized cells called laticifers, is a crucial part of a plant’s defense system against herbivory and pathogens. It consists of a broad spectrum of active compounds, which are beneficial not only for plants, but for human health as well, enough to mention the use of morphine or codeine from poppy latex.
latex
antiviral proteins
antimicrobial compounds
laticifers
latex-bearing plants
defense-related proteins
low-molecular compiunds
alkaloids
CRISPR/Cas9
Chelidonium majus
1. Introduction
Latex-bearing plants have a long history of benefiting human health and medicinal use in many different regions and cultures all over the world. Recent research suggests that the opium poppy (Papaver somniferum L.) was already in the process of domestication at the end of 4th millennium BC [1] and early domesticated ancestors of Cannabis sativa L. diverged ~10,000 years BC [2]. Those are two leading examples of laticiferous plant species used for therapies and together with Hevea brasiliensis Muli. Arg., which is the main and irreplaceable natural rubber source, have the best known and described latex composition. These complex fluids consist of different secondary metabolites, like terpenes, alkaloids, or phenolics, and jointly with a broad range of proteins are the first line of plant herbivore defense system. Another extensively studied laticiferous medicinal plant is Greater Celandine (Chelidonium majus L.), a relative of the opium poppy, which is a rich source of numerous biologically active compounds, used in traditional folk medicine as antiviral, antibacterial, antifungal, choleretic, and anticancer agents [3][4][5][6]. Many compounds of latex are active in both eukaryotic and prokaryotic organisms [7]. At present, when mankind is running out of antibiotics and other antimicrobial compounds, new cancer therapies are still needed, and to make matters worse, the scale of pest and microbial resistance is increasing, exploration of such rich natural deposits of active molecules is a very promising research direction.
2. Diversity and Role of Latex in Plant Physiology
Latex is a milky emulsion produced by complex secretory structures called laticifers. It is defined as a suspension of various particles (organic and inorganic) dispersed in a liquid with different refractive index. Depending on prevalent content and plant species studied, it can be milky white or yellowish, orange to brown or even colorless. However, it is more than a liquid. It is identified as a laticifer’s protoplast with mitochondria, plastids, endoplasmic reticulum, Golgi bodies, polyribosomes, and vacuoles [8]. Laticifers are latex-producing, highly specialized plant cells or connected cells, which are spread through the whole plant body in the form of linear tubes which can grow and elongate with plant organs. They can be found in almost every part of latescent plants, namely in the root, stem, leaf, sepal, petal, stamen, ovary, and stigma, or they can occur only in some particular organs. Latex occupies the whole volume of the laticifer system [9].
Latex was identified in at least 20,000 plant species belonging to 43 families of vascular plants. Most of them are Angiosperms (41 families), one family belongs to ferns, and one to gymnosperms [8][9][10]. In a great example of convergent evolution events in the plant kingdom, latex occurrences take place several times in phylogenetically unrelated orders. Laticifers developed in both monocotyledonous and dicotyledonous, in the basal clades (Ana-grade), magnoliids, monocots, basal eudicots, rosids, and asterides [11][12]. One morphotype of laticifers, articulated, which are fused chains of cells with intact, porous, or even absent terminal walls, form laticiferous vessels and were recorded in 27 families. The other main morphotype is called non-articulated, which occurs more rarely and is formed by a single plant cell with almost infinite growth potential. Both types of laticifers can extend not only longitudinally with the growth of organs, but also radically create branched networks of tubes. For the proper classification of the laticifer system, it is essential to use plant material with embryos or meristems. Only based on ontology can articulated and non-articulated morphotypes be distinguished (analysis of number of precursor cells and phase of laticifers development often coupled with analysis of laticifers enzymatic activity of pectinases and chitinases, with the latter active only in articulated laticifers [13]). Previously, cases of incorrect assignment to the appropriate laticifer types were described, e.g., for mulberry [14][15] or for Ficus montana Burm.f. and Maclura tinctoria L. [13]. Attempts were made to use types of laticifers as a diagnostic tool for some taxa, but it is more likely that different morphotypes will be found in different species within the same family [11].
Nowadays, it is well established that the biological role of latex is plant defense against herbivores and pathogens (bacteria, fungi, viruses) [10], but in 1989 Webster and Baulk concluded that the function of latex was unknown [16]. Many latex metabolites are stored within large vacuoles and are released after being physically damaged at the site of injury. Some of them act as toxic and dissuasive components. After mechanical disruption of plant tissue, latex is immediately released and is the first line of plant defense. Thanks to its inherent stickiness and coagulation properties, latex forms a barrier against pathogen invasion. Moreover, latex’s rapid coagulation and high viscosity can restrict herbivore movements, as well as immobilize mouthparts and other sense organs [17]. This strategy gives an advantage to latescent plants, especially in environments with high a herbivory rate, like tropical or subtropical forests [18].
3. Main Components of Latex
Taking into consideration the defense role of latex in plant development, it should not be surprising how complex and diverse the latex composition can be. Despite tremendous variability in latex components, which is dependent on the species, phase of development, external and internal stimuli, and stresses [19], two major groups of biologically active compounds can be distinguished, namely secondary metabolites and proteins. Many of those products are cytotoxic and it was suggested that laticifers evolved as sequestering compartments, which ensure the storage of such substances regardless of the vascular system. This solution provides a unique and preformed defense mechanism with almost immediate response to herbivory attack. The internal pressure of latex causes the secretion of concentrated active substances at the point of damage in a few seconds. In contrast, an inducible defense system needs hours or even days to synthesize and collect sufficient amounts of active substances to act against pathogens [20]. In the context of latex composition, it is worth noticing that a synergetic mode of action was established for some of its constituents, like terpenes associated with phenolic compounds [20][21] or different proteins exhibiting defense functions against insects or fungi [22][23].
Secondary metabolites are a heterogeneous group of chemical compounds not essential to vegetative growth, but for plant adaptation to changes in the external as well as internal environment. As mentioned before, the presence of specific metabolites in latex is a highly species-specific trait, but in general terpenes, phenolics, alkaloids, and cardenolides are present in most of the laticifer types.
One of the most abundant groups of secondary metabolites in plant latex are alkaloids. Those amino acid derivatives, which are highly bioactive and often toxic, serve eco-physiological functions in plants, providing better fitness to specific environmental niches [24]. Alkaloids for thousands of years have been used and abused by humans, even leading to military conflicts (like opium wars in the 19th century or ongoing drug wars in many countries). Those low molecular compounds were found amongst 35 families, mostly angiosperms, including Apocynaceae, Papaveraceae, and Moracea [8]. The best known and described example of laticifers rich in alkaloids is opium, namely the dried latex of P. somniferum used in folk and traditional medicine, as well as psychedelic drugs. Opium contains at least 20 alkaloids, such as morphine, papaverine, and codeine. Morphine may constitute up to 5% of fresh latex, and codeine up to 1% [25]. Latex of Chelidonium majus, a species closely related to P. somniferum, is also a rich source of bioactive alkaloids, e.g., chelidonine, sanguinarine, berberine or coptisine (chemical structures are depicted on Figure 1). Those isoquinoline alkaloids can reach up to 20% of fresh latex mass [26] and are known for their multiple pharmacological effects (antioxidant, anti-inflammatory, anticancer, anti-neurodegenerative, and antimicrobial). Although C. majus is related to P. somniferum, its latex does not contain morphine-like alkaloids, such as morphine or codeine, and therefore does not have sedative effects. Yet, some of the C. majus secondary metabolites, like berberine and chelidonine, as well as protein enriched extracts can have analgesic effects, similar to morphine [27]
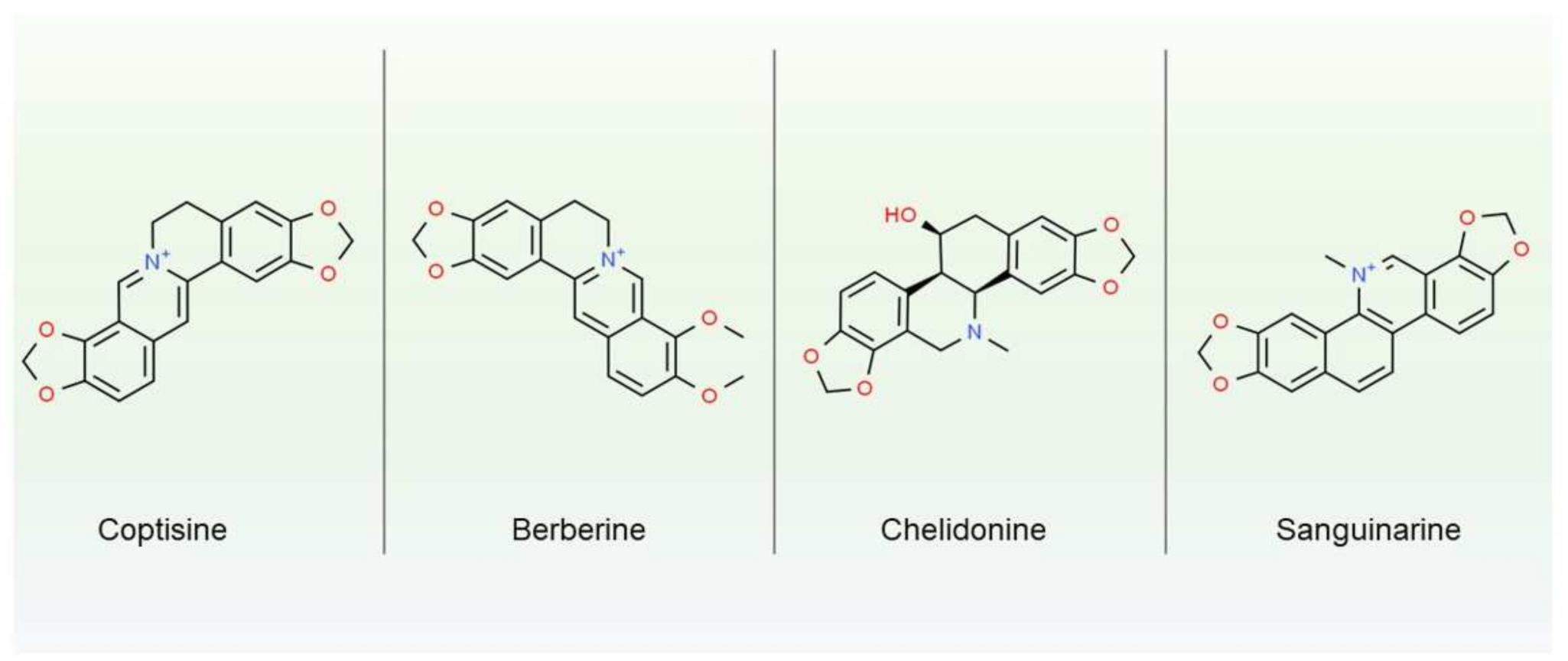
Figure 1. Chemical structure of four most studied alkaloids from Chelidonium majus.
Laticifers are not only a reservoir system for low-molecular weight defense compounds, but as mentioned earlier, constitute a living cell, and as one, has a distinct proteome. A wide range of both constitutive and inducible proteins are present in latex, with huge diversity between different plant species. We can distinguish some common protein functional groups prevalent in different laticiferous plant species, like proteases, protease inhibitors, lectins, oxidases, chitinases, or defense-related proteins.
The exact molecular mechanism responsible for many biomedical properties of laticiferous plant species is still not well established. Yet, the research community managed to define some interesting therapeutic activities and latex active compounds responsible for their occurrence. The advent of new high throughput sequencing technologies and the fast, efficient genome editing system of CRISPR/Cas9 gives the research community the tools necessary to fulfill the broadened gap between supply and demand for medicines of plant origin. One of the greatest constraints to working with latex-bearing plants is insufficient genome information. Exploration of the genome of the plant of interest enables the precise modification and avoidance of off-target mutations. Nevertheless, the application of CRISPR/Cas9 modification sheds a new light on the function of some genes in laticiferous plant species, as well as sets directions for the improvement of agronomically important traits. Moreover, it provides a modern basis for further exploration and pharmacological utilization of latex compounds.
References
- Jesus, A.; Bonhomme, V.; Evin, A.; Ivorra, S.; Soteras, R.; Salavert, A.; Antolín, F.; Bouby, L. A Morphometric Approach to Track Opium Poppy Domestication. Sci. Rep. 2021, 11, 9778.
- Ren, G.; Zhang, X.; Li, Y.; Ridout, K.; Serrano-Serrano, M.L.; Yang, Y.; Liu, A.; Ravikanth, G.; Nawaz, M.A.; Mumtaz, A.S.; et al. Large-Scale Whole-Genome Resequencing Unravels the Domestication History of Cannabis sativa. Sci. Adv. 2021, 7, eabg2286.
- Zielińska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s Ups and Downs—21 Centuries of Medicinal Uses of Chelidonium majus from the Viewpoint of Today’s Pharmacology. Front. Pharmacol. 2018, 9, 299.
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium maju—An Integrative Review: Traditional Knowledge versus Modern Findings. Complementary Med. Res. 2010, 17, 241–248.
- Maji, A.K.; Banerji, P. Chelidonium majus L. (Greater celandine—A Review on Its Phytochemical and Therapeutic Perspectives. Int. J. Herb. Med. 2015, 3, 10–27.
- Nawrot, J.; Wilk-Jędrusik, M.; Nawrot, S.; Nawrot, K.; Wilk, B.; Dawid-Pać, R.; Urbańska, M.; Micek, I.; Nowak, G.; Gornowicz-Porowska, J. Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties. Int. J. Environ. Res. Public Health 2020, 17, 1540.
- Salomé Abarca, L.F.; Klinkhamer, P.G.L.; Choi, Y.H. Plant Latex, from Ecological Interests to Bioactive Chemical Resources. Planta Med. 2019, 85, 856–868.
- Nawrot, R. (Ed.) Latex, Laticifers and Their Molecular Components—From Functions to Possible Applications; Advances in Botanical Research Series; Academic Press: Cambridge, MA, USA, 2020.
- Kekwick, R.G.O. Latex and Laticifers. In Encyclopedia of Life Sciences 2002; John Wiley & Sons: London, UK; New York, NY, USA, 2002.
- Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Laticifers, Latex, and Their Role in Plant Defense. Trends Plant Sci. 2019, 24, 553–567.
- Hagel, J.M.; Yeung, E.C.; Facchini, P.J. Got Milk? The Secret Life of Laticifers. Trends Plant Sci. 2008, 13, 631–639.
- Prado, E.; Demarco, D. Laticifers and secretory ducts: Similarities and differences. In Ecosystem Services and Global Ecology; InTech: London, UK, 2018; ISBN 9781789237382.
- Marinho, C.R.; Teixeira, S.P. Cellulases and Pectinases Act Together on the Development of Articulated Laticifers in Ficus montana and Maclura tinctoria (Moraceae). Protoplasma 2019, 256, 1093–1107.
- Kitajima, S.; Taira, T.; Oda, K.; Yamato, K.T.; Inukai, Y.; Hori, Y. Comparative Study of Gene Expression and Major Proteins’ Function of Laticifers in Lignified and Unlignified Organs of Mulberry. Planta 2012, 235, 589–601.
- Van Veenendaal, W.L.H.; Den Outer, R.W. Distribution and Development of the Non-Articulated Branched Laticifers of Morus nigra L. (Moraceae). Acta Bot. Neerl. 1990, 39, 285–296.
- Webster, C.C. Natural Rubber: Biology, Cultivation and Technology. Agric. Syst. 1994, 45, 233–235.
- Dussourd, D.E. Entrapment of Aphids and Whiteflies in Lettuce Latex. Ann. Entomol. Soc. Am. 1995, 88, 163–172.
- Agrawal, A.A.; Konno, K. Latex: A Model for Understanding Mechanisms, Ecology, and Evolution of Plant Defense against Herbivory. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 311–331.
- Nawrot, R.; Wołuń-Cholewa, M.; Goździcka-Józefiak, A. Nucleases Isolated from Chelidonium majus L. Milky Sap Can Induce Apoptosis in Human Cervical Carcinoma HeLa Cells but Not in Chinese Hamster Ovary CHO Cells. Folia Histochem. Cytobiol. 2008, 46, 79–83.
- Konno, K. Plant Latex and Other Exudates as Plant Defense Systems: Roles of Various Defense Chemicals and Proteins Contained Therein. Phytochemistry 2011, 72, 1510–1530.
- Wink, M. Plant Secondary Metabolism: Diversity, Function and Its Evolution. Nat. Prod. Commun. 2008, 3, 1205–1216.
- Souza, D.P.; Freitas, C.D.T.; Pereira, D.A.; Nogueira, F.C.; Silva, F.D.A.; Salas, C.E.; Ramos, M.V. Laticifer Proteins Play a Defensive Role against Hemibiotrophic and Necrotrophic Phytopathogens. Planta 2011, 234, 183–193.
- Ramos, M.V.; Grangeiro, T.B.; Freire, E.A.; Sales, M.P.; Souza, D.P.; Araújo, E.S.; Freitas, C.D.T. The Defensive Role of Latex in Plants: Detrimental Effects on Insects. Arthropod-Plant Interact. 2010, 4, 57–67.
- Nguyen, T.-D.; Dang, T.-T.T. Cytochrome P450 Enzymes as Key Drivers of Alkaloid Chemical Diversification in Plants. Front. Plant Sci. 2021, 12, 682181.
- Itenov, K.; Mølgaard, P.; Nyman, U. Diurnal Fluctuations of the Alkaloid Concentration in Latex of Poppy Papaver Somniferum Is due to Day–night Fluctuations of the Latex Water Content. Phytochemistry 1999, 52, 1229–1234.
- Tomè, F.; Colombo, M.L. Distribution of Alkaloids in Chelidonium majus and Factors Affecting Their Accumulation. Phytochemistry 1995, 40, 37–39.
- Mikołajczak, P.Ł.; Kędzia, B.; Ożarowski, M.; Kujawski, R.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Białas, W.; Gryszczyńska, A.; Buchwald, W.; Szulc, M.; et al. Evaluation of Anti-Inflammatory and Analgesic Activities of Extracts from Herb of Chelidonium majus L. Cent. Eur. J. Immunol. 2015, 40, 400–410.
More
Information
Subjects:
Biochemistry & Molecular Biology; Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.3K
Revisions:
2 times
(View History)
Update Date:
30 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





